Open Access Article
Open Access ArticleTriterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliata†
Jin-Kui Ouyang‡
a,
Li-Mei Dong‡a,
Qiao-Lin Xu*b,
Jing Wang§
c,
Shao-Bo Liua,
Tao Qiana,
Yun-Fei Yuanc and
Jian-Wen Tan *ac
*ac
aState Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou 510642, China. E-mail: jwtan@scau.edu.cn; Tel: +86-20-85280256
bGuangdong Provincial Key Laboratory of Silviculture, Protection and Utilization, Guangdong Academy of Forestry, Guangzhou 510520, China. E-mail: qlxu@sinogaf.cn
cGuangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China
First published on 5th December 2018
Abstract
Ten pentacyclic triterpenoids including a new multiflorane triterpene acid, 2α,3β,23-trihydroxymultiflor-7-en-28-oic acid (1), and a new lupane triterpene monoglucoside named akebiaoside C (2), were obtained from the leaves of Akebia trifoliata. Their structures were elucidated by extensive spectroscopic analysis, and they were all isolated from the leaves of A. trifoliata for the first time. These compounds, except 4 and 5, showed in vitro α-glucosidase inhibitory activity much stronger than acarbose. Especially, 2, 3, 6, 8 and 10 displayed in vitro α-glucosidase inhibitory activity with IC50 values from 0.004 to 0.081 mM, which were close or even more potent than corosolic acid (IC50 0.06 mM). Triterpenoids 1, 8 and 10 were further revealed to show moderate in vitro cytotoxic activity against human tumor A549, HeLa and HepG2 cell lines, with IC50 values ranging from 26.5 to 51.9 μM. Compound 9 selectively showed in vitro cytotoxicity toward HeLa and HepG2 cell lines, with IC50 values of 81.49 and 73.47 μM, respectively. These findings provided new data to support that the leaves of A. trifoliata are a rich source in bioactive triterpenoids highly valuable to be developed for medicinal usage.
Introduction
Akebia trifoliata, traditionally a medicinal plant in China, is naturally widely distributed in eastern Asia countries like Japan, Korea, and China.1 This perennial liana plant is capable of producing large edible fruits with tremendous potential as a new fruit crop.2 As a traditional Chinese folk medicine, A. trifoliata has been used as a diuretic and an antiphlogistic with a long history.3,4 In recent years, some phytochemical studies have been carried out on the stems and fruits of this plant, by which many chemical constituents including triterpenoids, phenolics and lignans were revealed, and some of them displayed significant bioactivities.5–11A. trifoliata is typically also a deciduous plant with most of its leaves reproducible and collectable in large scale annually, implicating that the leaves of A. trifoliata might potentially be a promising source for some bioactive chemicals. Meanwhile, few phytochemical studies have been conducted on the leaves of this plant in the past decades. Very recently, with the aim of clarifying potential bioactive chemicals in the leaves of A. trifoliata, we initiated a phytochemical investigation on the leaves of this plant, by which two new triterpene saponins were firstly identified.12 In continuation of this work, ten pentacyclic triterpenoids including a new multiflorane triterpene acid (1) and a new lupane triterpene monoglucoside (2) are here further obtained from the leaves of A. trifoliata (Fig. 1). Herein, we report the isolation and structural elucidation of these compounds, along with the tests of their in vitro α-glucosidase inhibitory activity and their cytotoxic activity against three human tumor cell lines.
Results and discussion
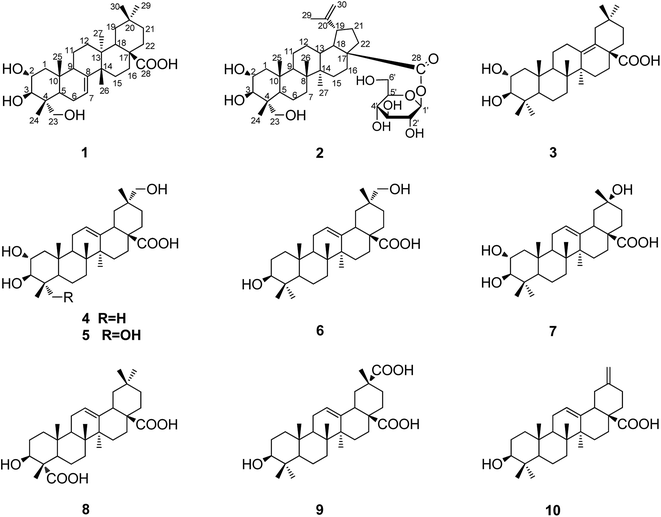
Compound 1 was obtained as a white amorphous powder with molecular formula C30H48O5 as determined by HR-ESI-MS, m/z 511.3378 [M + Na]+ (calcd for C30H48O5Na, 511.3394), which requires seven degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) showed signals recognizable for six tertiary methyls at δH 1.08 (3H, s), 1.07 (3H, s), 0.99 (3H, s), 0.95 (3H, s), 0.84 (3H, s) and 0.77 (3H, s), an oxymethylene at δH 3.45 (1H, d, J = 11.0 Hz, Ha-23) and 3.22 (1H, d, J = 11.0 Hz, Hb-23), two oxymethines at δH 3.68 (1H, m, H-2) and 3.36 (1H, d, J = 9.3 Hz, H-3), and an olefinic proton at δH 5.45 (1H, br.s, H-7). The 13C-NMR (DEPT) spectra (Table 1) supported the above analysis, which indicated the presence of 30 carbons including six methyl groups, ten methylenes [with one oxygenated at δC 66.0 (C-23)], six methines [including an olefinic methine at δC 119.3 (C-7) and two oxymethines at δC 69.5 (C-2) and 78.1 (C-3)], and seven quaternary carbons [including an olefinic quaternary carbon at δC 149.3 (C-8) and a carboxyl carbon at δC 180.4 (C-28)]. These above findings, accounted for two of the seven degrees of unsaturation, supported