Open Access Article
Open Access ArticleOrganic loading rate shock impact on extracellular polymeric substances and physicochemical characteristics of nitrifying sludge treating high-strength ammonia wastewater under unsteady-state conditions
Lei
Yang
 ab,
Yong-Xiang
Ren
*ab,
Ning
Chen
ab,
Shen
Cui
ab,
Xu-Hui
Wang
ab and
Qian
Xiao
ab
ab,
Yong-Xiang
Ren
*ab,
Ning
Chen
ab,
Shen
Cui
ab,
Xu-Hui
Wang
ab and
Qian
Xiao
ab
aKey Laboratory of Northwest Water Resource, Environment and Ecology, MOE, Engineering Technology Research Center for Wastewater Treatment and Reuse, Shaanxi, China. E-mail: ryx@xauat.edu.cn; Fax: +86 2982202729; Tel: +86 2982202509
bShaanxi Key Laboratory of Environmental Engineering, Xi'an University of Architecture and Technology, Xi'an 710055, China
First published on 13th December 2018
Abstract
Laboratory experimentation was used to investigate the impact of the organic loading rate shock on extracellular polymeric substances (EPSs) and the physicochemical characteristics of nitrifying sludge (NS) treating high-strength ammonia wastewater. The increased organic loading rates (OLRs) strongly influenced the stability of the NS with regard to nutrient removal, biomass–liquid separation, and surface properties, leading to the sludge system collapse at the OLR of 0.75 kg COD per kg MLVSS d. However, an incomplete recovery of the NS after the high OLRs shock was observed when decreasing the OLRs. In addition, the variations of OLRs resulted in relatively stable amounts of tightly bound EPS (TB-EPS), but a significant change in loosely bound EPS (LB-EPS). Both in LB-EPS and TB-EPS, the proteins (PN) contents and proteins to polysaccharides (PN/PS) ratios decreased with the increase in OLRs. Results from the excitation emission matrix spectra implied that the tryptophan PN-like substances were the major components in EPS at low OLRs, while the humic acid-like and fulvic acid-like substrates increased markedly at high OLRs. Furthermore, correlation analysis demonstrated that PN and the PN/PS ratio were the most important factors in determining the physicochemical properties of the NS. It was indicated that the PN could accurately reflect the sludge properties of the NS, and thus effectively change the surface properties of the sludge, contributing to the cohesion between the aggregates to maintain a stable structure.
1 Introduction
There are many different kinds of human activities that generate wastewater with large quantities of ammonium: petrochemical, pharmaceutical, fertilizer, and food industries, as well as leachates produced by urban solid waste disposal sites or waste from pig farms. An uncontrolled discharge of this type of waste can cause serious damage to the environment, primarily through eutrophication of the receiving waterbody.1 A biological nitrogen removal process (BNRP) is commonly applied in the field of high-strength ammonia wastewater treatment, which requires a combined two-step process: the conversion of ammonia to nitrate in an aerobic nitrification process and a subsequent nitrate to nitrogen gas process in anoxic denitrification.2 Due to the low growth rates of autotrophic nitrifying bacteria, which are vulnerable to high loads of ammonium and organic matter, it is an undisputed fact that the nitrification process is the rate-limiting step for nitrogen removal.1–3 Hence, the stability of nitrifying sludge (NS) would directly determine the performance of high-strength ammonia wastewater treatment systems.As BNRPs have been applied to the treatment of a wide range of industrial and municipal wastewaters with variable nutrient inputs, sewage treatment plants have to cope with the changes in the composition of wastewater and the daily fluctuation in flow. In practice, the control of activated sludge systems is carried out on the basis of the organic loading. The organic loading rate (OLR) is one of the most critical parameters for a wastewater nitrification process as it directly affects the nutrient removal performance and physiological properties of biomass in NS. Changes in the OLR may cause variations in the ratios of food to microorganism (F/M) or dissolved oxygen (DO) levels, which are two fundamental parameters associated with microbial behaviors.4 Accordingly, biomass characteristics (e.g., sludge concentration, floc structure, particle size, biomass–liquid separation, and surface properties) are also related to the variable microbial properties. Importantly, as the excreted and autolysis substances of microorganisms, they play important roles in microbial aggregates, extracellular polymeric substances (EPS) that strongly depend on microbial community and activity, and tend to display various characteristics under different OLRs.5–7
During the biological nitrogen removal process for the treatment of high-strength ammonia wastewater, high concentrations of ammonia may inhibit the microbial activity, affecting the characteristics of nutrient removal, bioflocculation, and the microbial community of the NS.8,9 Up to now, the effects of the influent OLR on the biological nitrification ability in various activated sludge processes have been studied intensively, but quite scare results have been obtained in terms of the influence of OLR shock on EPS production and the floc physicochemical properties of NS under a high-strength ammonia condition. Several studies have proved that the differences in the wastewater nutrient contents could influence the physiological properties of biomass and the chemical composition of the extracted EPS in conventional activated sludge (CAS).10–15 Liao et al.13 found that sludge flocs at the lower sludge retention times (SRTs) were much more irregular and more variable in size with time than those at higher SRTs, and the level of effluent suspended solids at low SRTs was higher than that at high SRTs. Li and Yang14 revealed that the loosely bound EPS (LB-EPS) contents decreased as the SRT lengthened, and the LB-EPS had a negative effect on bioflocculation and sludge–water separation. Durmaz and Sanin15 demonstrated that the fraction between the proteins (PN) and polysaccharides (PS) in EPS changed dramatically to favor the production of a higher amount of PS, and the CAS became much harder to dewater and settle as the COD/N ratio increased. On the contrary, Ye et al.6 pointed out that the amounts of EPS decreased with the increase in COD/N ratio, and the filterability and compactibility of CAS samples were improved considerably with the increase in COD/N ratio. However, Luo et al.7 observed that the flocculent sludge maintained a relatively stable structure and nitrification performance under different COD/N ratios, but the lowest COD/N ratio resulted in a sharp EPS reduction in flocculent sludge. Therefore, to date, the influence of OLRs on the EPS production and floc physicochemical characteristics of sludge is still unclear. Since the sludge properties of NS is significant different from CAS,16 and there are no studies available in the literature detailing the effects of influent OLRs on NS system, the influent OLR shock impact on NS system in disposing of high-strength ammonia wastewater is still a very important topic that needs further investigation.
