Open Access Article
Open Access ArticleInfluence of the acidity of solid catalyst HSO3-ZSM-5 on the hydrolysis of pretreated corncob†
Nguyen Hoang Chunga,
Le Quang Diena,
Thai Dinh Cuonga,
Nguyen Van Lieub and
Phan Huy Hoang *a
*a
aSchool of Chemical Engineering, Hanoi University of Science & Technology, No. 1, Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn; Tel: +84-4-38684955
bVietnam Institute for Building Materials, 235, Nguyen Trai Street, Hanoi, Vietnam
First published on 14th December 2018
Abstract
This work aimed to investigate the application of a solid acid catalyst, a replacement for mineral acids or enzymes, to biomass conversion for further applications. Sulfonated zeolite, HSO3-ZSM-5, was successfully synthesized and characterized by several analysis techniques. The obtained catalyst showed high activity and efficiency in the hydrolysis of pretreated corn cob. Moreover, the acidity of the zeolite product positively influenced the biomass conversion. The influences of reaction parameters such as catalyst loading, reaction time and temperature on the hydrolysis were also established. Under suitable conditions, a hydrolysis yield of ∼54% was achieved. This recyclable solid acid catalyst provided a promising potential for applications in many industrially important hydrolysis processes of biomass.
Introduction
Catalysts are generally classified into two classes. Homogeneous catalysts possess many advantages including high activity and great ability to diffuse in solutions. However, they also have many drawbacks such as difficulties in separation and recovery, corrosion hazards, severe reaction conditions, and environmental pollution. Using heterogeneous catalysts, which are recyclable at low energy consumption can be considered as a move toward a more environmentally sustainable process.1–5In the class of heterogeneous catalysts, zeolite materials have been studied and applied widely in many applications. Possessing many advantages such as high surface area, low-toxicity, and functional groups for grafting or attachment, zeolites have been used effectively in industry as ion exchangers, adsorbents, separators, and catalysts for chemical reactions.6–8 Among a variety of zeolites, ZSM-5 has been used most popularly. On the one hand, the material has strong acidity, high selectivity thanks to its special micro–meso porous structure. On the other hand, ZSM-5 is able to change its acidity and active site. The material, therefore, has become an effective catalyst, which is favorable for organic chemical conversions.9,10 Catalytic properties strongly depend on acidity and particles size of zeolites. It is well-known that zeolite acidity is reflected by the quantity and strength of active sites. Therefore, catalytic activity and selectivity of a zeolite can be enhanced by improving its acidity. This can be done by introducing functional groups to the structure of zeolite including surfaces and pores.11–13 The modified zeolites then show better catalytic activity, acidity, and selectivity in a series of importantly industrial reactions and processes. Yeong et al.11 successfully introduced 3-mercaptopropyltrimethoxysilane into the silicalite-1 pore-structure by using in situ deposition method and subsequently oxidized to sulfonic acid silicalite-1 membrane. The sulfonated silicalite-1 membrane was acting as a membrane with high selectivity and applicable to combined separation and reaction processes. Felice et al. synthesized faujasites with sulfonic groups attached, then applied it for water/methanol sorption and proton conductivity.12 The material showed increased proton conductivity and high selectivity in water/methanol sorption. It, therefore, can be conclude that functionalization of zeolites, especially with sulfonic groups would enhance several properties such as selectivity and catalytic activity. However, there has not been any publication regarding the application of sulfonation of ZSM-5 zeolite catalyst for biomass hydrolysis yet.