that 1 is a pentacyclic triterpenoid with three –OH groups, a trisubstituted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, and a COOH group in the molecule. Coupled with HSQC spectral analysis, the three OH groups can each be located at δC 69.5 (C-2), 78.1 (C-3) and δC 66.0 (C-23), respectively, and all the other 1H- and 13C-NMR spectral data can be assigned as shown in Table 1. In the 1H–1H COSY spectrum, the observation of proton spin-coupling correlations of H-2 (δH 3.68) with H2-1 (δH 1.95, 3.45) and H-3 (δH 3.36), and of H-6 (δH 2.08, 1.95) with H-5 (δH 5.45) and H-7 (δH 1.75), permitted to establish the structural fragments of C-1–C-2–C-3 and C-5–C-6–C-7 (Fig. 2). Besides, 1H–1H COSY correlation signals corresponding to the structural fragments of C-9–C-11–C-12, C-15–C-16, C-18–C-19 and C-21–C-22 were also presented (Fig. 2). In the HMBC spectrum, the exhibition of 1H–13C long-range correlations of H-5 (δH 1.75) with C-3 (δ 78.1), C-9 (δ 50.7), C-23 (δ 66.0), C-24 (δ 13.1) and C-25 (δ 15.2), of H-1 (δ 1.95, 1.03) with C-5 (δ 43.4) and C-25 (δ 15.2), of H-7 with C-9, and of H-9 (δ 2.29) with C-5, revealed the connections of C-4 with C-5, C-23, Me-24; C-10 (δ 37.0) with C-1 (δ 46.5), C-5, C-9, Me-25 (δ 15.2), and the connection of C-9 with C-8 (δ 149.3). The correlations of Me-26 with C-8, C-13, C-15, of H-18 with C-12, C-14, C-27, C-28, of H2-16 (δH 1.75) with C-18, C-22, C-28, and of H-15 with C-17, indicated the connections of C-13 with C-12, C-14, C-18, Me-27, the connections of C-14 with C-8, C-15, Me-26, and the connections of C-17 with C-16, C-18, C-22 and C-28. The observed HMBC correlations of H-18 with C-20, of H-21 with C-19, Me-29 and Me-30, indicated the linkages of C-20 with C-19, C-21, Me-29 and Me-30. Furthermore, the NOE correlations of H-2 with Me-24 (δH 0.77) and Me-25 (δH 0.84), and the presented proton spin-coupling constant of H-3 (JH-2,H-3 = 9.6 Hz) supported the α- and β-orientation of the –OH groups at C-2 and C-3, respectively.5 The NOE correlations of H-2 with Me-24, Me-25, and of H-5 with H-3, H2-23 and H-9, confirmed the α-orientation of H-5, H-9 and 23-CH2OH. The α-orientation of Me-27 and the β-orientation of H-18, Me-26, Me-30, were supported by significant NOE correlations of Me-27 with H-9, and of H-18 with Me-26, Me-30. Eventually, the whole structure of compound 1, as shown in Fig. 1, was established as 2α,3β,23-trihydroxymultiflor-7-en-28-oic acid.
C bond, and a COOH group in the molecule. Coupled with HSQC spectral analysis, the three OH groups can each be located at δC 69.5 (C-2), 78.1 (C-3) and δC 66.0 (C-23), respectively, and all the other 1H- and 13C-NMR spectral data can be assigned as shown in Table 1. In the 1H–1H COSY spectrum, the observation of proton spin-coupling correlations of H-2 (δH 3.68) with H2-1 (δH 1.95, 3.45) and H-3 (δH 3.36), and of H-6 (δH 2.08, 1.95) with H-5 (δH 5.45) and H-7 (δH 1.75), permitted to establish the structural fragments of C-1–C-2–C-3 and C-5–C-6–C-7 (Fig. 2). Besides, 1H–1H COSY correlation signals corresponding to the structural fragments of C-9–C-11–C-12, C-15–C-16, C-18–C-19 and C-21–C-22 were also presented (Fig. 2). In the HMBC spectrum, the exhibition of 1H–13C long-range correlations of H-5 (δH 1.75) with C-3 (δ 78.1), C-9 (δ 50.7), C-23 (δ 66.0), C-24 (δ 13.1) and C-25 (δ 15.2), of H-1 (δ 1.95, 1.03) with C-5 (δ 43.4) and C-25 (δ 15.2), of H-7 with C-9, and of H-9 (δ 2.29) with C-5, revealed the connections of C-4 with C-5, C-23, Me-24; C-10 (δ 37.0) with C-1 (δ 46.5), C-5, C-9, Me-25 (δ 15.2), and the connection of C-9 with C-8 (δ 149.3). The correlations of Me-26 with C-8, C-13, C-15, of H-18 with C-12, C-14, C-27, C-28, of H2-16 (δH 1.75) with C-18, C-22, C-28, and of H-15 with C-17, indicated the connections of C-13 with C-12, C-14, C-18, Me-27, the connections of C-14 with C-8, C-15, Me-26, and the connections of C-17 with C-16, C-18, C-22 and C-28. The observed HMBC correlations of H-18 with C-20, of H-21 with C-19, Me-29 and Me-30, indicated the linkages of C-20 with C-19, C-21, Me-29 and Me-30. Furthermore, the NOE correlations of H-2 with Me-24 (δH 0.77) and Me-25 (δH 0.84), and the presented proton spin-coupling constant of H-3 (JH-2,H-3 = 9.6 Hz) supported the α- and β-orientation of the –OH groups at C-2 and C-3, respectively.5 The NOE correlations of H-2 with Me-24, Me-25, and of H-5 with H-3, H2-23 and H-9, confirmed the α-orientation of H-5, H-9 and 23-CH2OH. The α-orientation of Me-27 and the β-orientation of H-18, Me-26, Me-30, were supported by significant NOE correlations of Me-27 with H-9, and of H-18 with Me-26, Me-30. Eventually, the whole structure of compound 1, as shown in Fig. 1, was established as 2α,3β,23-trihydroxymultiflor-7-en-28-oic acid.