Biological wastewater treatment plants are usually operated under unsteady-state conditions owing to the frequent variations in influent and process operation. Diurnal, seasonal, and irregular variations in flow rate and substrate strength and composition bring about changes to the NS system in OLRs. The unsteady operational condition often causes a serious deterioration in treatment performance and sludge–water separation.10 However, the variations of EPS production and sludge characteristics under unsteady-state OLR conditions have not been well characterized yet. The influences of EPS on the physicochemical properties of NS in relation to bioflocculation, sludge–water separation, and the sludge surface properties during unstable operation also need to be investigated. The assessment of NS stability and the correlation between EPS and the physicochemical properties under unsteady-state OLR conditions is necessary to provide valuable information to evaluate the BNRP performance and offer a guide to aid process design.
In order to develop an NS system for treating high-strength ammonia wastewater under variable influent conditions, this study is aimed at probing the impact of OLRs between 0 and 0.75 kg COD per kg MLVSS d on NS destabilization and exploring the recovery ability after high OLRs shock. The study covers an evaluation on reactor nutrient performance, an analysis of the key physical characteristic parameters, monitoring the main EPS components, and investigating the correlation between floc physicochemical properties of NS under unsteady-state OLR operational conditions. It is expected that the information derived from this study should be valuable for enhancing the nitrification performance and stability of NS.
2 Materials and methods
2.1 Reactor and operation
The experiment was carried out in a sequencing batch reactor (SBR), with a working volume of 20 L. The reactor was seeded with lab-grown autotrophic nitrifying sludge cultivated with synthetic inorganic ammonia oxidizing medium. The sludge in the reactor was well suspended by a mechanical stirrer and air diffuser. The mixed liquor suspended solids (MLSSs) concentration was kept at 2500–3000 mg L−1. The dissolved oxygen (DO) was supplied by an air pump from the bottom of the reactor and was controlled by a flow meter. The pH was controlled in the range of 7.5–8.0 and the temperature was maintained at 25 °C with a water circulation bath.The reactor was operated in a 12 h cycle mode, comprising five stages: 15 min for feeding, 180 min for anoxic conditions, 480 min for oxic conditions, 30 min for settling, and 15 min for drawing. The reactor was operated with a hydraulic retention time of 24 h. The synthetic high-strength ammonia wastewater was fed with different OLRs (0, 0.15, 0.3, 0.45, 0.75 kg COD per kg MLVSS d). The OLR was adjusted by modifying the influent COD/N in the feed (the influent ammonia concentrations were fixed at 200 mg L−1). Throughout this study, the reactor was subjected to nine different experimental conditions, as described in Table 1. The OLR was gradually increased from runs 1 to 5. Subsequently (runs 5 to 9), the OLR gradually decreased from 0.75 to 0 kg COD per kg MLVSS d. Each condition of specific OLR was operated for 4 weeks. The constituents (per liter) of the synthetic wastewater were as follows: 764.29 mg NH4Cl, 175.6 mg KH2PO4, 18 mg CaCl2, 60 mg MgSO4·7H2O, and 0.38 mL trace metal solution. The carbon content was adjusted by modifying the amount of sodium acetate in the feed. The trace metal solution contained (g L−1) FeCl3·6H2O (375), MnCl2·4H2O (30), ZnSO4·7H2O (30), H3BO3 (37.5), CuSO4·5H2O (7.5), KI (4.5), and EDTA (2500).
| Run | Influent NH4+–N (mg L−1) | Influent COD (mg L−1) | COD/N | OLR (kg COD per kg MLVSS d) | HRT (h) |
|---|---|---|---|---|---|
| 1 (0–30 d) | 200 | 0 | 0 | 0 | 24 |
| 2 (31–60 d) | 200 | 400 | 2 | 0.15 | 24 |
| 3 (61–90 d) | 200 | 800 | 4 | 0.3 | 24 |
| 4 (91–120 d) | 200 | 1200 | 6 | 0.45 | 24 |
| 5 (121–150 d) | 200 | 2000 | 10 | 0.75 | 24 |
| 6 (151–180 d) | 200 | 1200 | 6 | 0.45 | 24 |
| 7 (181–210 d) | 200 | 800 | 4 | 0.3 | 24 |
| 8 (211–240 d) | 200 | 400 | 2 | 0.15 | 24 |
| 9 (241–270 d) | 200 | 0 | 0 | 0 | 24 |
2.2 Physicochemical characterization of the nitrifying sludge
The morphology of the sludge was checked by microscopic examination (Nikon 90i, Japan) and scanning electron microscopy (SEM) (JSM-6510LV, Japan). The filamentous organism content was quantified as filament index using the method by Jenkins et al.17 The most probable number (MPN) technique was adopted to estimate the number of nitrifying bacteria, and the plate colony-counting method was applied to count the heterotrophic bacteria.18The floc size distribution was determined by a laser particle size distribution analyzer (LS230, Beckman Coulter, US). The flocculating ability (FA) of sludge was measured to indicate the performance of the sludge flocculation. The FA of sludge was determined as the reflocculation ability of sludge flocs after disruption, following the method of Jin et al.11 The settleability of the sludge was determined by the sludge volume index (SVI) according to the standard methods.19 MLSS and mixed liquor volatile suspended solids (MLVSS) measurements were carried out gravimetrically.19 The specific resistance of filtration (SRF) was used to evaluate the dewaterability of the flocs, as described by Li and Yang.14 The relative hydrophobicity (RH) of the sludge was measured as the adherence to hydrocarbons. Detailed procedures of the sludge hydrophobicity test are available elsewhere.11,20 For the zeta potential measurement, one milliliter of sludge was diluted to 100 mL with deionized water, and the zeta potential was obtained from a Nanosizer ZS instrument (Nano ZS90, Malvern Instruments Co., UK).
A two-step heat extraction method was adopted to extract the LB-EPS and tightly bound EPS (TB-EPS) from the sludge samples, as reported previously.14 The NS was harvested by centrifugation (4000 g, 5 min) in a 50 mL tube. The dewatered sludge pellet was resuspended in a 0.05% NaCl solution that had a similar salinity to its original sludge of 50 mL. The NaCl solution for dilution was pre-heated to 70 °C to ensure that the sludge suspension reached an immediate warm temperature of 50 °C. The sludge suspension was immediately sheared by a vortex mixer for 1 min, followed by centrifugation at 4000g for 10 min to separate the solids and supernatant. The collected supernatant was regarded as the LB-EPS of the sludge sample. The residual sludge pellet left in the centrifuge tube was resuspended in a 0.05% NaCl solution to its original volume of 50 mL, then heated at 60 °C for 30 min, and finally centrifuged at 4000g for 15 min. The collected supernatant was regarded as the TB-EPS extraction of the sludge.