Energy demand has been raising rapidly, while traditional natural resources are becoming exhausted. Facing this urgent circumstance, biomass can be considered as a sustainable alternative source, which is also environmentally friendly, for energy.2,5 Recently, there have been many efforts focusing on the use of catalysts and solvents to improve the efficiency in hydrolysis and/or conversion of lignocellulosic biomass. Zhang et al.14 have successfully produced heteromolybdic acid-based catalysts, which effectively hydrolyzed and converted cellulose into glycolic acid. Heterogeneous cesium hydrogen phosphotungstate-supported Au catalyst (Au/Cs2HPW12O40) developed by Zhang et al.15 showed high selectivity in the hydrolysis and conversion of cellulose into gluconic acid. In another work,16 it was able to transform raw biomass to over 67% of formic acid by using Keggin-type vanadium-substituted phosphomolybdic acid catalyst. Among a variety of solid acid catalysts, sulfonated solid catalyst has also been studied widely.2,3,5,17–24 For examples, Akiyama et al.2 has successfully synthesized a polymer attached with porous sulfonic acid groups and applied it for cellulose hydrolysis reactions. The new catalyst possessed good resistance to vapor at high temperature; high acidity for the cellulose hydrolysis. Furthermore, Onda et al.18 showed potential results in the hydrolysis of cellulose to glucose using solid acid catalyst. Saw dust, a biomass residue, was used by Liu et al.20 as a carbon source for production of solid acid catalyst based on magnetic porous carbon by a combination of pyrolysis and sulfonation. Their obtained catalyst showed high catalytic activity in sugar hydrolysis.
This work aims to apply sulfonated ZSM-5 zeolite catalyst for hydrolysis reaction of lignocellulosic biomass. Influences of acidity of the HSO3-ZSM-5 on efficiency of the biomass hydrolysis are evaluated. Furthermore, other factors affecting the lignocellulose hydrolysis yield including reaction time and temperature are also investigated. The synthesized HSO3-ZSM-5 zeolite shows high acidity and catalytic activity in the biomass hydrolysis. These outstanding properties are achieved thanks to the zeolitic structure itself as well as strong sulfonic acid groups that are introduced by sulfonation.
Experimental
Materials
Used chemicals were all analytical grades originated from Sigma-Aldrich including tetraethyl orthosilicate (TEOS); tetra propyl ammonium hydroxide (TPAOH); potassium hydroxide (KOH); sodium aluminate (NaAlO2); cetyltrimethyl ammonium bromide (CTAB – C19H42BrN); 3-mercaptopropyl trimethoxysilane (MPTS); hydrogen peroxide (H2O2).Dry corncob collected from the North of Vietnam was used for preparation of biomass feedstock. The corncob was subject to ethanol extraction following the procedure described in T 204 cm-97 TAPPI standard. The extraction lasted for 6 h to ensure that all extractives were dissolved and removed. The pretreated corncob was stored at room temperature for further investigations.
Synthesis of sulfonated zeolite HSO3-ZSM-5
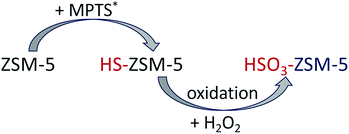
ZSM-5 zeolite was synthesized by using templating agents according to Hoang et al.8 In addition, cetyl trimethylammonium bromide (CTAB – C19H42BrN) was used as a surfactant to obtain mesoporous structure of zeolite. The resulted ZSM-5 was then subject to –HS attachment reaction before oxidizing these –HS groups to –HSO3 in the final product. The synthesis consists of two stages as described in Scheme 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, and 1
2.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Toluene was used as a solvent. The mixtures were then heated and stirred within 6 h. After this time, the solid part was separated from the liquid phase by centrifugation. It was then washed for several times with ethanol and distilled water or until the catalyst gained its white color. Finally, the catalyst was dried at 90 °C to remove all the remaining water.