| No. | δC (1) | δH (1) | No. | δC (1) | δH (1) |
|---|---|---|---|---|---|
| a Recorded at 600 MHz for 1H- and at 100 MHz for 13C-NMR data, δ in ppm and J in Hz. | |||||
| 1 | 46.5 CH2 | 1.93 (m), 1.03 (m) | 16 | 31.8 CH2 | 2.15 (m), 1.62 (m) |
| 2 | 69.5 CH | 3.68 (m) | 17 | 44.4 CH2 | — |
| 3 | 78.1 CH | 3.36 (d, 9.6) | 18 | 42.6 CH | 2.56 (m) |
| 4 | 44.2 C | — | 19 | 36.5 CH2 | 1.41 (m), 1.22 (m) |
| 5 | 43.2 CH | 1.75 (m) | 20 | 29.4 C | — |
| 6 | 24.8 CH2 | 2.08 (m), 1.95 (m) | 21 | 35.1 CH2 | 1.49 (m), 1.48 (m) |
| 7 | 119.3 CH | 5.45 (br.s) | 22 | 34.7 CH2 | 1.38 (m), 1.21 (m) |
| 8 | 149.3 C | — | 23 | 66.0 CH2 | 3.45 (d, 10.8), 3.22 (d, 10.8) |
| 9 | 50.7 CH | 2.29 (m) | 24 | 13.1 CH3 | 0.77 (s) |
| 10 | 37.0 C | — | 25 | 15.2 CH3 | 0.84 (s) |
| 11 | 18.7 CH2 | 1.62 (m), 1.53 (m) | 26 | 29.5 CH3 | 1.07 (s) |
| 12 | 38.3 CH2 | 1.73 (m), 1.61 (m) | 27 | 26.1 CH3 | 1.08 (s) |
| 13 | 37.9 C | — | 28 | 180.4 C | — |
| 14 | 42.2 C | — | 29 | 33.8 CH3 | 0.95 (s) |
| 15 | 34.5 C | 1.81 (m), 1.72 (m) | 30 | 31.7 CH3 | 0.99 (s) |
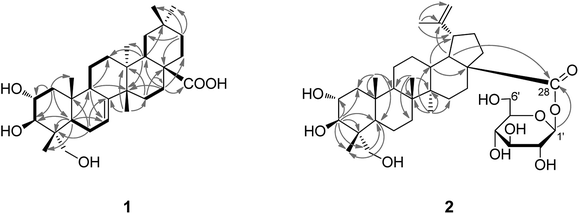
Compound 2, C36H58O10 (positive HR-ESI-MS showed [M + Na]+ m/z 673.3916, calcd for C36H58O10Na 673.3922) was also obtained as a white powder. The 1H and 13C NMR spectra of 2 showed one sugar anomeric proton at δH 6.42 (Glc I-1) and an anomeric carbon at δC 95.3, suggesting the existence of a sugar moiety in the structure. Acid hydrolysis of 2 with 2 N HCl released the sugar unit from the molecule, which was identified to be a D-glucose as determined by GC-MS analysis of its chiral derivatives (see Experimental part). The detailed 1H- and 13C-NMR assignments of the D-glucose moiety in 2 (as listed in Table 2) were established by interpretation of combined HSQC and HMBC data. Apart from the signals due to the D-glucopyranose moiety, the remaining signals in the 1H NMR spectrum for the aglycone of 2 were readily recognized for five tertiary methyls at δH 1.71 (3H, s), 1.15 (3H, s), 1.03 (3H, s), 0.97 (3H, s) and 0.92 (3H, s), two olefinic protons at δH 4.86, (1H, br.s) and 4.73 (1H, br.s), two oxymethine protons at δH 4.25 (1H, m) and 4.21 (1H, d, J = 9.3 Hz), and two protons for a hydroxymethylene group at δH 4.20 and 3.70 (each 1H, d, J = 10.4 Hz). The 13C NMR spectrum indicated, besides the signals for the glucose moiety, 30 carbons for the aglycone unit, including five methyls, eleven methylenes [including an exomethylene at δC 109.9 (C-30), and a hydroxymethylene at δC 66.2 (C-23)], seven methines (including two oxygenated methines at δC 69.0 and 78.0), and seven quaternary carbons (including an olefinic quaternary carbon at δC 150.7 and a carboxyl carbon at δC 174.8). By comparison, it was found that the 1H- and 13C-NMR spectroscopic data (Table 2) of the aglycone of 2 were closely related to those of known compound hovenic acid (i.e. 2α,3β,23-trihydroxylup-20(29)-en-28-oic acid).13 These findings supported 2, as shown in Fig. 1, to be a monodesmoside saponin of 2α,3β,23-trihydroxylup-20(29)-en-28-oic acid with a D-glucose moiety linked at C-28 14. This deduction was consistent with the molecular formula of 2, and well supported by the 2D NMR spectroscopic data. Coupled with HSQC and HMBC spectral analysis, the whole 1H- and 13C-NMR spectral data of 2 were assigned as shown in Table 2. In