The three-dimensional (3D) excitation-emission matrix (3D-EEM) spectra of EPS were measured using a fluorescence luminescence spectrometer (FP-6500, Jasco Corporation, Japan). To obtain the EEM spectra of EPS, the emission (Em) and excitation (Ex) wavelengths were increased at 5 nm increments from 240 to 570 nm and from 220 to 480 nm, respectively. The Em and Ex slits were set at 5 nm, and the scanning speed was kept at 1000 nm min−1 for all the samples.
2.3 Chemical analysis
The levels of NH4+–N, NO2−–N, NO3−–N, TN, and COD were measured according to the standard methods.19 The DO concentration was measured by a DO meter (HQ 30d, HACH, US). Both the LB-EPS and TB-EPS extractions were analyzed for total organic carbon (TOC), PN, PS and DNA. TOC was measured by a TOC analyzer (TOC-L CPN, Shimadzu, Japan). PN was analyzed by an adaptation of the Lowry method using casein as the standard, PS was determined using the anthrone method with a glucose standard, and DNA was detected by the diphenylamine colorimetric method.212.4 Statistical analysis
The results are presented here as the mean ± SD (standard deviation). All the statistical analyses were carried out with the software SPSS version 19.0 (IBM Corporation, Armonk, NY, USA).3 Results and discussion
3.1 Effect of organic loading rate shock on nutrient removal
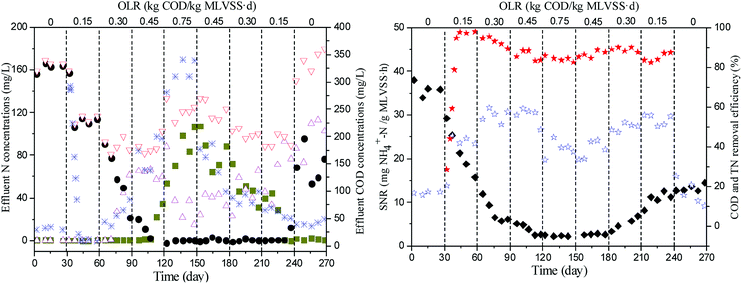
The SBR performance regarding nutrient removal under various OLRs is illustrated in Fig. 1. Since the sludge was cultured with inorganic ammonium oxidizing medium for nearly one year upon operation, the COD concentrations in effluent were minimal. During the early transitional phase after an organic substance was imposed, the effluent COD concentrations were significantly increased. With the acclimation of the biomass under the new process conditions for 10 days, the effluent COD concentrations dropped substantially to about 10 mg L−1, and the COD removal efficiency increased to above 97%. With the increasing COD/N ratio, the effluent COD concentrations rose up to 339.24 mg L−1 at the OLR of 0.75 kg COD per kg MLVSS d. When the original loading conditions resumed after the shock load, the COD concentrations in the effluent decreased gradually from 340.12 mg L−1 to 35.96 mg L−1, but did not recover to the original level of the inorganic condition. The higher effluent COD concentrations under the carbon-limited influent condition might result from cellular lysis.22 During the whole process, the percentage of COD removal was relatively stable at 80%, indicating the variation of OLRs resulted in a slight effect on the COD removal.Throughout the operation, the effluent NH4+–N and NO2−–N were negligible and TN was mainly in the form of NO3−–N in the initial stage. An elevated concentration of nitrite was detected in the effluent when the OLR increased to above 0.30 kg COD per kg MLVSS d, and considerable nitrification inhibition was observed after the shock load of 0.75 kg COD per kg MLVSS d. During the period of decreasing OLRs, the effluent NH4+–N gradually decreased, accompanied with the accumulation of NO2−–N, but NO3−–N production was just partially restored even under inorganic conditions. The accumulation of NH4+–N and NO2−–N under high OLRs was mainly due to the limitation of DO. This may have resulted from the heterotrophs outcompeting the autotrophs for oxygen and space due to their higher specific growth rates and growth yields and the subsequent washing out of the autotrophs.23 The bacteria counting results demonstrated that a sharp decline of nitrifying bacteria was observed during increasing the OLRs (Table 2). Compared with ammonia-oxidizing bacteria (AOB), nitrite-oxidizing bacteria (NOB) were more susceptible to OLR shock. This phenomenon might be due to the higher oxygen affinity constant of NOB. The long-term oxygen stress led to a loss of activity in nitrite oxidation rather than ammonia oxidation.24
| Run | Heterotrophic bacteriaa | Ammonia-oxidizing bacteriab | Nitrite-oxidizing bacteriab |
|---|---|---|---|
| a The unit is cfu mg−1 MLVSS. b The unit is cells per mg MLVSS. | |||
| 1 | 0.45 × 105 | 1.46 × 104 | 1.93 × 104 |
| 2 | 0.65 × 105 | 1.25 × 104 | 1.34 × 104 |
| 3 | 0.38 × 106 | 0.98 × 104 | 0.44 × 104 |
| 4 | 1.12 × 106 | 0.75 × 104 | 2.16 × 102 |
| 5 | 0.86 × 106 | 0.56 × 104 | 0.64 × 102 |
| 6 | 1.48 × 106 | 0.62 × 104 | 0.96 × 102 |
| 7 | 0.62 × 106 | 0.87 × 104 | 1.67 × 102 |
| 8 | 1.18 × 105 | 0.96 × 104 | 7.25 × 102 |
| 9 | 0.53 × 105 | 1.15 × 104 | 0.37 × 104 |
During the operation, the specific nitrification rate decreased dramatically from 38.06 to 2.23 mg NH4+–N g−1 MLVSS h with the OLRs increasing from 0 to 0.75 kg COD/kg MLVSS d, but just recovered to 14.58 mg NH4+–N g−1 MLVSS h when the OLR was reduced to 0. Furthermore, simultaneous denitrification occurred at all operation stages, whereby the denitrification rate improved markedly after the addition of organic carbon with the OLRs increasing from 0 to 0.30 kg COD per kg MLVSS d, but seriously deteriorated as the OLRs increased to 0.75 kg COD per kg MLVSS d. These results indicated that sufficient electron donors were necessary for denitrification, but an overload of organic substrates under the high-strength ammonia condition would cause the inhibition of nitrification, which would significantly affect the activities of the heterotrophic and autotrophic microorganisms. Numerous reports have indicated that the accumulation of ammonia or nitrite not only brings damage to nitrifying bacteria, but also inhibits the growth of denitrifying bacteria. The main causes are believed to be the inhibitory effects of free ammonia and free nitrous acid, which can affect the metabolic processes of microorganisms.8,9,25
3.2 Effect of organic loading rate shock on the morphology of NS
The typical morphology of sludge flocs under different OLRs are shown in Fig. 2. The sludge floc was in a regular and dense structure at low OLRs, and changed to a scattered floc form with the increase in OLRs. No filamentous microorganisms were found at the initial OLRs of 0 and 0.15 kg COD per kg MLVSS d, whereas high levels of filamentous microorganisms existed under OLRs of 0.45 and 0.75 kg COD per kg MLVSS d. The deficiency of DO at high OLRs might be the main contributing factor to the excessive proliferation of filamentous microorganisms.26 Moreover, the mean size of the sludge flocs varied between 48 and 144 µm (Table 3). With the increase in OLRs, the floc size increased slightly from 102.03 ± 1.15 to 108.68 ± 2.42 µm at the OLR of 0.15 kg COD per kg MLVSS d, and then decreased rapidly to 47.65 ± 1.92 µm at the OLR of 0.75 kg COD per kg MLVSS d. Up to now, the results from the literature are contradictory regarding the impact of OLR on floc size distribution.12,13,27,28 Previous studies showed that the floc size and F/M were positively7,12,27 or negatively28 correlated or even that there was no clear relationship.6,13 This discrepancy might result from the distinct operating conditions, types of configuration, and the ranges of OLRs tested. | ||
