Continuous flow oxidation of benzylic and aliphatic alcohols using bleach: process improvement by precise pH adjustment in flow with CO2†
Abstract
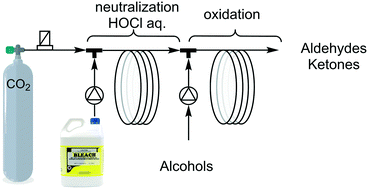
An improved method for the oxidation of benzylic and aliphatic alcohols using concentrated bleach (1.6 M NaOCl) using 2-step continuous flow microreactors is reported herein. Enhancement of the reaction rate was obtained by on-line pH adjustment. In the first step, the pH of the bleach was precisely decreased to pH 8.5 using CO2. Such pH is a compromise between oxidative properties, chemical stability and unwanted precipitation. The aqueous bleach solution was subsequently used for the oxidation of benzyl alcohols in EtOAc using catalytic amounts of TBAB (n-Bu4N+Br−) in a segmented flow microreactor. The total conversion of benzyl alcohols was obtained in less than 1 min using 2 eq. of NaOCl at r.t. without any metal catalysts. Selective benzyl alcohol oxidation (>98%) was demonstrated on primary and secondary alcohols. For aliphatic alcohols, 0.1 mol% TEMPO was used as a co-catalyst in synergy with TBAB and a total conversion with high selectivity (>98%) was achieved in less than 2 min.


 Please wait while we load your content...
Please wait while we load your content...