the HMBC spectrum, the 1H–13C long-range correlations of H-3 (δH 4.21) with C-1, C-2, C-4, C-5, C-24 and C-23 evidenced the direct linkage of C-4 with Me-24 and C-4 with C-23, and supported the location of a hydroxyl group at each of C-2, C-3 and C-23. The HMBC correlations of H3-25 with C-1, C-5, C-9, C-10, of H3-26 with C-7, C-8, C-9, C-14, and of H3-27 with C-8, C-13, C-14, C-15, supported the locations of Me-25 at C-10, Me-26 at C-8, and Me-27 at C-14, respectively. The HMBC correlations of H3-29 with C-19, C-20, C-30, of H-18 with C-20 and C-28, indicated the connections of C-20 with Me-29, C-19 and C-30, and the connection of C-17 with C-28. The 1H–13C long-range correlation of H-1′ (δH 6.42) with C-28 (δC 174.8) confirmed the glycoside linkage of the D-glucose moiety with the aglycone at C-28. Besides, the β-anomeric configuration of the D-glucose moiety was indicated by the coupling constant of 3JH1′,H2′ (8.2 Hz).14,15 The presented proton spin-coupling constant of H-3 (3JH-2,H-3 = 9.3 Hz) supported the α- and β- configurations of the –OH groups at C-2 and C-3, respectively.5 The stereochemistry of the 23-CH2OH group at C-4 was deduced as the α-configuration from the NOE correlation between H-2 and Me-24 in the NOESY spectrum. The α-iso-propenyl group at the C-19 position was evidenced by the observation of NOE correlations between H-13 (δH 2.64) and H-19 (δH 3.38). Therefore, the whole structure of compound 2 was identified as 2α,3β,23-trihydroxylup-20(29)-en-28-oic acid-O-β-D-glucopyranosyl ester, trivially named akebiaoside C.
| No. | δC (2) | δH (2) | No. | δC (2) | δH (2) |
|---|---|---|---|---|---|
| a Recorded at 500 MHz for 1H- and at 150 MHz for 13C-NMR data, δ in ppm and J in Hz. | |||||
| 1 | 48.0 CH2 | 2.34 (m), 1.32 (m) | 19 | 47.3 CH2 | 3.38 (m) |
| 2 | 69.0 CH | 4.25 (m) | 20 | 150.7 C | — |
| 3 | 78.0 CH | 4.21 (d, 9.3) | 21 | 30.7 CH2 | 2.10 (m), 1.41 (m) |
| 4 | 43.5 C | — | 22 | 36.7 CH2 | 2.17 (m), 1.47(m) |
| 5 | 47.8 CH | 1.75 (m) | 23 | 66.2 CH2 | 4.20 (d, 10.4), 3.70 (d, 10.4) |
| 6 | 18.3 CH2 | 1.68 (m), 1.41 (m) | 24 | 14.0 CH3 | 1.03 (s) |
| 7 | 34.1 CH2 | 1.52 (m), 1.31 (m) | 25 | 18.0 CH3 | 0.97 (s) |
| 8 | 41.1 C | — | 26 | 16.3 CH3 | 1.15 (s) |
| 9 | 50.8 CH | 1.53 (m) | 27 | 14.7 CH3 | 0.92 (s) |
| 10 | 38.4 C | — | 28 | 174.8 C | — |
| 11 | 21.1 CH2 | 1.47 (m), 1.22 (m) | 29 | 19.2 CH3 | 1.71 (s) |
| 12 | 25.8 CH2 | 1.83 (m), 1.11 (m) | 30 | 109.9 CH2 | 4.86 (br.s), 4.73 (br.s) |
| 13 | 38.2 C | 2.64 (m) | 1′ | 95.3 CH | 6.42 d (8.2) |
| 14 | 42.7 C | — | 2′ | 74.2 CH | 4.18 (m) |
| 15 | 30.0 CH2 | 2.02 (m), 1.16 (m) | 3′ | 78.7 CH | 4.30 (m) |
| 16 | 32.1 CH2 | 2.63 (m), 1.46 (m) | 4′ | 70.9 CH | 4.36 (m) |
| 17 | 56.8 C | — | 5′ | 79.3 CH | 4.05 (m) |
| 18 | 49.7 CH | 1.70 (m) | 6′ | 62.0 CH2 | 4.46 (m), 4.41 (m) |
The eight known compounds were identified as 2α,3β-dihydroxyolean-13(18)-en-28-oic acid (3),16 2α,3β,29-trihydroxyolean-12-en-28-oic acid (4),11 stachlic acid A (5),17 mesembryanthemoidigenic acid (6),18 2α,3β,20α-trihydroxy-29-norolean-12-en-28-oic acid (7),19 gypsogenic acid (8),20 serratagenic acid (9),21 and akebonoic acid (10),22 by comparison of their NMR and MS spectral data to those reported in literatures. These compounds were all obtained from the leaves of A. trifoliata for the first time.