| Fig. 2 The SEM images of nitrifying sludge flocs under different organic loading rates (R1–R9 represent the operation of run 1 to run 9). | ||
| Run | Mean floc size (µm) | FA (%) | SVI (mL g−1) | SRF (1010 m kg−1) |
|---|---|---|---|---|
| 1 | 102.3 ± 1.5 | 52.4 ± 2.1 | 37.1 ± 1.6 | 0.6 ± 0.1 |
| 2 | 108.6 ± 2.4 | 43.4 ± 1.4 | 48.8 ± 2.8 | 1.8 ± 0.3 |
| 3 | 69.3 ± 2.1 | 35.2 ± 3.2 | 60.6 ± 3.1 | 31.2 ± 3.7 |
| 4 | 50.2 ± 1.1 | 29.8 ± 2.2 | 105.3 ± 5.4 | 174.2 ± 13.2 |
| 5 | 47.7 ± 1.9 | 24.3 ± 1.4 | 219.9 ± 9.7 | 506.5 ± 34.1 |
| 6 | 59.3 ± 2.6 | 22.8 ± 1.9 | 312.6 ± 24.2 | 352.4 ± 22.4 |
| 7 | 89.5 ± 1.7 | 27.9 ± 2.1 | 246.8 ± 16.7 | 81.6 ± 7.4 |
| 8 | 132.5 ± 2.8 | 35.7 ± 1.7 | 195.5 ± 11.2 | 28.5 ± 4.2 |
| 9 | 143.2 ± 3.4 | 48.6 ± 2.9 | 123.6 ± 7.8 | 13.2 ± 2.7 |
When the OLRs were decreasing, the contents of filamentous microorganisms decreased, the mean floc size increased, and the sludge floc structure became regular and compacted. At the end of the investigation, the size of the sludge floc rose up to 143.26 ± 3.44 µm at the OLR of 0, but a certain extent of filamentous microorganisms still existed in the sludge sample. Previous study suggested that floc morphology was related to both substrate and DO gradient.29 Liao et al.13 found that the irregular floc morphology was related to both substrate and oxygen diffusion limitation, and smooth flocs predominated when substrate and oxygen gradients were not important. In this study, the sludge presented a large size and compact structure at low OLRs, the substrate was limited for the growth of sludge and starved conditions produced a more stable biomass. This is consistent with nitrifying organisms that are known to grow in dense microcolonies that generally form the strongest fraction of the flocs.16 While at high OLRs, the low DO with a high F/M created a DO diffusion limitation in flocs and favored the growth of heterotrophic bacteria that formed more irregular floc morphology. The continuous increase of OLRs could also cause large disturbance to the growth and adhesion properties of nitrifying organisms, and finally led to the breakup of sludge into smaller flocs. It can be inferred that the stability of nitrifying organisms to the OLRs shock under high-strength ammonia condition is important for floc structure.
3.3 Effect of organic loading rate shock on sludge flocculation, settleability and dewaterability
The flocculation of sludge was evaluated by the FA at the end of each phase. The change of OLRs resulted in marked variations in flocculation, and poorer sludge flocculation was observed with the increase in OLRs (Table 3). The FA decreased from 52.42 ± 2.14% to 22.86 ± 1.89% and then recovered to 48.64 ± 2.19% with the change in OLRs. This result was in agreement with previous findings that a lower F/M apparently resulted in a better sludge flocculation and effluent clarification, and an overload of organic substrates would lead to the deterioration of the sludge flocculation ability.14 However, Ye et al.6 stated a different viewpoint, where a seriously deteriorated flocculation of sludge occurred when a low COD/N ratio was imposed, and the sludge flocculation ability could be restored completely as the COD/N ratio returned back to the original level. In this study, the incomplete recovery of sludge flocculation was observed when the OLRs decreased. This phenomenon might have been due to the special high-strength ammonia environment, which severely affected the microbial metabolisms under the dynamic OLRs operational conditions.The settleability of sludge is desirable at the early phase (shown in Table 3). However, with the increasing OLRs, the sludge became worse in settleability, and the occurrence of sludge bulking was also observed. The SVI increased dramatically to 312.65 ± 20.89 mL g−1 at an OLR of 0.75 kg COD per kg MLVSS d. When reducing the OLRs, the settleability of the sludge was enhanced greatly, with the SVI decreasing gradually to around 120–130 mL g−1, but it still failed to get back to its original levels. Generally, a higher degree of settleability is related with the strongly flocculated flocs of activated sludge. Very small flocs or big flocs with a high number of filamentous microorganisms all could lead to a relatively poor settleability.6 Besides, a poor settleability of sludge could also be caused by the excessive production of carbonaceous polymers, which physically prevent the cells from forming close contact.30 In this study, the dispersed flocs and excessive carbohydrate production were all observed at high OLRs (Table 3 and Fig. 3), which might be the combination causes for the deteriorated sludge settleability at high OLRs.
 | ||
| Fig. 3 Effect of organic loading rate shock on the EPS components of nitrifying sludge (■ PN; ○ PS; ▲ DNA; ◇ TOC; ★ PN/PS). | ||
As shown in Table 3, the dewaterability of the sludge was excellent at low OLRs, but did not change significantly when the OLRs increased from 0 to 0.30 kg COD per kg MLVSS d, while the SRF increased by two orders of magnitude as the OLR increased to 0.45 kg COD per kg MLVSS d. When decreasing the OLRs, the SRF values recovered gradually, but the sludge was still difficult to dehydration, even at lower OLRs. The results were consistent with other reports. For example, Durmaz and Sanin15 stated that SRF decreased slightly and the filterability of CAS did not change significantly when the COD/N ratio slightly increased, but when the COD/N ratio increased further, SRF increased about hundred times. Ye et al.6 found that the sludge dewaterability deteriorated whenever the COD/N ratio increased or decreased, but more significantly as the COD/N ratio decreased. In addition, it was reported that the dewaterability of sludge might be determined by the distribution of the floc size, whereby particles in the range of 1–100 µm had the greatest negative influence on the dewaterability of the activated sludge, and the dewaterability declined with the increase in the concentration of particles in this size range.31 The increased small particles observed in this study at high OLRs might be the major contributing factor for the decline in the dewaterability of NS.