3. Toluene was used as a solvent. The mixtures were then heated and stirred within 6 h. After this time, the solid part was separated from the liquid phase by centrifugation. It was then washed for several times with ethanol and distilled water or until the catalyst gained its white color. Finally, the catalyst was dried at 90 °C to remove all the remaining water.Biomass hydrolysis catalyzed by HSO3-ZSM-5 zeolite
The hydrolysis was conducted in a 150 mL autoclave reactor. 0.5 g of pretreated corncob was weighed and placed in the reactor. A certain amount of the catalyst and water were added to reach desired solid/liquid ratios. The reactor was placed in an oil bath, which was set at 100–130 °C and maintained at the reaction temperature for a certain period (4–8 h).After the hydrolysis, the reactor was cooled down to room temperature, and then the hydrolysate was separated from reaction mixture using filter papers. Reducing sugars of the hydrolysate were analyzed with HPLC. The solid residue was washed, dried for determination of dry yield of the hydrolysis.
Structural characterizations
X-ray diffraction (XRD) patterns of all samples were recorded with a D8-Advance X-ray Diffractometer equipped with a scintillation counter detector with Cu-Kα radiation. Other parameters were wavelength k = 1.5406 Å, voltage 30 kV, current intensity 25 mA, scanning angle 2θ changed from 5° to 50°, scanning rate 2° min−1, room temperature 25 °C. Scanning electron microscope/energy dispersive X-ray spectroscopy (SEM-EDS) were performed with FE-SEM JEOL JSM-7600F (Japan). Sample was subject to several steps of preparation including dispersion in ethanol, drying, coating onto sample holder, coating with a very thin layer of gold onto surface. Infrared spectra (IR) of samples were recorded with an FTIR NICOLET 6700 NRX RAMAN MODULE – THERMO using KBr pellet method (1 mg of sample per 100 mg of KBr). Brunauer–Emmett–Teller (BET) analysis using a Quantachrome NOVA 4200 E Porosimeter analyzer was used to determine mesoporous properties of the samples. NH3-temperature programmed desorption (TPD) was used to determine the amount and strength of the acid sites with Micrometrics 2900 instrument with a thermal conductivity detector. Approx. 0.20 g of sample was saturated with NH3 at 120 °C, then flushed with helium to remove all physically adsorbed NH3. Finally, the desorption of NH3 was carried out at temperature ranging from 100 to 550 °C with heating rate of 10 °C min−1.Basic components of the pretreated corncob were characterized following TAPPI methods.
After the hydrolysis reaction, the hydrolysate was centrifuged to recover the supernatant. Total reducing sugars (TRS) were analyzed with an HPLC (High Performance Liquid Chromatography) Agilent 1200 equipped with G1322A vacuum pump, Aminex HPX-87P column. Working conditions were controlled as follows: temperature 5–50 °C, humidity 5–96%, and using GLP/GMP compliance as standard. The stationary phase was in lead form with particle size of 9 μm, 8% cross linkage; mobile phase was ionized water which was filtered through a 0.45 μm filter and degassed, working well at pH 5–9. Obtained data were analyzed with Agilent 2D LC ChemStation software (G2170BA). The measurements were conducted at the Laboratory of Food Technology (Hanoi University of Science and Technology).
Results and discussion
Synthesis and characterization of solid acid catalysts HSO3-ZSM-5 with different acidity
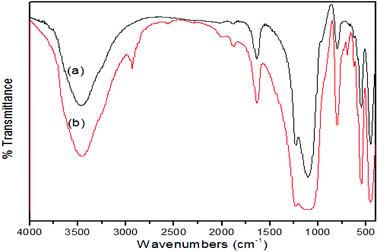
Mesoporous ZSM-5 zeolite was synthesized by a hydrothermal method and then the obtained zeolite was subject to a 2-phase reaction to attach HSO3– onto its structure. The reaction resulted in a sulfonated zeolite (HSO3-ZSM-5), whose structure was characterized by XRD, IR spectroscopy, while EDS technique helped confirm the presence of HSO3 groups based on S content of the zeolite. XRD patterns (Fig. S1, ESI†) of the mesoporous HSO3-ZSM-5 zeolite particles show an MFI-type zeolitic structure with high crystallinity and well-defined diffraction peaks of a high structural order. The as-obtained HSO3-ZSM-5 particles after the sulfonation are pure without any other non-zeolitic phases as seen from the XRD pattern. There are almost no alterations on structure and crystallinities of the zeolite as confirmed by XRD characterization.According to the IR spectra (Fig. 1), the sample possesses all specific vibration spectra of a zeolite structure. The broad peak at around 3440 cm−1 is OH stretching of water while the peak at around 1635 cm−1 is bending mode of OH of water absorbed in the channels of zeolites.10 The asymmetric stretching of Si–O–Si or Si–O–Al bonds with frequency band at 1099 cm−1, the most prominent in the IR spectra is also observed.8 In addition, the band observed at around 450 cm−1 and at 550 cm−1 are assigned to internal vibrations of TO (tetrahedrons) and vibrations of the secondary building units, respectively.8 As seen in Fig. 1B, there are extra new peaks with broadband around 2400–2900 cm−1 assignable to the strong hydrogen bond between SO3H groups1 and new peaks at around 1050 cm−1 and 650 cm−1 which is assigned to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching and S–O stretching, respectively.12 This proves that sulfonic groups were successfully introduced into the surfaces of the ZSM-5 zeolite forming HSO3-ZSM-5.