These isolated triterpenoids were evaluated for their α-glucosidase inhibitory activity, with acarbose and corosolic acid used as two reference compounds. The resulting IC50 values, as listed in Table 3, indicated that all the compounds, except 4 and 5, showed stronger the α-glucosidase inhibitory activity than acarbose (IC50 0.409 mM). Especially, compounds 2, 3, 6, 8 and 10 displayed the α-glucosidase inhibitory activity with IC50 values ranging from 0.004 to 0.081 mM, which were close or even more potent than corosolic acid (IC50 0.06 mM). The results suggested that these compounds from the leaves of A. trifoliata, at least for 2, 3, 6, 8 and 10, were effective α-glucosidase inhibitors valuable to be developed as effective hypoglycemic agents for diabetes chemotherapy.23 Comparison of the chemical structures and the α-glucosidase inhibitory activity of 6 versus 4 indicated that the addition of a hydroxyl group at C-2 had an obviously negative effect on the α-glucosidase inhibitory activity of the oleanane type triterpenes.
| Compounds | IC50 (mM) | Compounds | IC50 (mM) |
|---|---|---|---|
| a Values represent mean ± SD (n = 3) based on three individual experiments. | |||
| 1 | 0.109 ± 0.003 | 6 | 0.042 ± 0.002 |
| 2 | 0.015 ± 0.001 | 7 | 0.367 ± 0.003 |
| 3 | 0.021 ± 0.002 | 8 | 0.081 ± 0.003 |
| 4 | 0.503 ± 0.004 | 9 | 0.342 ± 0.002 |
| 5 | 0.592 ± 0.007 | 10 | 0.009 ± 0.001 |
| Acarbose | 0.409 ± 0.006 | Corosolic acid | 0.060 ± 0.002 |
Compounds 1–10 were further tested for their in vitro cytotoxicity against human cancer cell lines A549, HeLa and HepG2, using a microdilution titre technique as described in the Experimental section. The resulting IC50 values are displayed in Table 4, compared to adriamycin as positive control. Compounds 1, 8 and 10 were found to show moderate cytotoxicity against all the three cancer cell lines, with IC50 values ranging from 26.5 to 51.9 μM. Compound 9 showed weak cytotoxicity toward HeLa and HepG2 cell lines, with IC50 values 81.49 and 73.47 μM, respectively. While, no obvious cytotoxic activity was detected for the other compounds in this bioassay. Comparison of the chemical structures and the cytotoxic activity of 5 versus arjunolic acid12 indicated a negative effect on the cytotoxicity of the oleanane type triterpenes when the Me-29 group was replaced by a –CH2OH group.
| Compounds | A549 | HeLa | HepG2 |
|---|---|---|---|
| a Values represent mean ± SD (n = 3) based on three individual experiments. | |||
| 1 | 27.58 ± 3.24 | 31.45 ± 2.38 | 38. 52 ± 5.63 |
| 2–7 | >100 | >100 | >100 |
| 8 | 26.54 ± 7.52 | 43.63 ± 8.41 | 35.67 ± 7.50 |
| 9 | >100 | 81.49 ± 16.50 | 73.47 ± 0.90 |
| 10 | 48.77 ± 8.56 | 27.82 ± 7.53 | 51.94 ± 5.37 |
| Adriamycin | 0.68 ± 0.06 | 0.48 ± 0.07 | 1.25 ± 0.04 |
A. trifoliata is a liana plant widely distributed in Eastern Asia countries. As traditionally a medicinal plant, also a rapidly developing economic plant commercially for fruits in China, A. trifoliata has now been developed and cultivated in large scale in many places of China, including Hunan, Hubei, Jiangxi, Shaanxi, and Chongqing provinces.1,3 Previously, phytochemical studies of this plant were mainly focused on the stems and fruits, by which structurally diverse triterpenes, triterpene saponins, and some other type of chemicals were identified. However, few studies were conducted on the leaves,12 though the leaves of this plant were annually collectable in large scale. In a recent study, we have identified two new triterpene saponins from the leaves of A. trifoliata. The present findings further indicated that the leaves of this plant is rich in bioactive natural products valuable to be developed for medicinal usage. Among the chemicals here identified, 1 is a new multiflorane type triterpene. To the best of our knowledge, this is the first time for a multiflorane type triterpene isolated from A. trifoliata, suggesting that more so far unidentified triterpenoids would still exist in the leaves of A. trifoliata worthy of further investigation.
Materials and methods
General experimental procedures
Optical rotations were obtained on a Perkin-Elmer 341 polarimeter (Perkin-Elmer, Waltham, MA, USA) with MeOH as solvent. The 1D and 2D Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker Advance 600 instrument or Bruker Ascend-500 spectrometer (Bruker BioSpin GmbH). The positive and negative ESI-MS were collected on a MDS SCIEX API 2000 LC/MS/MS instrument (Applied Biosystems, Foster City, CA, USA) after the test solutions were directly injected into the ESI source by a syringe pump. HR-ESI-MS spectra were obtained on a Bruker maXis mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) in positive-ion mode. Preparative HPLC was conducted using a CXTH P3000 HPLC pump and a UV 3000 UV-Vis Detector with a Fuji-C18 column (10 μm to 100 A, ChuangXinTongHeng Science And Technology Co., Ltd, Beijing, China); the performance of MPLC (medium pressure liquid chromatography) achieved by using a CXTH P3000 HPLC pump, with a UV 3000 UV-Vis Detector and a ODS column (40 × 2.5 cm i.d, 50 μM, YMC Co. Ltd., Kyoto, Japan).For column chromatography (CC), silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), YMC ODS-A (50 μm, YMC Co. Ltd., Kyoto, Japan), and Sephadex LH-20 (Pharmacia Fine Chemical Co. Ltd., Uppsala, Sweden) were used. Those analytical grade petroleum ether (b.p. 60–90 °C), MeOH, AcOEt, CHCl3, acetone, and n-butanol were purchased from Tianjin Fuyu Fine Chemical Industry Co. (Tianjin, China); HPLC grade MeOH was obtained from J&K Chemical Ltd. (Beijing, China); MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), pyridine-d5, DMSO-d6, and α-glucosidase were purchased from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, MO, USA). RPMI-1640 medium and fetal calf serum were from Gibco BRL (Gaithersburg, MD, USA). p-Nitrophenyl-α-D-glucopyranoside (PNPG) and acarbose were from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and adriamycin was from Pfizer Italia SRL (Roma, Italy).
Plant materials
The leaves of Akebia trifoliata, collected in August 2014 at Sangzhi, Hunan Province in China, was authenticated by Prof. Fuwu Xing at the South China Botanical Garden, Chinese Academy of Sciences (CAS), and a voucher specimen (no. 20140815) was deposited at the Laboratory of Bioorganic Chemistry of South China Botanical Garden, CAS.Extraction and isolation
Powders of air-dried leaves of A. trifoliata (3.5 kg) were extracted with 95% EtOH at room temperature for three times (each time 10 L for 2 days). The EtOH extracts were next combined and concentrated in vacuo to provide a dark brown residue, which was suspended in 3 L H2O and then sequentially extracted by petroleum ether (3 L × 3), EtOAc (3 L × 3) and n-butanol (n-BuOH, 3 L × 3). The petroleum ether and EtOAc layers were evaporated in vacuo to yield a petroleum ether-soluble (31 g) and EtOAc-soluble (168 g) fractions. The petroleum ether-soluble fraction was subjected to silica gel CC (100 cm × 10.5 cm i.d.) using a gradient of petroleum ether–acetone (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–0
0–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) to provide nine fractions (E1–E9). Fraction E4 (1.7 g) was further applied to a silica gel column using petroleum ether–acetone (10
100, v/v) to provide nine fractions (E1–E9). Fraction E4 (1.7 g) was further applied to a silica gel column using petroleum ether–acetone (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–6
1–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) elution and then using MPLC eluted with MeOH–H2O (9.5
1) elution and then using MPLC eluted with MeOH–H2O (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v/v) to yield 10 (2.2 mg). Fraction E6 (290 mg) was purified by a Sephadex LH-20 column (160 × 3 cm i.d.) chromatography eluted with pure acetone to provide compound 3 (2.8 mg). Fraction E8 (2.2 g) was passed through an MCI gel column (60 × 6 cm i.d.) for depigmentation. The resultant methanolic eluate (1.3 g) of E8 was sequentially separated by MPLC using a gradient of MeOH–H2O (6
0.5, v/v) to yield 10 (2.2 mg). Fraction E6 (290 mg) was purified by a Sephadex LH-20 column (160 × 3 cm i.d.) chromatography eluted with pure acetone to provide compound 3 (2.8 mg). Fraction E8 (2.2 g) was passed through an MCI gel column (60 × 6 cm i.d.) for depigmentation. The resultant methanolic eluate (1.3 g) of E8 was sequentially separated by MPLC using a gradient of MeOH–H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4–10
4–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, v/v) to obtain eight subfractions (E8-1–E8-8). The subfraction E8-5 was purified by silica gel CC eluted with CHCl3–MeOH (10
0, v/v) to obtain eight subfractions (E8-1–E8-8). The subfraction E8-5 was purified by silica gel CC eluted with CHCl3–MeOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–9
0–9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford compounds 6 (3.2 mg) and 9 (2.1 mg).
1, v/v) to afford compounds 6 (3.2 mg) and 9 (2.1 mg).
The EtOAc-soluble fraction was subjected to silica gel CC (100 cm × 10.5 cm i.d.) eluted with CHCl3–MeOH (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3–0
3–0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) to obtain ten fractions (F1–F10). Fraction F5 (7.1 g), obtained on elution with CHCl3/MeOH of 85
100, v/v) to obtain ten fractions (F1–F10). Fraction F5 (7.1 g), obtained on elution with CHCl3/MeOH of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (v/v), was further subjected to silica gel CC (80 × 5 cm i.d.) eluted with CHCl3–MeOH of increasing polarity (98
15 (v/v), was further subjected to silica gel CC (80 × 5 cm i.d.) eluted with CHCl3–MeOH of increasing polarity (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–90
2–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) to obtain six subfractions (F5-1–F5-6). Subfraction F5-3 (1.57 g) was separated by MPLC eluted with MeOH–H2O (60
10, v/v) to obtain six subfractions (F5-1–F5-6). Subfraction F5-3 (1.57 g) was separated by MPLC eluted with MeOH–H2O (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–100
40–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, v/v) system at a flow rate of 10 mL min−1, and further purified by a Sephadex LH-20 column (150 cm × 2.5 cm i.d) eluted with MeOH to afford compound 8 (2.5 mg). Fraction F5-5 (2.3 g) was separated by MPLC eluted with MeOH–H2O (30
0, v/v) system at a flow rate of 10 mL min−1, and further purified by a Sephadex LH-20 column (150 cm × 2.5 cm i.d) eluted with MeOH to afford compound 8 (2.5 mg). Fraction F5-5 (2.3 g) was separated by MPLC eluted with MeOH–H2O (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70–80
70–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) at a flow rate of 10 mL min−1 to obtain subfractions F5-5-1–F5-5-6. Subfraction F5-5-5 was purified by preparative HPLC with a Fuji-C18 column (10 μm to 100 A) eluted with MeOH–H2O (73
20, v/v) at a flow rate of 10 mL min−1 to obtain subfractions F5-5-1–F5-5-6. Subfraction F5-5-5 was purified by preparative HPLC with a Fuji-C18 column (10 μm to 100 A) eluted with MeOH–H2O (73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27, v/v) at a flow rate of 8 mL min−1 to afford compounds 4 (tR 53 min, 2 mg) and 1 (tR 100 min, 2.4 mg). Fraction F7 (22 g), obtained on elution with CHCl3–MeOH (60
27, v/v) at a flow rate of 8 mL min−1 to afford compounds 4 (tR 53 min, 2 mg) and 1 (tR 100 min, 2.4 mg). Fraction F7 (22 g), obtained on elution with CHCl3–MeOH (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v), was further subjected to silica gel CC (100 cm × 10.5 cm i.d) eluted with a gradient of CHCl3–MeOH (90
40, v/v), was further subjected to silica gel CC (100 cm × 10.5 cm i.d) eluted with a gradient of CHCl3–MeOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–60
10–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) to obtain six subfractions (F7-1–F7-6). Subfraction F7-3 (2.8 g) was further separated by MPLC using a gradient of MeOH/H2O (65
40, v/v) to obtain six subfractions (F7-1–F7-6). Subfraction F7-3 (2.8 g) was further separated by MPLC using a gradient of MeOH/H2O (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35–70
35–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) to afford compound 5 (16 mg) and 7 (2 mg). Fraction F9 (3.1 g), obtained on elution with CHCl3–MeOH (1
30, v/v) to afford compound 5 (16 mg) and 7 (2 mg). Fraction F9 (3.1 g), obtained on elution with CHCl3–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), was subjected to a silica gel column (80 cm × 5 cm i.d), eluted with a gradient of CHCl3–MeOH (9
1, v/v), was subjected to a silica gel column (80 cm × 5 cm i.d), eluted with a gradient of CHCl3–MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to give subfractions F9-1–F9-8. The fraction F9-4 (0.6 g) was separated by a silica gel column (80 × 7.5 cm i.d.) eluted with CHCl3–MeOH (98
5, v/v) to give subfractions F9-1–F9-8. The fraction F9-4 (0.6 g) was separated by a silica gel column (80 × 7.5 cm i.d.) eluted with CHCl3–MeOH (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–90
2–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) to yield four sub-fractions (F9-4-1–F9-4-4). Subfraction F9-4-4 was first separated by MPLC with elution system of MeOH/H2O (25
10, v/v) to yield four sub-fractions (F9-4-1–F9-4-4). Subfraction F9-4-4 was first separated by MPLC with elution system of MeOH/H2O (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75–80
75–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) at a flow rate of 10 mL min−1, and further purified by a Sephadex LH-20 column (150 cm × 2.5 cm i.d) eluted with 20% CHCl3 in methanol (v/v) to afford compounds 2 (5 mg).
20, v/v) at a flow rate of 10 mL min−1, and further purified by a Sephadex LH-20 column (150 cm × 2.5 cm i.d) eluted with 20% CHCl3 in methanol (v/v) to afford compounds 2 (5 mg).
Acid hydrolysis for the sugar unit of 2
Powders of 2.8 mg compound 2 was dissolved in 4 mL 2 M HCl and heated at 90 °C for 2 h. After cooling, the reaction mixture was extracted three times (each 4 mL) with EtOAc. The aqueous layer was then evaporated in vacuo to dryness to give a sugar-containing residue, which was reacted with L-cysteine methyl ester hydrochloride in C5H5N at 60 °C for 2 h. Subsequently, N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) was added and stirred under reflux at 60 °C for 10 h. The supernatant was then analyzed by GC-MS technique using a GCMS-QP2010 PLUS instrument, equipped with a HP-5ms capillary column (30 m, 0.25 mm ID), conditioned at a constant helium flow rate of 46.5 cm s−1, 1 μL injection volume, injector temperature at 230 °C, temperature program as 2 °C min−1 to 180 °C, then 20 °C min−1 to 280 °C. Electron ionization mode was set at 70 eV. The sugar unit derived from the hydrolysis of 2 was confirmed to be D-glucose by comparison of the retention time of the derivative with that of authentic D-glucose derivative (tR 11.852 min) prepared via the same process.α-Glucosidase inhibition assay
The α-glucosidase inhibitory activity of compounds 1–10 were tested by using a method as we recently described in the literature,12 with both acarbose and corosolic acid utilized as reference compounds. The resulting IC50 values of the tested compounds were listed in Table 3.Cytotoxic assay
The cytotoxic activity of compounds 1–10 against human tumor A549, HeLa and HepG2 cell lines were assayed by using 96 well plates according to a literature MTT method with slight modification.24 In brief, the cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. Each well of 96-well cell culture plates was seeded with 100 μL adherent cells (5 × 104 cell per mL) and placed in an atmosphere with 5% CO2 at 37 °C for 24 h to form a monolayer on the flat bottoms. Subsequently, in each well, the supernatant was removed and 100 μL fresh medium and 100 μL medium containing one of the test compounds was added. Then the plate was incubated in 5% CO2 atmosphere at 37 °C. After 3 days, 20 μL MTT at concentration 5 mg mL−1 in DMSO was added into each well and incubated for 4 h. Carefully, the supernatant in each well was removed and 150 μL DMSO was added. Then the plate was vortex shaken for 15 min to dissolve blue formazan crystals. The OD (optical density) value of each well was tested on a Genois microplate reader (Tecan GENios, Männedorf, Switzerland) at 570 nm. All the tests were conducted by three individual experiments and adriamycin was applied as a positive control. In a test, for each of the tumor cell lines, each of the test compounds was set at concentrations 50, 25, 12.5, 6.25, 3.125, 1.5625 μg mL−1. The inhibitory rate of tumor cell growth was calculated by the formula: inhibition rate (%) = (ODcontrol − ODtreated)/ODcontrol × 100%, and the IC50 values were calculated by SPSS 16.0 statistic software. The three tumor cell lines were purchased from the Kunming Institute of Zoology, CAS. The resulting IC50 values listed in Table 4 were based on three individual experiments and represented as means ± standard deviation (SD).Conclusions
Ten pentacyclic triterpenoids, including a new multiflorane triterpene acid 1 and a new lupane triterpene monoglucoside 2, were obtained from the leaves of A. trifoliata. Their structures were elucidated by extensive spectroscopic and chemical means. All the compounds were isolated from the leaves of A. trifoliata for the first time. These compounds, except 4 and 5, were found to show the in vitro α-glucosidase inhibitory activity much stronger than acarbose. Especially, compounds 2, 3, 6, 8 and 10 displayed in vitro α-glucosidase inhibitory activity with IC50 values close or even more potent than corosolic acid. Furthermore, compounds 1, 8, 9 and 10 selectively showed in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines. The present study support that the leaves of A. trifoliata is a highly valuable source rich in bioactive chemicals worthy to be developed in medicinal field.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work was supported by the National Natural Science Foundation of China (31470422, 31500291 and 31270406), the Natural Science Foundation of Guangdong Province (2014A030313742), and the Science and Technology Project of Guangdong Province (to J.-W. Tan).References
- L. Li, X. Yao, C. Zhong, X. Chen and H. Huang, HortScience, 2010, 45, 4–10 Search PubMed.
- Z. Wang, C. Zhong, F. Bu, D. Peng, J. Peng and F. Yuan, Agric. Sci. Technol., 2005, 6, 12–18 Search PubMed.
- L. Li, X.-Z. Chen, X.-H. Yao, H. Tian and H.-W. Huang, Wuhan Zhiwuxue Yanjiu, 2010, 28, 497–506 Search PubMed.
- Jiangsu New Medical College, Dictionary of Chinese Herbal Medicines, Shanghai Science and Technology Press, Shanghai, China, 1995, p. 169 Search PubMed.
- Y. Mimaki, M. Kuroda, A. Yokosuka, H. Harada, M. Fukushima and Y. Sashida, Chem. Pharm. Bull., 2003, 51, 960–965 CrossRef CAS PubMed.
- H. Gao and Z. Wang, Phytochemistry, 2006, 67, 2697–2705 CrossRef CAS PubMed.
- S. Iwanaga, T. Warashina and T. Miyase, Chem. Pharm. Bull., 2012, 60, 1264–1274 CrossRef CAS.
- Y. Wang, J. Lu and R.-C. Lin, Chin. Tradit. Herb. Drugs, 2004, 35, 495–498 CAS.
- S.-G. Guan, W.-B. Yu and S.-H. Guan, Lishizhen Med. Mater. Med. Res., 2010, 21, 905–906 CAS.
- S. H. Guan, J. M. Xia, Z. Q. Lu, G. T. Chen, B. H. Jiang, X. Liu and D. A. Guo, Magn. Reson. Chem., 2008, 46, 186–190 CrossRef CAS PubMed.
- J. Wang, H. Ren, Q.-L. Xu, Z.-Y. Zhou, P. Wu, X.-Y. Wei, Y. Cao, X.-X. Chen and J.-W. Tan, Food Chem., 2015, 168, 623–629 CrossRef CAS PubMed.
- Q.-L. Xu, J. Wang, L.-M. Dong, Q. Zhang, B. Luo, Y.-X. Jia, H.-F. Wang and J.-W. Tan, Molecules, 2016, 21, 962 CrossRef PubMed.
- K. Yoshikawa, Y. Kondo, E. Kimura and S. Arihara, Phytochemistry, 1998, 49, 2057–2060 CrossRef CAS.
- H.-G. Luo, L. Ma and L.-Y. Kong, Bioorg. Med. Chem., 2008, 16, 2912–2920 CrossRef PubMed.
- D. Wang, G. P. Zhou, Y. S. Yang, Y. L. Su and T. F. Ji, Chin. Chem. Lett., 2009, 20, 833–835 CrossRef CAS.
- N. Zeng, Y. Shen, L.-Z. Li, W.-H. Jiao, P.-Y. Gao, S.-J. Song, W.-S. Chen and H.-W. Lin, J. Nat. Prod., 2011, 74, 732–738 CrossRef CAS PubMed.
- J.-H. Yang, Y.-S. Wang, R. Huang, S.-D. Luo, H.-B. Zhang and L. Li, Helv. Chim. Acta, 2006, 89, 2830–2835 CrossRef CAS.
- F. Guo, S. Lin and Y. Li, Chin. J. Med. Chem., 2005, 15, 294–296 CAS.
- J. Wang, Q.-L. Xu, M.-F. Zheng, H. Ren, T. Lei, P. Wu, Z.-Y. Zhou, X.-Y. Wei and J.-W. Tan, Molecules, 2014, 19, 4301–4312 CrossRef PubMed.
- K. Hidaka, M. Ito, Y. Matsuda, H. Kohda, K. Yamasaki and J. Yamahara, Phytochemistry, 1987, 26, 2023–2027 CrossRef CAS.
- Z.-Y. Ji, Y.-X. Yang, F.-F. Zhuang, F.-L. Yan and C.-H. Wang, Zhongyaocai, 2015, 38, 510–513 CAS.
- A. Ikuta and H. Itokawa, Phytochemistry, 1986, 25, 1625–1628 CrossRef CAS.
- T. Ieyama, M. D. P. T. Gunawan-Puteri and J. Kawabata, Food Chem., 2011, 128, 308–311 CrossRef CAS PubMed.
- X. Xu, H. Xie, J. Hao, Y. Jiang and X. Wei, Food Chem., 2010, 123, 1123–1126 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08894b |
| ‡ These authors contributed equally to this work. |
| § Presently work at Shenzhen Boton Flavor and Fragrances Co., Ltd, Shenzhen 518051, China. |
| This journal is © The Royal Society of Chemistry 2018 |