The results mentioned above indicate that a significant shock of OLRs has an adverse impact on the performance of the sludge–water separation of NS. Different from the easier recovery of CAS treating municipal wastewater, the sludge–water separation properties of NS treating high-strength ammonia wastewater are difficult to restore after a high OLR shock. It is thus necessary to maintain a stable operation of an NS system with a minimum variation in process conditions, which is important for the favorable performance of nutrient removal and biosolids–water separation.
3.4 Effect of organic loading rate shock on EPS production and the components
The structure of bound EPS is generally depicted by a two layer model: the inner layer consists of TB-EPS, which has a definite shape and is bound stably to microbial cells, while the outer layer comprises LB-EPS, which is a loose and dispersible slime layer without an obvious edge.32 Fig. 3 shows that the shift of OLRs resulted in considerable variations in the constituents of EPS. The LB-EPS contents changed significantly with the variation of OLRs, which kept at a higher level at low OLRs, but then decreased with the increase in OLRs. Compared to LB-EPS, the changes of TB-EPS during the operation were less significant, and stabilized at an average content of 50 mg g−1 MLVSS. The change of the LB-EPS content appeared to be more significant than that of the TB-EPS, which was in agreement with the other observations.6,14,33 As is well known, metabolic products are more readily disturbed in the outer layer of the cells, thus influencing the yield of LB-EPS.14 In the present study, the increased OLRs strongly inhibited the microbial activities under the high-strength ammonia condition, causing significant changes in LB-EPS secretion as active responses to the shock of OLRs. TB-EPS accounting for more than 80% of the total EPS was reported to be responsible for the cell adhesion and attachment in the inner floc structure through strong interactions.30,32 Due to the special structural characteristics of EPS, the TB-EPS contents could remain relatively stable as the culture conditions changed.As PN and PS are thought to be the major components of EPS, their contents were determined during our investigations. For both TB-EPS and LB-EPS, PN was the predominant component at lower OLRs, while PS was the major constituent at higher OLRs. In TB-EPS, PN decreased and PS increased with the increasing OLRs, while afterwards PN increased and PS decreased with the gradual recovery of the OLRs. Moreover, the variation of PN in LB-EPS was consistent with TB-EPS, which decreased with the increasing OLRs, while PS was independent of the OLRs. No significant differences in DNA contents in TB-EPS were observed, compared to those closely related to the OLRs in LB-EPS, which indicated that DNA was more likely to bind on the cell surface.34 The DNA and PN contents in LB-EPS in the same trends showed that a large amount of the content was accumulated at lower OLRs, especially in the final stage, in which the increased lysis of the microorganisms occurred at a low F/M. The presence of DNA in EPS mainly resulted from cell lysis, which indicated indirectly that cell lysis might be one of the mechanisms contributing to the presence of PN in LB-EPS. In addition, no correlation was observed between PN and DNA contents in TB-EPS; thereby it was likely that the production of PN in TB-EPS might be mainly due to the secretion of microbial cells.
Compared with the irregular changes of the PN and PS contents, the PN/PS ratio in LB-EPS or TB-EPS decreased with the increasing OLRs, indicating that lower OLRs tended to produce EPS with a higher PN/PS ratio, and that excessive influent carbon substrates might lead to a decrease in the PN/PS ratio. This was consistent with previous findings that sludge growing on wastewater with a low F/M tended to produce EPS with a high PN/PS ratio.16,34 In addition, some recent studies found that the structures of EPS in nitrifier flocs had special characteristics compared to the conventional biofilm and activated sludge, whereby the content of PN was much higher than that of the PS.16 The present study demonstrated that the ratio of PN/PS was significantly affected by the nutrient substance, and supported the viewpoint that bacteria tended to convert excess carbon substrates into intracellular storage compounds, like PS, in EPS at high OLRs; whereas at low OLRs, the majority of the carbon source was used in biomass synthesis instead of PS production, and intracellular PN was released due to increased lysis and endogenous respiration.27,34
The variations in EPS observed in this study under unsteady-state conditions appeared to be related directly to the dynamic changes in the OLRs. For instance, some researchers have found that a decrease in OLR was positively related to the total EPS quantity,15,35 while others have suggested that EPS was negatively related to the OLR,36 or even independent of the OLR.34 Such inconsistent or even controversial conclusions might be partly attributed to the different system types and evaluation methods used in these studies and the unknown mechanisms controlling EPS production in sludge flocs. There is no doubt that the external environmental conditions exhibit significant effects on EPS production and composition. Specifically in term of feedwater characteristics, the operational parameters (pH, temperature, DO, SRT, HRT, F/M, and MLSS) and the presence of exogenous toxic substances are important factors affecting the production of EPS.32 Although the inconsistent results were always obtained from different studies, many previous studies have indicated that the EPS abundance of the sludge became more stable if the change was favorable to the microbial community. In contrast, when unfavorable process changes were imposed, the EPS production would change significantly.10 Therefore, it is well demonstrated that the stable operation of NS system under dynamic OLRs operational conditions is important to microbial EPS secretion, in terms of responding to the excellent performance of sludge–water separation.