O symmetric stretching and S–O stretching, respectively.12 This proves that sulfonic groups were successfully introduced into the surfaces of the ZSM-5 zeolite forming HSO3-ZSM-5.
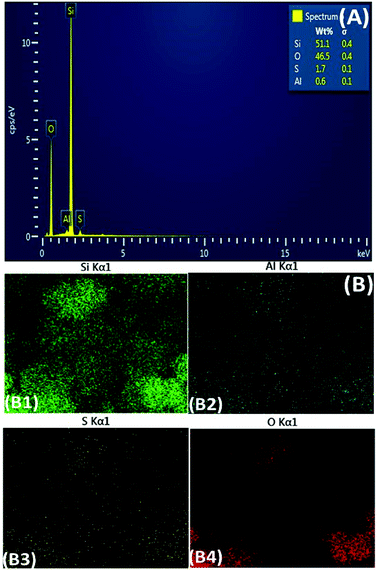
These results are once again confirmed by EDS data as shown in Fig. 2. According to Fig. 2(A), 0.5% of S is present in the sample, while no S is detected in the non-modified zeolite (data not shown). Even distribution of all elements, especially S observed in Fig. 2(B) indicates that S has been successfully and effectively introduced to the zeolite structure with high dispersion (Fig. 2, B3).
N2 adsorption and desorption isotherms of the HSO3-ZSM-5 zeolite (Fig. S2, ESI†) indicate a relatively large diameter of the mesopore. The BET surface area and pore diameter of the HSO3-ZSM-5 zeolite (468 m2 g−1 and 5.6 nm, respectively) are slightly lower than those of the non-modified ZSM-5 zeolite (487 m2 g−1 and 5.8 nm, respectively). This suggests that the bulky sulfonic groups are successfully incorporated on the HSO3-ZSM-5 pore surface and they do not change the pore structure of the zeolite.
Effect of sulfonation temperature
Temperature mainly influences any chemical process. In this work, influence of temperature of the sulfonation was investigated by changing the treatment temperature while maintaining other conditions. The obtained product, HSO3-ZSM-5, was elementally analyzed using energy-dispersive X-ray spectroscopy (EDS) for determination of S (in HSO3) attached on ZSM-5 particles. On the other hand, NH3-TPD was utilized to investigate the concentration and strength of acid sites by the amount of desorbed NH3 and the location of desorption peak. The results are presented in Table 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite mass ratio was 1
zeolite mass ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reaction time was 6 h)
1 and reaction time was 6 h)
| Sample | Temperature, °C | S content, % | Acidity, mmol g−1 |
|---|---|---|---|
| M1 | 50 | 0.1 | 0.66 |
| M2 | 60 | 0.6 | 1.52 |
| M3 | 70 | 0.5 | 1.40 |
According to the results shown in Table 1, when increasing temperature from 50 °C to 60 °C, sulfur content in the product is enhanced from 0.1% to 0.6%, respectively. This is obvious since increased temperature supports the reaction making more MPTS attached on the zeolite surfaces. However, when temperature reaches 70 °C, S content reduces to 0.5%. This can be explained by the fact that high temperature causes solvent (toluene) to evaporate, which does not favor the reaction by reducing the contact of the agent to the zeolite surfaces. Suitable reaction temperature is, therefore, chosen at 60 °C. On the other hand, the acidity is directly proportional to the amount of HSO3 groups, which is reflected in S content. The highest acidity of 1.52 mmol g−1 is obtained when conducting the synthesis at 60 °C. Lower or higher temperature leads to reduction in this parameter. Sample M2 was, therefore, chosen for further investigation.
