Open Access Article
Open Access ArticlePhosphine-mediated enantioselective [1 + 4]-annulation of Morita–Baylis–Hillman carbonates with 2-enoylpyridines†
Tao Wang‡
a,
Pengfei Zhang‡ba,
Wenjun Li‡ *c and
Pengfei Li
*c and
Pengfei Li *a
*a
aDepartment of Chemistry and Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China. E-mail: lipf@sustc.edu.cn; flyli1980@gmail.com
bSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, 150080, China
cDepartment of Medicinal Chemistry, School of Pharmacy, Qingdao University, Qingdao, Shandong 266021, China. E-mail: liwj@qdu.edu.cn
First published on 12th December 2018
Abstract
This work describes a phosphine-catalyzed asymmetric [1 + 4] annulation of Morita–Baylis–Hillman carbonates with 2-enoylpyridines for constructing the enantiomerically enriched 2,3-dihydrofuran motif. In the presence of (−)-1,2-bis[(2R,5R)-2,5-dimethylphospholano]benzene, a series of Morita–Baylis–Hillman carbonates reacted with 2-enoylpyridines smoothly to afford a wide range of optically active 2,3-dihydrofurans featuring pyridine motifs in high yields with excellent asymmetric induction.
Recently Morita–Baylis–Hillman (MBH) carbonates have been proved to be diverse reaction partners for the synthesis of a wide variety of carbo- and heterocyclic compounds.1 In particular, MBH carbonates have been successfully employed as C1 synthons in many cyclization reactions for the construction of diverse heterocycles.2 In terms of [1 + 4] annulations between MBH carbonates and α,β-unsaturated carbonyl compounds, Zhang et al. firstly reported a PPh3 catalyzed [1 + 4] annulation of MBH carbonates with activated α,β-unsaturated ketones (enones) to furnish a series of racemic 2,3-dihydrofurans.3 The substituent (e.g., alkyne moiety) at the α-position of the enone was necessary to improve the reactivity of the enone by lowering the energy of the LUMO, which was critical for obtaining a high yield. Two years later, Huang et al. realized the catalyst dosage controlling the product distribution between 2,3-dihydrofurans and biaryls from the phosphine mediated [1 + 4] annulation of MBH carbonates and β,γ-unsaturated α-keto esters.4 In 2014, Shi et al. reported a tunable phosphine-triggered cascade reaction of MBH carbonates and 3-acyl-2H-chromen-2-ones for the synthesis of diverse chromenones.5 Different from [1 + 4] annulation, He et al. disclosed that the PBu3-mediated reactions between MBH carbonates and chalcones underwent either [3 + 2] or [2 + 2 + 1] annulations depending on the substituent variation of both reactants.6 However, in sharp contrast, the reports on enantioselective catalytic annulation of MBH carbonate as C1-synthon are very limited.7 The landmark study reported by Shi and co-workers presented thiourea-phosphines that were efficient catalysts for the asymmetric [1 + 4] annulation of MBH carbonates with activated α,β-unsaturated ketones, although the reaction of MBH carbonates bearing an electron-donating substituent on their aromatic group remained a challenge (Scheme 1A).8 In 2016, Ouyang and Chen et al. remarkably disclosed the first highly enantio- and diastereoselective [1 + 2] annulation reactions of MBH carbonates and 2-alkylidene-1H-indene-1,3(2H)-diones (Scheme 1B).9 Despite their elegant examples, the organocatalytic asymmetric annulations of MBH carbonates as C1-synthons are still far from well-developed.10
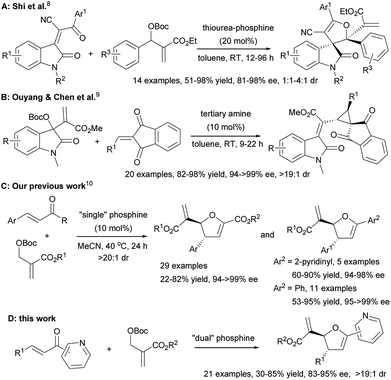 | ||
| Scheme 1 Limited examples of enantioselective annulation of MBH carbonates with α,β-unsaturated ketones. | ||
Very recently, we overcame the restriction to successfully develop a chiral phosphine catalysed [1 + 4] annulation of MBH carbonates with electron-deficient olefins for constructing optitally active 2,3-dihydrofurans (Scheme 1C).11 Furthermore, several cases of 2-enoylpyridines furnished the corresponding products in 60–90% yield with 94–98% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Although the ubiquitous nature of 2-enoylpyridines in enantioselective reactions,12 the systematic study on asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines has not been reported so far. Therefore, developing efficient strategy for the asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines is highly desirable. Herein, we report comprehensive results from the phosphine-catalyzed asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines (Scheme 1D).