3.5 EEM spectra of EPS
For further analysis on EPS, 3D-EEM spectroscopy was applied to study the change in the fluorescent expression of LB-EPS and TB-EPS extracted from NS with the variation of OLRs (as shown in Fig. 4). It can be obviously observed from the 3D-EEM results that not only the major fluorescence substances but also the peak locations and fluorescence intensities of the EPS samples regularly change, regardless of LB-EPS and TB-EPS. | ||
| Fig. 4 EEM spectra of LB-EPS and TB-EPS extracted from nitrifying sludge under different organic loading rates (R1–R9 represent the operation of run 1 to run 9). | ||
Four fluorescence peaks (peaks A, B, C, and D) could be readily identified from the 3D-EEM spectra of LB-EPS. Peak A and peak B were observed at Ex/Em wavelengths of 230–240/330–355 nm and 280–290/330–360 nm, respectively, which were assigned to aromatic PN-like substances and tryptophan PN-like substances. Peak C was identified at an Ex/Em of 350/445 nm, which was related to humic acid-like substances. Peak D was located at 275–285/450 nm, representing the fulvic acid-like substances.37,38 At the initial stage with the OLR of 0, the aromatic PN-like substances (peak A) were found to be the prominent peak in the EEM spectra of LB-EPS, and gradually disappeared as the COD/N increased to 4. The tryptophan PN-like substances (peak B) showed few variations in LB-EPS under all OLRs, except for high fluorescence intensities observed at the final cultural period at the OLR of 0. This result was consistent with the photometric data that PN in LB-EPS decreased with the increase in OLRs, which indicated that more PN would be synthesized under carbon-limited conditions. The fluorescence intensities of the humic acid-like substances (peck C) strengthened with the increase in OLRs, peaking at an OLR of 0.75 kg COD per kg MLVSS d, and then gradually decreasing. The fourth peak, for fulvic acid-like substances (peak D), was only detected in the EEM spectrum of LB-EPS at high OLRs. Previous studies showed that LB-EPS plays a more important role in the flocculability of activated sludge, since it might function as the primary surface for cell flocculation and attachment.14 Accordingly, the deteriorated flocculability caused by excessive F/M might be closely correlated with the destroyed LB-EPS structures.
Five peaks with different fluorescence intensities were readily identified from the EEM spectra of TB-EPS. A similar fluorescence signal about peaks A and B was observed in different OLRs conditions of TB-EPS, suggesting that some constituents of the PN-like substances were universal in NS, and that the organic loads did not change the PN-like substances fluorescent expression of TB-EPS significantly. The predominance of PN in EPS may be due to the presence of a large quantity of exoenzymes, whereby the easy degradation and uptake of readily biodegradable organic substrates gives rise to a high level of exoenzymes in the EPS matrix.14 In terms of the fluorescent intensity, peak B was found to be the primary peak in the EEM spectra of TB-EPS, which implied that these tryptophan PN-like substances played an important role in the bioaggregate.39 However, the fluorescence intensities of humic acid-like substances (peck C) and fulvic acid-like substances (peck D) became stronger as the OLRs increased, and then gradually weakened with the decrease in OLRs. Besides the four peaks observed in LB-EPS, a fifth peak (peak E, Ex/Em = 320/380 nm) was also detected in the EEM spectrum of TB-EPS, which might be one of intermediate products corresponding to visible marine humic acids.38 Peak E appeared at an OLR of 0.15 kg COD per kg MLVSS d and gradually strengthened with the increase in OLRs. Compared with the PN-like substances, this suggested that the structures of humic acid-like substances in TB-EPS were significantly affected by the high levels of organic substrates. Humic compounds are considered as an important component of EPS in activated sludge.21 Some researchers suggested that humic compounds in EPS were mainly adsorbed from the wastewater and hydrolysis process.40 Previous studies demonstrated that these humic acid-like substances and fulvic acid-like substances were likely to deteriorate to sludge flocculation, which reasonably interpreted the deterioration of flocculability under high OLRs.12,21,41 The increase in humic acid-like substances in the operation of NS might be used as an important indicator of floc structure deterioration since it could affect the bioflocculation.
In this study, the 3D-EEM spectroscopy results qualitatively described the change in the fluorescent expression of EPS with the variation of OLRs. It was clearly demonstrated that the tryptophan PN-like substances were the major components in EPS at low OLRs, while high levels of humic acid-like and fulvic acid-like substrates were produced at high OLRs. However, spectrally overlapping fluorescence components could be identified, thus affecting the acquirement of their relative concentrations determined by the fluorescence intensities of the EEMs. Further study should be conducted to discuss the quantitative information on the individual EPS components by a parallel factor analysis. Besides, some other spectroscopy techniques should also be used to elucidate the functional groups and element compositions in EPS.
3.6 Effect of organic loading rate shock on the surface properties of sludge flocs
Microbial cells and sludge flocs carry negative charges due to the ionization of the anionic functional groups, such as carboxylic, sulfate, and phosphate.26 The relationship between the OLR and zeta potential of sludge at neutral pH is shown in Fig. 5. The zeta potential became more negative with the increase in OLRs, whereby a significantly higher negative value of zeta potential (−36.16 ± 0.78 mV) was found for sludge at a high OLR of 0.75 kg COD per kg MLVSS d, but the value shot up to −26.68 ± 1.24 mV at an OLR of 0. Liao et al.34 also found that the charge of sludge surfaces became more negative at lower SRT conditions. A deeper interpretation of the results of the OLRs versus the surface charge of sludge may involve the variation of EPS, since the amino groups in PN carry positive charges and can neutralize some of the negative charges from carboxyl and phosphate groups.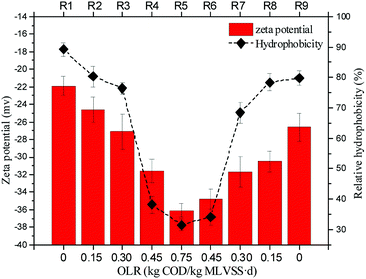 | ||
| Fig. 5 Effect of organic loading rate shock on the zeta potential and on the relative hydrophobicity of nitrifying sludge. | ||
Hydrophobic interactions result from the behavior of particles or molecules incapable of interacting electrostatically or establishing hydrogen bonds with water, which are therefore drawn together when plunged in an aqueous phase.42 As shown in Fig. 4, the highest hydrophobicity peaked at 89.25 ± 2.15% at the initial stage with the OLR of 0. When increasing the OLR, the RH decreased significantly to 31.54 ± 1.92% at an OLR of 0.75 kg COD per kg MLVSS d, and then recovered to 79.83 ± 2.44% with the decrease of OLR to 0. A similar phenomenon was also found in other studies. Liao et al.34 observed that sludge at low OLRs were significantly more hydrophobic than that at high OLRs. Durmaz and Sanin15 found the hydrophobicity of sludge decreased from 86% to 64% when the COD/N ratio increased from 9 to 43. The RH of the sludge expressed in this study, as an average of the hydrophobicity of polymeric compounds and the bacterial cells, indicated the presence of both hydrophobic and hydrophilic groups in the sludge polymers. Under carbon-limited conditions, the amount of nitrogen in the influent was higher than the stoichiometrically required nitrogen by the microorganisms, which could utilize this excess nitrogen in the synthesis of PN, resulting in a higher hydrophobicity, while the excess carbon in the influent at high OLRs was used for PS production, which then coats the cell surfaces, covering the hydrophobic sites and lowering the measured cell surface hydrophobicity.30