Effect of MPTS amount
Influences of MPTS dosages on the sulfonation was also investigated by adjusting the amount of MPTS used while other conditions were kept constant. For this purpose, M2 sample obtained from the previous section was subject to sulfonation using different MPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite ratios. The processes resulted in corresponding products namely M2-1, M2-2, M2-3, and M2-4. Table 2 presents S content in correlation with MPTS amount of samples.
zeolite ratios. The processes resulted in corresponding products namely M2-1, M2-2, M2-3, and M2-4. Table 2 presents S content in correlation with MPTS amount of samples.
| Sample | MPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite mass ratio zeolite mass ratio |
S content, % | Acidity, mmol g−1 |
|---|---|---|---|
| M2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.6 ± 0.05 | 1.52 |
| M2-1 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.9 ± 0.05 | 1.87 |
| M2-2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.2 ± 0.05 | 2.13 |
| M2-3 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7 ± 0.05 | 2.89 |
| M2-4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.4 ± 0.05 | 2.45 |
As seen from Table 2, increasing MPTS dosage, i.e. MPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite ratio, results in a significant enhancement of S content of the HSO3-ZSM-5. When using the same amount of MPTS and zeolite (1
zeolite ratio, results in a significant enhancement of S content of the HSO3-ZSM-5. When using the same amount of MPTS and zeolite (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), S content of the product is approx. 0.6%. Doubling the MPTS amount, i.e. 2
1 ratio), S content of the product is approx. 0.6%. Doubling the MPTS amount, i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the content of this element rises to 1.2%. S content of the HSO3-ZSM-5 zeolite reaches its highest value of 1.7% when the amount of MPTS is 2.5 times greater than the zeolite. This can be explained based on a well-known theory of reaction dynamics, which states that concentration of a reactant (i.e. dosage) is directly proportional to reaction rate as well as efficiency of the reaction. However, when the ratio is 3
1 ratio, the content of this element rises to 1.2%. S content of the HSO3-ZSM-5 zeolite reaches its highest value of 1.7% when the amount of MPTS is 2.5 times greater than the zeolite. This can be explained based on a well-known theory of reaction dynamics, which states that concentration of a reactant (i.e. dosage) is directly proportional to reaction rate as well as efficiency of the reaction. However, when the ratio is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, S content reduces to 1.6%. This indicates that the amount of S in the zeolite may reach a saturation level. Once this state is reached, more reactant added will not provide any enhancement to S content in the zeolite. On the other hand, this sometimes might create negative effect.
1, S content reduces to 1.6%. This indicates that the amount of S in the zeolite may reach a saturation level. Once this state is reached, more reactant added will not provide any enhancement to S content in the zeolite. On the other hand, this sometimes might create negative effect.
The dependency of the HSO3-ZSM-5 acidity on S content is once again confirmed. The acidity is directly proportional to S content in the zeolite and reaches its highest value of 2.89 mmol g−1 at the MPTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite ratio of 2.5
zeolite ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Lignocellulose hydrolysis
HSO3-ZSM-5 zeolite catalysts with different contents of sulfonic group corresponding to different acidities were selected to investigate their influences on biomass hydrolysis. The pretreated corncob containing of 30.1% of cellulose, 38.6% of pentosan, and 19.3% of lignin was used as lignocellulosic biomass in the hydrolysis experiment. The results regarding S content and yield of reducing sugar are presented in Table 3.| Catalyst sample code | M2 | M2-1 | M2-2 | M2-3 | M2-4 |
| S content, % | 0.6 | 0.9 | 1.2 | 1.7 | 1.4 |
| Reducing sugar yield, % | 49.3 | 51.4 | 52.8 | 54.1 | 53.2 |
According to Table 3, sulfur content (i.e. the amount of HSO3– groups attached to the ZSM-5 zeolite particles) clearly affects the hydrolysis yield. When S content of the catalyst increases from 0.6% to 1.7%, the yield of reducing sugars also increases from 49.4% to 54.1%, respectively. The reducing sugar yield reaches its highest value of 54.1% when S content is 1.7%. This is obvious since S content is directly proportional to the amount of HSO3 groups attached to the ZSM-5 particles, which improves acidity of the sulfonated catalyst. As a result, the hydrolysis of glycosidic linkages in the lignocellulosic material is improved significantly. Sample M2-3 with 1.7% of S was, therefore, selected as the catalyst for hydrolyzing the biomass in further investigations.
In order to investigate the effect of catalyst loading on the yield of reducing sugar, three experiments were carried out at three catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) biomass ratios of 0.5
biomass ratios of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1.5
1, and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The obtained results show that the higher loading of catalyst the higher reducing sugar yield could be achieved. In particular, the yield of sugar is 38.9%, 54.1%, and 55.0%, respectively. When increasing the amount of catalyst from 1
1. The obtained results show that the higher loading of catalyst the higher reducing sugar yield could be achieved. In particular, the yield of sugar is 38.9%, 54.1%, and 55.0%, respectively. When increasing the amount of catalyst from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1.5
1 to 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the yield of sugar is slightly increased. However, due to economic issues, 1
1 ratio, the yield of sugar is slightly increased. However, due to economic issues, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of catalyst loading is chosen for next experiments. Moreover, two reactions were carried out under similar reaction conditions for comparison: one without catalyst and one with parent ZSM-5 zeolite catalyst. The results show that only a small amount of the corncob is hydrolyzed in the absence of catalyst as well as in presence of parent ZSM-5 zeolite. When using original ZSM-5 catalyst, ∼19% of reducing sugars are achieved. Without catalyst, the corncob biomass is partly hydrolyzed (about 8% of reducing sugars are obtained) due to dissolution of low molecular weight hemicelluloses.
1 ratio of catalyst loading is chosen for next experiments. Moreover, two reactions were carried out under similar reaction conditions for comparison: one without catalyst and one with parent ZSM-5 zeolite catalyst. The results show that only a small amount of the corncob is hydrolyzed in the absence of catalyst as well as in presence of parent ZSM-5 zeolite. When using original ZSM-5 catalyst, ∼19% of reducing sugars are achieved. Without catalyst, the corncob biomass is partly hydrolyzed (about 8% of reducing sugars are obtained) due to dissolution of low molecular weight hemicelluloses.
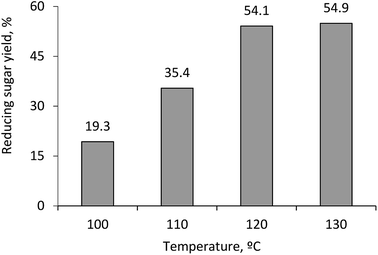
Normally, temperature positively affects reaction yield as increased temperature results in increased reaction rate. In this work, influences of reaction temperature of the corncob hydrolysis producing single sugars was also studied. The results are illustrated in Fig. 3.
As can be seen from Fig. 3, when increasing temperature from 100 °C to 130 °C, the reducing sugar yield increases from 19.3% to 54.9%. The highest yield of reducing sugar is 54.9% when conducting the reaction at 130 °C. However, when temperature is increased for 10 °C from 120 to 130 °C, the yield of reducing sugar only enhances from 54.1% to 54.9%, i.e. approx. 0.8%. Based on economic benefits and technical issues, 120 °C was chosen as an appropriate temperature for the hydrolysis of the corn cob catalyzed by the synthesized HSO3-ZSM-5.
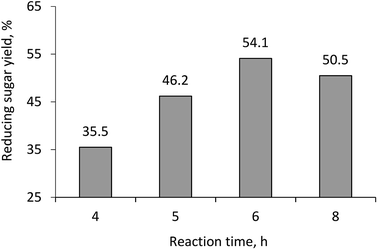
Reaction time is one of important factors that affects any chemical process. To investigate the influences of reaction time on the reducing sugar yield of the biomass hydrolysis, the reaction time was varied from 4 to 8 h. According to the results illustrated in Fig. 4, the reducing sugar yield is almost proportional to the time when other conditions such as temperature, catalyst dosage and solid/liquid ratio were kept constant. The yield corresponding to the time of 4, 5, and 6 h were 35.5, 46.2, 54.1%, respectively. However, when the hydrolysis was conducted for longer time of 8 h, the yield tends to slightly decrease from 54.1% to 50.5%. It can be explained by the fact that the reducing sugars are partly degraded and converted into other substances when lengthening the reaction time.18 Therefore, 6 h was chosen as suitable reaction time for the hydrolysis.
Interestingly, besides glucose, xylose and arabinose were also obtained after the hydrolysis of corncob biomass. It is can be explained that hemicellulose of corncob contains various polysaccharides such as xylan and arabinan. Under conditions of temperature: 120 °C; time: 6 h; and catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) biomass ratio: 1
biomass ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of glucose, xylose and arabinose in reducing sugars was determined as 23, 28.7 and 2.4%, respectively.
1, the yield of glucose, xylose and arabinose in reducing sugars was determined as 23, 28.7 and 2.4%, respectively.
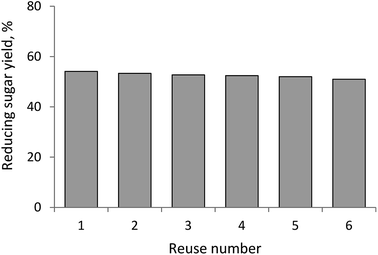
Furthermore, sample M2-3 was subject to several runs of biomass hydrolysis to evaluate its recycling ability. Results (Fig. 5) show that after the sixth run, the obtained amount of reducing sugars is about 52%, quite close to the first run with about 54% of reducing sugars. This indicates that the synthesized catalyst can be recycled for several times without losing its catalytic activity.
Conclusions
In processing of lignocellulosic materials for production of valuable products, the hydrolysis of carbohydrates such as cellulose, hemicelluloses forming monosaccharides or single sugars is regarded as a necessary first step. The single sugars can be further catalytically converted into a variety of intermediates, chemicals, and fuels. In this work, solid acid catalyst as an alternative to mineral acids or enzymes in biomass conversions was studied. HSO3-ZSM-5 was successfully synthesized by sulfonation of ZSM-5 zeolite catalyst and applied to the hydrolysis of pretreated corn cob. The obtained solid acid catalyst shows high catalytic activity and effectively influences the biomass hydrolysis. Furthermore, proper conditions for the hydrolysis using HSO3-ZSM-5 as catalyst have been established as temperature: 120 °C; time: 6 h; solid/liquid ratio: 1/20; catalyst dosage: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under these conditions, the yield of reducing sugars of the hydrolysis catalyzed by HSO3-ZSM-5 is ∼54%. The as-synthesized catalyst, which is a recyclable solid acid catalyst provides a promising potential for applications in many industrially important hydrolysis processes of biomass.
1. Under these conditions, the yield of reducing sugars of the hydrolysis catalyzed by HSO3-ZSM-5 is ∼54%. The as-synthesized catalyst, which is a recyclable solid acid catalyst provides a promising potential for applications in many industrially important hydrolysis processes of biomass.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.03-2015.15.Notes and references
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787 CrossRef CAS PubMed.
- G. Akiyama, R. Matsuda, H. Sato, M. Takata and S. Kitagawa, Adv. Mater., 2011, 23, 3294 CrossRef CAS PubMed.
- S. Van de Vyver, L. Peng, J. Geboers, H. Schepers, F. de Clippel, C. J. Gommes, B. Goderis, P. A. Jacobs and B. F. Sels, Green Chem., 2010, 12, 1560 RSC.
- J. Pang, A. Wang, M. Zheng and T. Zhang, Chem. Commun., 2010, 46, 6935 RSC.
- D. Lai, L. Deng, Q. Guo and Y. Fu, Energy Environ. Sci., 2011, 4, 3552 RSC.
- P. H. Hoang and L. Q. Dien, Chem. Eng. J., 2015, 262, 140 CrossRef CAS.
- P. H. Hoang and B. Nguyen Xuan, RSC Adv., 2015, 5, 78441 RSC.
- P. H. Hoang, N. T. Nhung and L. Q. Dien, AIP Adv., 2017, 7, 105311 CrossRef.
- P. H. Hoang and N. V. Long, Cellul. Chem. Technol., 2017, 51, 447 CAS.
- G. Giannetto, et al., Zeolites, 1997, 19, 169 CrossRef CAS.
- Y. F. Yeong, A. Z. Abdullah, A. L. Ahmad and S. Bhatia, J. Eng. Sci. Technol., 2008, 3, 87 Search PubMed.
- V. Felice, S. Ntais and A. C. Tavares, Microporous Mesoporous Mater., 2013, 169, 128 CrossRef CAS.
- I. K. Mbaraka, D. R. Radu, V. S. Y. Lin and B. H. Shanks, J. Catal., 2003, 219, 329 CrossRef CAS.
- J. Zhang, X. Liu, M. Sun, X. Ma and Y. Han, ACS Catal., 2012, 2, 1698 CrossRef CAS.
- J. Zhang, X. Liu, M. N. Hedhili, Y. Zhu and Y. Han, ChemCatChem, 2011, 3, 1294 CrossRef CAS.
- J. Zhang, M. Sun, X. Liu and Y. Han, Catal. Today, 2014, 233, 77 CrossRef CAS.
- M. Sasaki, Z. Fang, Y. Fukushima, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2000, 39, 2883 CrossRef CAS.
- A. Onda, T. Ochi and K. Yanagisawa, Green Chem., 2008, 10, 1033 RSC.
- Y. Xiong, Z. Zhang, X. Wang, B. Liu and J. Lin, Chem. Eng. J., 2014, 235, 349 CrossRef CAS.
- W.-J. Liu, K. Tian, H. Jiang and H.-Q. Yu, Sci. Rep., 2013, 3, 2419 CrossRef PubMed.
- L. Hu, L. Lin, Z. Wu, S. Zhou and S. Liu, Appl. Catal., B, 2015, 174–175, 225 CrossRef CAS.
- M. Goswami, S. Meena, S. Navatha, K. N. Prasanna Rani, A. Pandey, R. K. Sukumaran, R. B. N. Prasad and B. L. A. Prabhavathi Devi, Bioresour. Technol., 2015, 188, 99 CrossRef CAS PubMed.
- F. Guo, Z. Fang, C. C. Xu and R. L. Smith, Prog. Energy Combust. Sci., 2012, 38, 672 CrossRef CAS.
- S. Morales-delaRosa, J. M. Campos-Martin and J. L. G. Fierro, Chem. Eng. J., 2012, 181–182, 538 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09190k |
| This journal is © The Royal Society of Chemistry 2018 |