1 dr. Although the ubiquitous nature of 2-enoylpyridines in enantioselective reactions,12 the systematic study on asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines has not been reported so far. Therefore, developing efficient strategy for the asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines is highly desirable. Herein, we report comprehensive results from the phosphine-catalyzed asymmetric [1 + 4] annulation of MBH carbonates with 2-enoylpyridines (Scheme 1D).
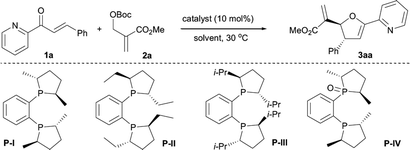
To achieve better yields without compromising the asymmetric induction of the [1 + 4] annulation of MBH carbonates and 2-enoylpyridines, we revisited the prototypical catalyst system (Table 1). Choosing the [1 + 4] annulation of 2-(methoxycarbonyl)allyl tert-butyl carbonate 2a and 3-phenyl-1-(pyridin-2-yl)prop-2-en-1-one 1a as model reaction, we screened a series of phosphine catalysts.13 It was found that (−)-1,2-bis[(2R,5R)-2,5-dimethylphospholano]benzene P-I mediated reaction generated the better results, furnishing the desired product 3aa in 79% yield with 91% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entry 1). Increasing the steric hindrance of catalyst resulted in lowering yields and enantioselectivities (Table 1, entries 2 and 3). The investigations of reaction media indicated that solvent affected the reaction in terms of yield and stereoselectivity (Table 1, entries 5–9), and MeCN was more suitable for the transformation to afford the product 3aa in 77% yield with 94% ee and >19
1 dr (Table 1, entry 1). Increasing the steric hindrance of catalyst resulted in lowering yields and enantioselectivities (Table 1, entries 2 and 3). The investigations of reaction media indicated that solvent affected the reaction in terms of yield and stereoselectivity (Table 1, entries 5–9), and MeCN was more suitable for the transformation to afford the product 3aa in 77% yield with 94% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entry 10). Further optimization of the conditions including reaction temperature, ratio of reactants, and reaction time enabled the formation of 3aa in 81% yield with 95% ee and >20
1 dr (Table 1, entry 10). Further optimization of the conditions including reaction temperature, ratio of reactants, and reaction time enabled the formation of 3aa in 81% yield with 95% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entries 11–14).
1 dr (Table 1, entries 11–14).
| Entry | Cat. | Solvent | t (h) | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: unless noted, a mixture of 1a (0.2 mmol), 2a (0.24 mmol), and catalyst (10 mol%) in the solvent (1.0 mL) was stirred at 30 °C for the time given.b Isolated yield.c dr = diastereomeric ratio, determined by 1H NMR.d Enantiomeric excess (ee) of major enantiomer, determined by chiral HPLC analysis.e Performed at 40 °C.f Performed at 0 °C.g 2a (0.3 mmol) was used. | ||||||
| 1 | P-I | CH2Cl2 | 24 | 79 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 2 | P-II | CH2Cl2 | 24 | 55 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−78 |
| 3 | P-III | CH2Cl2 | 24 | 28 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−48 |
| 4 | P-IV | CH2Cl2 | 24 | 40 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 5 | P-I | CHCl3 | 24 | 56 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 6 | P-I | THF | 24 | 38 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 7 | P-I | Toluene | 24 | 65 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 |
| 8 | P-I | EtOAc | 24 | 51 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 9 | P-I | (CH2Cl)2 | 24 | 71 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 10 | P-I | MeCN | 24 | 77 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 11e | P-I | MeCN | 24 | 69 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 12f | P-I | MeCN | 24 | 57 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 13g | P-I | MeCN | 24 | 69 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 14 | P-I | MeCN | 48 | 81 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
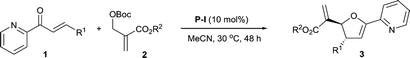
With the optimal reaction conditions determined, we then investigated the substrate scope of this asymmetric [1 + 4] annulation and the results were summarized in Table 2. It was found that the ester group of the MBH carbonates affected the yield of the reaction without any discernible impact on the asymmetric induction. Increasing the size of the ester group led to a decrease in the yield (Table 2, entries 1–3). The substrate scope of 2-enoylpyridines 1 was examined by reactions with MBH carbonate 2a. Importantly, this catalytic strategy was applicable to various substituted 2-enoylpyridines bearing different types of substituents. Both electron-withdrawing (F, Cl, Br, CF3) and electron-donating (MeO) groups were well tolerated to afford the corresponding products 3ba–ja in 75–85% yields with 91–94% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, entries 4–12). Substrates 3-(4-nitrophenyl)-1-(pyridin-2-yl)prop-2-en-1-one 1k and 1-(pyridin-2-yl)-3-p-tolylprop-2-en-1-one 1l were found to react with 2a slowly to furnish 3ka in 30% yield with 91% ee (Table 2, entry 13) and 3la in 52% yield with 92% ee (Table 2, entry 14), respectively. Pleasingly, 3-(naphthalen-3-yl)-1-(pyridin-2-yl)prop-2-en-1-one 1m reacted with 2a smoothly to afford product 3ma in 85% yield with 83% ee and >19
1 dr (Table 2, entries 4–12). Substrates 3-(4-nitrophenyl)-1-(pyridin-2-yl)prop-2-en-1-one 1k and 1-(pyridin-2-yl)-3-p-tolylprop-2-en-1-one 1l were found to react with 2a slowly to furnish 3ka in 30% yield with 91% ee (Table 2, entry 13) and 3la in 52% yield with 92% ee (Table 2, entry 14), respectively. Pleasingly, 3-(naphthalen-3-yl)-1-(pyridin-2-yl)prop-2-en-1-one 1m reacted with 2a smoothly to afford product 3ma in 85% yield with 83% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, entry 15). In addition, the heteroaromatic 1n-o were also compatible to afford the corresponding adducts 3na-oa in 60–63% yield with 94% ee and >19
1 dr (Table 2, entry 15). In addition, the heteroaromatic 1n-o were also compatible to afford the corresponding adducts 3na-oa in 60–63% yield with 94% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, entries 16 and 17). Notably, adduct 3pa was obtained in 64% yield with 92% ee and >19
1 dr (Table 2, entries 16 and 17). Notably, adduct 3pa was obtained in 64% yield with 92% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr from the annulation of 2a with 5-phenyl-1-(pyridin-2-yl)penta-2,4-dien-1-one (Table 2, entry 18). Importantly, the annulation of aliphatic 2-enoylpyridine 1q was also compatible and afforded the desired product 3qa in 61% yield with 92% ee (Table 2, entry 19). It was found that 2-(methoxycarbonyl)-1-phenylallyl tert-butyl carbonate was not compatible and no desired product was obtained (Table 2, entry 20). Taken altogether, our results demonstrate that catalyst P-I is broadly applicable in the asymmetric [1 + 4] annulation of MBH carbonates and 2-enoylpyridines.
1 dr from the annulation of 2a with 5-phenyl-1-(pyridin-2-yl)penta-2,4-dien-1-one (Table 2, entry 18). Importantly, the annulation of aliphatic 2-enoylpyridine 1q was also compatible and afforded the desired product 3qa in 61% yield with 92% ee (Table 2, entry 19). It was found that 2-(methoxycarbonyl)-1-phenylallyl tert-butyl carbonate was not compatible and no desired product was obtained (Table 2, entry 20). Taken altogether, our results demonstrate that catalyst P-I is broadly applicable in the asymmetric [1 + 4] annulation of MBH carbonates and 2-enoylpyridines.
| Entry | R1 | R2 | 3 | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: unless noted, a mixture of 1 (0.2 mmol), 2 (0.24 mmol), and P-I (10 mol%) in MeCN (1.0 mL) was stirred at 30 °C for 48 h.b Isolated yield.c dr = diastereomeric ratio, determined by 1H NMR.d Enantiomeric excess (ee) of major enantiomer, determined by chiral HPLC analysis.e Instead of 2a, 2-(methoxycarbonyl)-1-phenylallyl tert-butyl carbonate was used. | ||||||
| 1 | Ph | Me | 3aa | 81 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 2 | Ph | Et | 3ab | 72 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 3 | Ph | Bn | 3ac | 54 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 4 | 2-BrC6H4 | Me | 3ba | 76 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 5 | 2-MeOC6H4 | Me | 3ca | 75 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 6 | 3-ClC6H4 | Me | 3da | 82 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 7 | 3-BrC6H4 | Me | 3ea | 79 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 8 | 3-MeOC6H4 | Me | 3fa | 79 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 9 | 4-FC6H4 | Me | 3ga | 79 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 10 | 4-ClC6H4 | Me | 3ha | 80 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 11 | 4-BrC6H4 | Me | 3ia | 78 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 12 | 4-CF3C6H4 | Me | 3ja | 85 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 13 | 4-NO2C6H4 | Me | 3ka | 30 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 14 | 4-MeC6H4 | Me | 3la | 52 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 15 | 2-Naphthyl | Me | 3ma | 85 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 |
| 16 | 2-Thienyl | Me | 3na | 63 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 17 | 2-Pyridinyl | Me | 3oa | 60 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 |
| 18 | PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
Me | 3pa | 64 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 19 | Me | Me | 3qa | 61 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 20e | Ph | Me | 3ad | — | — | — |
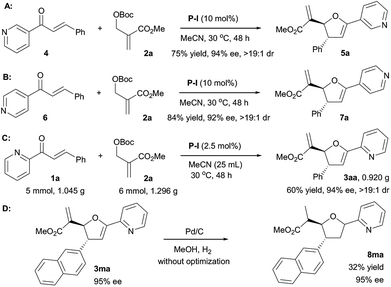
In order to further explore the scope of the [1 + 4] annulation, other α,β-unsaturated pyridinyl ketones were also surveyed (Scheme 2). Under the standard conditions, 3-phenyl-1-(pyridin-3-yl)prop-2-en-1-one 4 reacted with MBH carbonate 2a smoothly to afford the product 5a in 75% yield with 94% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2A). The P-I mediated [1 + 4] annulation of MBH carbonate 2a with 3-phenyl-1-(pyridin-4-yl)prop-2-en-1-one 6 also furnished the desired 7a in 84% yield with 92% ee and >19
1 dr (Scheme 2A). The P-I mediated [1 + 4] annulation of MBH carbonate 2a with 3-phenyl-1-(pyridin-4-yl)prop-2-en-1-one 6 also furnished the desired 7a in 84% yield with 92% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2B). Notably, these results indicated that the pyridinyl group of α,β-unsaturated ketones has no effect on the stereoselectivity of the reaction. To highlight the synthetic potential of the catalytic system, we also evaluated the gram-scale synthesis of 3aa. In the presence of P-I with a loading of 2.5 mol% in MeCN of 25 mL at 30 °C for 48 h, 5.0 mmol of 1a (1.045 g) reacted smoothly with 6.0 mmol of 2a (1.296 g), affording 3aa in 60% yield (0.920 g) with 94% ee and >19
1 dr (Scheme 2B). Notably, these results indicated that the pyridinyl group of α,β-unsaturated ketones has no effect on the stereoselectivity of the reaction. To highlight the synthetic potential of the catalytic system, we also evaluated the gram-scale synthesis of 3aa. In the presence of P-I with a loading of 2.5 mol% in MeCN of 25 mL at 30 °C for 48 h, 5.0 mmol of 1a (1.045 g) reacted smoothly with 6.0 mmol of 2a (1.296 g), affording 3aa in 60% yield (0.920 g) with 94% ee and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2C). As mentioned in our previous work,10 the product 3ma could be hydrogenated and tetrahydrofuran 8ma was obtained in 32% yield with 95% ee (Scheme 2D).
1 dr (Scheme 2C). As mentioned in our previous work,10 the product 3ma could be hydrogenated and tetrahydrofuran 8ma was obtained in 32% yield with 95% ee (Scheme 2D).
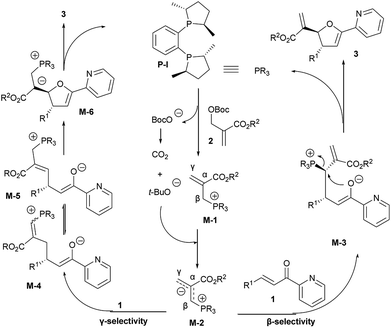
The absolute configurations of chiral 2,3-dihydrofurans 3 were confirmed according to the products from the [1 + 4] annulation between MBH carbonates and α,β-unsaturated ketones under the same conditions.10 Accordingly, two proposed reaction pathways were shown in Scheme 3. An initial SN2 attack of nucleophilic chiral phosphine catalyst P-I on the MBH carbonate 2 triggered the elimination of the leaving group (BocO−) delivering the chiral phosphonium salt intermediate M-1, which was then deprotonated by an in situ-generated base (BocO− = CO2 + t-BuO−) to give chiral allylic phosphorus ylide M-2. The β-selective reaction of M-2 with 2-enoylpyridine 1 furnished intermediate M-3, followed by cyclization to generate the desired product 3.9 Alternatively, the ylide M-2 reacted with 1 via γ-selectivity to afford intermediate M-4, which interconverts with intermediate M-5.2b,2d,7b Then an intramolecular Michael addition of M-5 afforded intermediate M-6. Finally, M-6 engaged in elimination of the chiral phosphine P-I to give the annulation product 3.
In conclusion, we have successfully developed an efficient organocatalytic asymmetric [1 + 4] annulation of MBH carbonates and 2-enoylpyridines. With (−)-1,2-bis[(2R,5R)-2,5-dimethylphospholano]benzene as catalyst, the reactions proceed well to furnish a series of chiral 2,3-dihydrofurans featuring pyridine motifs in high yields and excellent stereoselectivities. Importantly, the reaction was successfully extended to other α,β-unsaturated pyridinyl ketones without compromising the yields and asymmetric induction.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21871128), Special Funds for the Development of Strategic Emerging Industries in Shenzhen (JCYJ20170817110526264), the National Key Research and Development Program of China (2016YFA0501403), and the Shenzhen Nobel Prize Scientists Laboratory Project (C17783101).Notes and references
- For selected examples, see: (a) B. Tan, N. R. Candeias and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 4672 CrossRef CAS PubMed; (b) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMedfor some reviews, see: (c) V. Declerck, J. Martinez and F. Lamaty, Chem. Rev., 2009, 109, 1 CrossRef CAS PubMed; (d) G.-N. Ma, J.-J. Jiang, M. Shi and Y. Wei, Chem. Commun., 2009, 5496 RSC; (e) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (f) P. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC.
- (a) P. Xie, Y. Huang and R. Chen, Org. Lett., 2010, 12, 3768 CrossRef CAS PubMed; (b) J. Tian, R. Zhou, H. Sun, H. Song and Z. He, J. Org. Chem., 2011, 76, 2374 CrossRef CAS PubMed; (c) R. Zhou, C. Duan, C. Yang and Z. He, Chem.–Asian J., 2014, 9, 1183 CrossRef CAS PubMed; (d) Y. Lei, X.-N. Zhang, X.-Y. Yang, Q. Xu and M. Shi, RSC Adv., 2015, 5, 49657 RSC; (e) Z. Qin, W. Liu, D. Wang and Z. He, J. Org. Chem., 2016, 81, 4690 CrossRef CAS PubMed.
- Z. Chen and J. Zhang, Chem.–Asian J., 2010, 5, 1542 CrossRef CAS PubMed.
- (a) P. Xie, Y. Huang and R. Chen, Chem.–Eur. J., 2012, 18, 7362 CrossRef CAS PubMed; (b) P. Xie, E. Li, J. Zheng, X. Li, Y. Huang and R. Chen, Adv. Synth. Catal., 2013, 355, 161 CrossRef CAS; (c) P. Xie, J. Yang, J. Zheng and Y. Huang, Eur. J. Org. Chem., 2014, 1189 CrossRef CAS.
- W. Yuan, H.-F. Zheng, Z.-H. Yu, Z.-L. Tang and D.-Q. Shi, Eur. J. Org. Chem., 2014, 583 CrossRef CAS.
- R. Zhou, J. Wang, H. Song and Z. He, Org. Lett., 2011, 13, 580 CrossRef CAS PubMed.
- (a) X.-N. Zhang, H.-P. Deng, L. Huang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 8664 RSC; (b) H. Li, J. Luo, B. Li, X. Yi and Z. He, Org. Lett., 2017, 19, 5637 CrossRef CAS PubMed; (c) F. Jiang, G.-Z. Luo, Z.-Q. Zhu, C.-S. Wang, G.-J. Mei and F. Shi, J. Org. Chem., 2018, 83, 10060 CrossRef CAS PubMed.
- F.-L. Hu, Y. Wei and M. Shi, Chem. Commun., 2014, 50, 8912 RSC.
- G. Zhan, M.-L. Shi, Q. He, W.-J. Lin, Q. Ouyang, W. Du and Y.-C. Chen, Angew. Chem., Int. Ed., 2016, 55, 2147 CrossRef CAS PubMed.
- For asymmetric [4 + 1]-annulations of electron-deficient olefins with different partners, see: (a) M.-W. Chen, L.-L. Cao, Z.-S. Ye, G.-F. Jiang and Y.-G. Zhou, Chem. Commun., 2013, 49, 1660 RSC; (b) B. Wu, M.-W. Chen, Z.-S. Ye, C.-B. Yu and Y.-G. Zhou, Adv. Synth. Catal., 2014, 356, 383 CrossRef CAS; (c) X.-L. Jiang, S.-J. Liu, Y.-Q. Gu, G.-J. Mei and F. Shi, Adv. Synth. Catal., 2017, 359, 3341 CrossRef CAS; (d) X.-L. Lian, A. Adili, B. Liu, Z.-L. Tao and Z.-Y. Han, Org. Biomol. Chem., 2017, 15, 3670 RSC.
- Y. Cheng, Y. Han and P. Li, Org. Lett., 2017, 19, 4774 CrossRef CAS PubMed.
- For a comprehensive review, see: (a) G. Desimoni, G. Faita and P. Quadrelli, Chem. Rev., 2014, 114, 6081 CrossRef CAS PubMedfor selected organocatalytic examples, see: (b) N. Molleti, N. K. Rana and V. K. Singh, Org. Lett., 2012, 14, 4322 CrossRef CAS PubMed; (c) N. Molleti, S. Allu, S. K. Ray and V. K. Singh, Tetrahedron Lett., 2013, 54, 3241 CrossRef CAS; (d) N. Molleti and V. K. Singh, Org. Biomol. Chem., 2015, 13, 5243 RSC; (e) S. Mukherjee, S. Mondal, A. Patra, R. G. Gonnade and A. T. Biju, Chem. Commun., 2015, 51, 9559 RSC; (f) Z.-H. Wang, Z.-J. Wu, X.-Q. Huang, D.-F. Yue, Y. You, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Chem. Commun., 2015, 51, 15835 RSC; (g) Z.-H. Wang, Z.-J. Wu, D.-F. Yue, Y. You, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Biomol. Chem., 2016, 14, 6568 RSC; (h) B. Cui, Y. Chen, J. Shan, L. Qin, C. Yuan, Y. Wang, W. Han, N. Wan and Y. Chen, Org. Biomol. Chem., 2017, 15, 8518 RSC.
- For reviews on phosphine catalysis, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (c) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (d) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (e) S.-X. Wang, X. Han, F. Zhong, Y. Wang and Y. Lu, Synlett, 2011, 19, 2766 Search PubMed; (f) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (g) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (h) Y. Xiao, Z. Sun, H. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (i) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed; (j) H. Li and Y. Lu, Asian J. Org. Chem., 2017, 6, 1130 CrossRef CAS; (k) H. Ni, W.-L. Chan and Y. Lu, Chem. Rev., 2018, 118, 9344 CrossRef CAS PubMed; (l) H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09453e |
| ‡ The two authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2018 |