3.7 Relationships between EPS and floc physical properties
To further understand the effect of OLR on the characteristics of NS, statistical analysis was used to compare the relationships among EPS, surface properties, and floc physical properties. No matter in LB-EPS or TB-EPS, the experimental data revealed that the sludge flocculation, settleability, and dewaterability were significantly correlated with PN and the PN/PS ratio (ANOVA, P < 0.05), but had no obvious correlations with the PS, DNA, and total EPS content (ANOVA, P > 0.05). These results suggest that PN was probably more important than PS for bioflocculation and the settling property of the sludge, and that the increased PN and PN/PS ratio resulted in an improved flocculation, settleability, and dewaterability. The decisive role of PN in flocculation, settleability, and dewaterability was consistent with previous findings.6,14,20,31 Higgins and Novak31 concluded that the bound PN was of more significance than PS to sludge dewaterability and settling, as they found that the degradation of PN by proteolytic enzymes resulted in the disintegration of sludge and a deteriorated dewaterability. Zhu et al.20 indicated that the increase in PN/PS ratio could effectively enhance the cohesion between the aggregates, and maintain a dense, stable structure. On the contrary, Ye et al.6 found that excessive PN in LB-EPS would impact bioflocculation and the settling property of the activated sludge and there were no correlations between the floc physical properties and TB-EPS in their studies.The results from this study suggest that the constituents of EPS play an important factor in controlling biomass–liquid separation. The other possible explanation would be based on the potentially significant difference in microbial communities under different OLRs. Seetha et al.43 reported that the shock loads changed the biodiversity and the dominant bacterial types of the reactor from Gram-positive rods to Gram-negative oval-shaped bacteria. The potential relationship between microbial communities and EPS production in NS needs further studies using molecular tools in the future to develop new insights in bioflocculation.
As there are many charged functional groups in EPS, their content and composition influence the surface charge and hydrophobicity of sludge flocs.5 It was found that the PN content was the most important factor in determining the surface properties of flocs than any other constituents. The statistical analysis showed that the PN content in LB-EPS and TB-EPS had significant positive correlations with the zeta potential and RH (ANOVA, P < 0.05). This finding could be related to the unique functional groups in PN. Most of the PN is formed by hydrophobic amino acids (glycine, alanine, etc.), which have important functions on the surface hydrophobicity of sludge. Many researchers confirmed that the positively charged amino group in PN could neutralize the negative charges of PS, uronic acid, and carboxylic acid in DNA, phosphate groups, etc., thereby reducing the sludge surface charge and enhancing the sludge surface hydrophobicity.12,13,44 In addition, PN bonding with the metal ions also reduced cell surface electronegativity, thus contributing to microbial aggregation.20 Therefore, the PN in sludge EPS could accurately reflect the sludge properties, and effectively change the surface properties of sludge, thus contributing to cohesion between the aggregates to maintain a dense, stable structure.
Due to the impact of EPS, the surface charge and the hydrophobicity of flocs consequently affect the bioflocculation and settling. Statistical analyses indicated that the zeta potential and RH were negatively correlated with the SVI and SRF (ANOVA, P < 0.05), but positively correlated with the FA (ANOVA, P < 0.05). Previous studies found that the surface properties were crucial in controlling the flocculation, settleability, and dewaterability of sludge flocs.11,13,20,44 According to thermodynamic theory, the Gibbs free energy in the sludge surface decreases with the increase in cell surface hydrophobicity, thus enhancing the flocculation ability of sludge flocs.45 Moreover, the increase in surface negative charges of bacterial cells would lead to strong electrostatic repulsion among microorganisms, and therefore cause a weaker bonding between the cells.11 Therefore, flocs with high negative surface charges and low hydrophobicity would be weak in floc strength and poor in aggregation properties.
4 Conclusions
The performances of nitrification and biomass–liquid separation of NS seriously deteriorated due to an overload of organic substrates in disposing the high-strength ammonia wastewater, and could not be recovered completely by decreasing the OLRs. The shift of OLRs resulted in considerable variations in the EPS constituents. The LB-EPS contents decreased with the increase in OLRs, but the TB-EPS change was less significant. For both the LB-EPS and TB-EPS, PN was the predominant component at low OLRs, and PS was the major constituent at high OLRs, otherwise the PN/PS ratio decreased with the increase in OLRs. Moreover, 3D-EEM spectroscopy revealed that the tryptophan PN-like substances were the major components in EPS at low OLRs, while high levels of humic acid-like and fulvic acid-like substrates were produced at high OLRs. Furthermore, statistical analysis showed that PN and the PN/PS ratio were in significant correlation with the sludge surface properties, flocculation, settleability, and dewaterability. It was indicated that PN in EPS was the most important factor determining the physicochemical properties of flocs, which effectively changed the surface properties and bioflocculation of NS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study is supported by the Natural Science Basic Research Program of Shaanxi Province (Grant No. 2018JQ5157), the Scientific Research Program of Shaanxi Provincial Education Department (Grant No. 17JS079), and the Science and Technology Co-ordination Innovation Plan of Shaanxi Province, China (2015KTZDSF01-06).Notes and references
- J. Carrera, J. A. Baeza, T. Vicent and J. Lafuente, Water Res., 2003, 37, 4211–4221 CrossRef CAS PubMed.
- L. Racz, T. Datta and R. Goel, Bioresour. Technol., 2010, 101, 6454–6460 CrossRef CAS PubMed.
- X. W. Zhang, J. Zhang, Z. Hu, H. J. Xie, D. Wei and W. K. Li, RSC Adv., 2015, 5, 61345–61353 RSC.
- B. Wu, S. Yi and A. G. Fane, Bioresour. Technol., 2011, 102, 6808–6814 CrossRef CAS PubMed.
- Y. Liu and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2003, 33, 237–273 CrossRef CAS.
- F. X. Ye, Y. F. Ye and Y. Li, J. Hazard. Mater., 2011, 188, 37–43 CrossRef CAS PubMed.
- J. H. Luo, T. W. Hao, L. Wei, H. R. Mackey, Z. Q. Lin and G. H. Chen, Water Res., 2014, 62, 127–135 CrossRef CAS PubMed.
- Q. Yao, D. C. Peng, B. Wang, Y. Y. Chen, J. Q. Li, Q. D. Zhao and B. B. Wang, J. Biosci. Bioeng., 2017, 124, 319–326 CrossRef CAS PubMed.
- J. W. Wu, Q. Yang, W. Luo, J. Sun, Q. X. Xu, F. Chen, J. W. Zhao, K. X. Yi, X. L. Wang, D. B. Wang, X. M. Li and G. M. Zeng, Chem. Eng. J., 2018, 336, 28–37 CrossRef CAS.
- S. F. Yang and X. Y. Li, Process Biochem., 2009, 44, 91–96 CrossRef CAS.
- B. Jin, B. M. Wilén and P. Lant, Chem. Eng. J., 2003, 95, 221–234 CrossRef CAS.
- B. M. Wilén, D. Lumley, A. Mattsson and T. Mino, Water Res., 2008, 42, 4404–4418 CrossRef PubMed.
- B. Q. Liao, I. G. Droppo, G. G. Leppard and S. N. Liss, Water Res., 2006, 40, 2583–2591 CrossRef CAS PubMed.
- X. Y. Li and S. F. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- B. Durmaz and F. D. Sanin, Environ. Technol., 2003, 24, 1331–1340 CrossRef CAS PubMed.
- Z. W. Liang, W. H. Li, S. Y. Yang and P. Du, Chemosphere, 2010, 81, 626–632 CrossRef CAS PubMed.
- D. Jenkins, M. G. Richard and G. T. Daigger, Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems, IWA Publishing, UK, 2004 Search PubMed.
- Z. G. Li, Y. M. Luo and Y. Teng, Soil and environmental microbiological method, Science Press, Beijing, 2008 Search PubMed.
- N. E. P. A. Chinese, Water and Wastewater Monitoring Methods, Chinese Environmental Science Publishing House, Beijing, 4th edn, 2002 Search PubMed.
- L. Zhu, M. L. Lv, X. Dai, Y. W. Yu, H. Y. Qi and X. Y. Xu, Bioresour. Technol., 2012, 107, 46–54 CrossRef CAS PubMed.
- B. Frølund, R. Palmgren, K. Keiding and P. Nielsen, Water Res., 1996, 30, 1749–1758 CrossRef.
- A. C. Texier and J. Gomez, Can. J. Microbiol., 2004, 50, 943–949 CrossRef CAS PubMed.
- H. S. Lee, S. J. Park and T. I. Yoon, Process Biochem., 2002, 38, 81–88 CrossRef CAS.
- Z. M. Fu, F. L. Yang, F. F. Zhou and Y. Xue, Bioresour. Technol., 2009, 100, 136–141 CrossRef CAS PubMed.
- Y. Zhou, A. Oehmen, M. Lim, V. Vadivelu and W. J. Ng, Water Res., 2011, 45, 4672–4682 CrossRef CAS PubMed.
- B. Tansel, J. Environ. Manage., 2018, 205, 231–243 CrossRef CAS PubMed.
- L. Hao, S. N. Liss and B. Q. Liao, Water Res., 2016, 89, 132–141 CrossRef CAS PubMed.
- A. D. Andreadakis, Water Res., 1993, 27, 1707–1714 CrossRef CAS.
- A. M. P. Martins, J. J. Heijnen and M. C. M. van Loosdrecht, Appl. Microbiol. Biotechnol., 2003, 62, 586–593 CrossRef CAS PubMed.
- T. T. More, J. S. S. Yadav, S. Yan, R. D. Tyagi and R. Y. Surampalli, J. Environ. Manage., 2014, 144, 1–25 CrossRef CAS PubMed.
- M. J. Higgins and J. T. Novak, J. Environ. Eng. Div. (Am. Soc. Civ. Eng.), 1997, 123, 479–485 CrossRef CAS.
- Y. H. Shi, J. H. Huang, G. M. Zeng, Y. L. Gu, Y. N. Chen, Y. Hu, B. Tang, J. X. Zhou, Y. Yang and L. X. Shi, Chemosphere, 2017, 180, 396–411 CrossRef CAS PubMed.
- C. Hong, Y. X. Si, Y. Xing, Z. Q. Wang, Q. Qiao and M. Liu, RSC Adv., 2015, 5, 23383–23390 RSC.
- B. Q. Liao, D. G. Allen, I. G. Droppo, G. G. Leppard and S. N. Liss, Water Res., 2001, 35, 339–350 CrossRef CAS PubMed.
- A. P. Miqueleto, C. C. Dolosic, E. Pozzi, E. Foresti and M. Zaiat, Bioresour. Technol., 2010, 101, 1324–1330 CrossRef CAS PubMed.
- D. T. Sponza, Enzyme Microb. Technol., 2003, 32, 375–385 CrossRef CAS.
- D. Wei, T. Yan, K. Y. Zhang, Y. Chen, N. Wu, B. Du and Q. Wei, Bioresour. Technol., 2017, 240, 171–176 CrossRef CAS PubMed.
- D. Wei, B. Du, J. Zhang, Z. Hu, S. Liang and Y. R. Li, Bioresour. Technol., 2015, 190, 474–479 CrossRef CAS PubMed.
- B. B. Wang, D. C. Peng, Y. P. Hou, H. J. Li, L. Y. Pei and L. F. Yu, Water Res., 2014, 58, 1–8 CrossRef CAS PubMed.
- B. B. Wang, X. T. Liu, J. M. Chen, D. C. Peng and F. He, Water Res., 2018, 129, 133–142 CrossRef CAS PubMed.
- W. J. Zhang, S. W. Peng, P. Xiao, J. He, P. Yang, S. W. Xu and D. S. Wang, RSC Adv., 2015, 5, 1282–1294 RSC.
- F. Jorand, F. P. Boue-Bigne, J. C. Block and V. Urbain, Water Sci. Technol., 1998, 37, 307–315 CrossRef CAS.
- N. Seetha, R. Bhargava and P. Kumar, Bioresour. Technol., 2010, 101, 3060–3066 CrossRef CAS PubMed.
- L. L. Yan, Y. Liu, Y. Wen, Y. Ren, G. X. Hao and Y. Zhang, Bioresour. Technol., 2015, 179, 460–466 CrossRef CAS PubMed.
- X. M. Liu, G. P. Sheng, H. W. Luo, F. Zhang, S. J. Yuan, J. Xu, R. J. Zeng, J. G. Wu and H. Q. Yu, Environ. Sci. Technol., 2010, 44, 4355–4360 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |