Open Access Article
Open Access ArticleSulfur–oleyl amine platelet derivatives with liquid crystalline behavior†
Vasileios Tzitzios *ad,
Konstantinos Dimosb,
Ioannis Lelidis
*ad,
Konstantinos Dimosb,
Ioannis Lelidis c,
Nikos K. Boukosd,
Vijay S. Wadia,
Georgia Basinaa,
George Nounesise and
Saeed M. Alhassan
c,
Nikos K. Boukosd,
Vijay S. Wadia,
Georgia Basinaa,
George Nounesise and
Saeed M. Alhassan *a
*a
aDepartment of Chemical Engineering, Khalifa University of Science and Technology, Petroleum Institute, P. O. Box 2533, Abu Dhaabi, United Arab Emirates. E-mail: v.tzitzios@inn.demokritos.gr; vasileios.tzitzios@ku.ac.ae; saeed.alkhazraji@ku.ac.ae
bCambridge Graphene Centre, University of Cambridge, Cambridge CB3 0FA, UK
cDepartment of Physics, National and Kapodistrian University of Athens, 15784 Athens, Greece
dInstitute of Nanoscience and Nanotechnology, NCSR Demokritos, 15310, Athens, Greece
eBiomolecular Physics Laboratory, NCSR Demokritos, 15310 Athens, Greece
First published on 12th December 2018
Abstract
A novel sulfur-based platelet derivative was synthesized by reacting elemental sulfur with oleyl amine. The sulfur–oleyl amine (S–OA) derivative has an ionic salt form, layered morphology and forms a highly lamellar structure. Polarized optical microscopy (POM) clearly shows the birefringent lyotropic liquid crystalline behavior of the S–OA platelets dispersions.
Sulfur is among the most abundant elements in the earth's crust and the tenth most common element, by mass, in the universe. Egyptians were using sulfur to bleach cotton fabric, Greeks and Romans utilized it as an antiseptic and topical pharmaceutical, while the Chinese were using sulfur in explosives. In the modern age, a large excess of elemental sulfur is produced as a by-product in the petroleum refining industry, from the hydrodesulfurization process, leading the research community to extensively investigate the conversion of elemental sulfur to valuable products. Up to now, the most important uses of elemental sulfur are the production of sulfuric acid and fertilizer. However, the existing applications have a relatively low sulfur demand with seven million tons annual excess; which is stored in powder form or as solid bricks in massive deposits.1 Although sulfur has some very interesting properties such as high electrochemical capacity,2–4 and high refractive index,5 the synthesis of stable and high sulfur content materials is still in its early stage of development. Subsequently, the development of novel chemistry and processing methodologies for elemental sulfur utilization is of great importance.
Up to now, the synthesis of stable sulfur based polymeric materials in one of the most challenging topics for sulfur utilization, and during the last decade a great effort has been made though the inverse vulcanization of elemental sulfur in the presence of divinylic species as a cross linker.6,7 On the other hand, an alternative approach for sulfur utilization is the usage of amine–elemental sulfur complexes for the synthesis of metal sulfides nanoparticles.8 Until now, a plethora of metal sulfide nanoparticles, which are promising candidates for many technological areas, such as semiconductors, have been successfully synthesized utilizing mainly amine-elemental sulfur chemistry.9,10
Here, we are presenting the formation of a sulfur–oleyl amine (S–OA) platelet derivative following a facile low temperature methodology. The material was prepared unintentionally while we were working on the synthesis of metal sulfides utilizing amine-elemental sulfur solutions as a sulfur source. After months we observed the formation of a goldish precipitate that behaved similarly to other platelet materials which show liquid crystalline behaviour such as graphene oxide, and more.11–14 To the best of our knowledge this is the first example of a sulfur/organic hybrid platelet derivative which furthermore shows liquid crystalline behaviour.
Sulfur–oleyl amine solutions were prepare by adding 100–600 mg of elemental sulfur powder (Aldrich, reagent grade) in 5 mL of oleyl amine (Aldrich >98% primary amine) in a 20 mL vial. The mixture was heated up to 100 °C under gentle magnetic stirring, until a clear dark red solution was formed. Subsequently, the samples remained at room temperature under ambient conditions in a dark place, until a goldish precipitate was formed. The precipitate was collected by discarding the supernatant and washing by hexane. The washed S–OA derivative was finally dispersed in hexane or in toluene and stored in a vial. Optical images of the S–OA goldish precipitate form the oleyl amine/sulfur solution, as well in hexane after the material purification are presented in Fig. S1, in the ESI.† It is worth to mention that the sulfur to oleyl amine molecular ratio is very crucial for the precipitation of the S–OA derivative. The material was precipitated only when the S to OA molecular ratio is higher than 0.8, while in the case of the S to OA molecular ratio is smaller than 0.8 the solution remain clear dark red without any sign of precipitation. It is worth to mention that the procedure is very slow, and the first sign of the goldish S/OA derivative precipitation appears since the first two weeks, but a significant precipitate was formed only after 3 months. Concerning the physicochemical behaviour, the hybrid material is insoluble in most common non-polar, such as hexane and toluene and quite soluble in polar such as ethanol and acetone.
The XRD pattern of the corresponded S–OA derivative is presented in Fig. 1a. The pattern shows clearly the formation of a well formed lamellar structure. The five definite diffraction peaks can be indexed as a periodic lamellar structure with a layer spacing of ∼3.8 nm, value which is close to the width of an oleyl amine bilayer confirming indirectly that the platelets are surface modified with oleyl amine molecules.15 The XRD pattern at higher angles (Fig. S2 in the ESI†) shows a broad peak around 20 degrees which indicates the amorphous nature of the S–OA derivative, with the presence of some low intensity narrow peaks. The diffractions that are indicated with an asterisk belong to the monoclinic elemental sulfur (JCPDS no 77-0145), with the most characteristic at 23.04° which corresponds to the (222) lattice.16 The elemental sulfur presence was also confirmed by the TEM studies, that clearly show the formation of elemental sulfur nanoparticles on the surface of the platelet S–OA derivative (Fig. 1b and S3†). These sulfur dots are spherical in the shape with 5 nm mean diameter, their origin is probably connected to the oxidation processes, but their population is very small. Furthermore, the TEM images, show that the platelets are in the sub-micron size regime and are mostly irregular in shape. The platelet structure of the S–OA derivatives was additionally confirmed by AFM studies (Fig. 1c and S4†). In fact, AFM height analysis is in very good agreement with the XRD results regarding the layer spacing of the platelets, revealing a mean ∼4 nm platelet thickness.
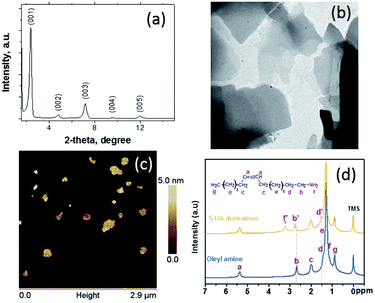 | ||
| Fig. 1 XRD pattern (a), TEM image (b), AFM image (c), and 1H NMR (d) of the S–OA platelet derivatives. | ||
The distribution of S in the platelet material is also characterized by elemental mapping analysis which is presented in Fig. 2. From the images it is clear that both C and S were uniformly distributed with-in the whole area indicating a homogeneous S distribution in the hybrid platelet S–OA derivatives.
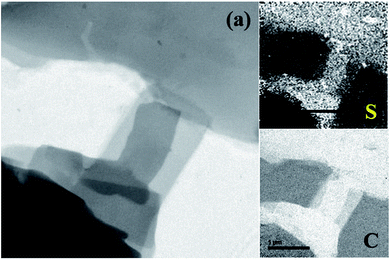 | ||
| Fig. 2 TEM image of the S–OA platelets derivatives (a) and the corresponding sulfur (S) and carbon (C) elemental mapping analysis images. | ||
Fig. 1c, shows the 1H NMR spectra of oleyl amine and S–OA derivatives. The majority of chemical shifts remain largely un-changed in contrast with the N–H protons, however, show a large drift from 1.17 ppm in oleyl amine to 3.22 ppm in the S–OA derivatives. This behaviour shows clearly the high degree of the amine protonation suggesting the formation of alkylammonium polysulfides.8 It was also observed that the α-CH2 protons adjacent to the amine functional groups at 2.61 ppm were influenced by the protonation and show slightly up field chemical shift (peak b), however the methyl and other methylene group peaks appeared without any change. It is interesting to note that the unchanged alkene peak at 5.31 ppm suggests the non-reactivity of sulfur with the double bond of the amine molecule at the specific temperature range. Additionally, the FT-IR spectroscopy studies, in agreement with the 1H NMR data, show that the oleyl amine double bond remains intact and a shifting of the N–H vibration at lower wavenumber indicating that the oleyl amine molecule interacts through the amine group (Fig. S5†).
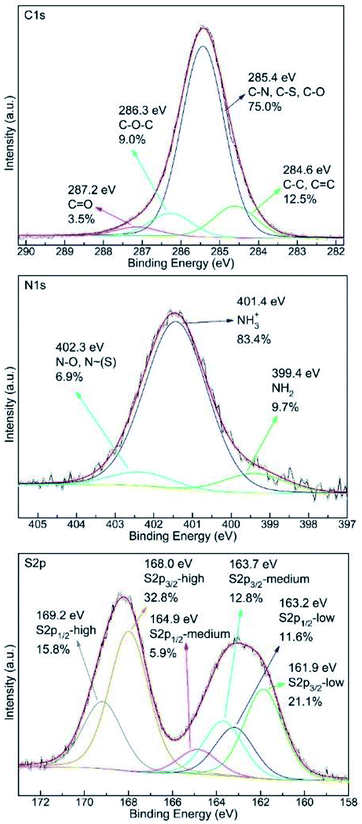
The C1s, N1s and S2p XPS spectra of the S–OA platelet derivative are shown in Fig. 3. Interpretation of these spectra is rather hard and kind of speculative given the XRD, TEM and AFM data. Nevertheless, all three spectra imply partial oxygen contamination of the material probably by incorporating dissolved oxygen during the heat treatment at 100 °C or during the interaction with atmospheric oxygen with time because the overall procedure take place under air atmosphere. Of high importance is the dominance of protonated amine groups in the nitrogen spectrum that suggests the ionic nature of the derivative. In addition, the high binding energy contribution can be attributed to moieties containing electronegative elements as oxygen and/or sulfur. Furthermore, S2p spectrum analysis is even more complicated as its proper fitting requires the presence of at least three different sulfur species and amongst them a low binding energy component at 161.9 eV which indicates the presence of negatively charged sulfur atoms. The medium binding energy component at 163.7 eV originates from elemental sulfur whereas the high binding energy contribution suggests the presence of oxidized species.17 The oxidized species probably have the structure of ammonium thiosulfate and they are formed via oxidation by humid air according to the reaction (1).17
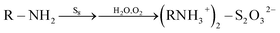 | (1) |
From the mechanistic point of view, it is well known for decades that amines have a great tendency to dissolve elemental sulfur producing colouring solutions. The initial stage involves the nucleophilic attack through the long pair electron of the nitrogen atom and consequently the sulfur ring opening and the formation of an amine–polysulfide complex according to the first step of the following reaction. This step is completely reversible. In a second step (reaction (2)), proposed by Davis and Nakshbendi,18 the amine–polysulfide complex reacts with a second amine molecule leading to the formation of an open chain alkylammonium N-polythioamine salt with a N–S bond.
| R–NH2 + S8 ⇄ RNH2+–S8− → (RNH3+)(RNH–S8−) | (2) |
In their work, they presented data from secondary amines and short chain aliphatic primary amines such as n-butylamine. More recent, Jordan W. Thomson et al.8 in their work which focuses on the octylamine–sulfur reaction, showed that at low temperatures, sulfur–alkylamine solutions exist mainly as alkylammonium polysulfides and upon heating the polysulfide ions react with excess alkylamine producing thioamide with simultaneous liberation of H2S. The H2S production is common for both primary and secondary amines. Ethylenediamine is producing H2S even at room temperature, while for octylamine the H2S evolution takes place upon heating at 130 °C. In our work the reaction of elemental sulfur with the oleyl amine takes place at low temperatures (<100 °C), in order to avoid the formation of thioamide or the formation of oleyl amine–sulfur polymer, through the double bond opening, which were synthesized at much higher, up to 180 °C, temperatures.19 Having into account the ionic nature of the S–OA derivatives from the N1s XPS data we are speculating that these layered derivatives have the salt structure of the second step of the reaction (2) product. From the best of our knowledge, this platelet S–OA derivative is synthesized for the first time. Furthermore, it is the first time that an amine sulfur derivative was isolated from the reaction mixture in a quite pure powder form. Previous studies focused on sulfur–amine reactions suffered from the inability to isolate the products from the reaction mixture.
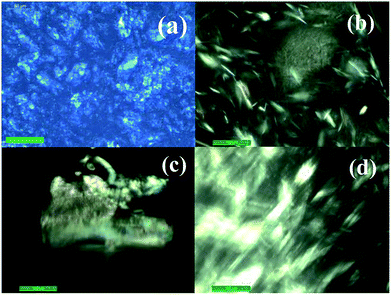
The birefringence and textures of S–OA platelet dispersions in toluene were examined by polarized optical microscopy (POM) operated in transmission mode under a Leica DM2500P microscope, by looking at a drop of the dispersion on a glass slide or in capillary tube. Images were captured with a Leica DFC420 digital image acquisition camera using crossed polarizers. Representative POM micrographs are given in Fig. 4. POM micrographs clearly show the birefringent lyotropic LC behaviour of S–OA platelet dispersions in toluene.
Summarizing, here we are presenting for the first time the synthesis and isolation of a sulfur–oleyl amine platelet derivative. The material was synthesized under mild conditions, at low temperatures, and has an ionic alkylammonium polysulfide nature. Furthermore, the platelet S–OA derivatives shows lyotropic liquid crystalline behavior up to 100 °C.
Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Kutney, Sulfur. History, Technology, Applications and Industry, ChemTec Publishing, 2007 Search PubMed.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500 CrossRef CAS PubMed.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904 CrossRef CAS PubMed.
- F. Wu, J. Chen, R. Chen, S. Wu, L. Li, S. Chen and T. Zhao, J. Phys. Chem. C, 2011, 115, 6057 CrossRef CAS.
- R. Okutsu, S. Ando and M. Ueda, Chem. Mater., 2008, 20, 4017 CrossRef CAS.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. Jun Ji, P. T. Dirlam, R. S. Glass, J. Jae Wie, N. A. Nguyen, B. W. Guralnick, J. Park, Á. Somogyi, P. Theato, M. E. Mackay, Y. E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518 CrossRef CAS PubMed.
- J. J. Griebela, R. S. Glassa, K. Charb and J. Pyun, Prog. Polym. Sci., 2016, 58, 90 CrossRef.
- J. W. Thomson, K. Nagashima, P. M. Macdonald and G. A. Ozin, J. Am. Chem. Soc., 2011, 133, 5036 CrossRef CAS PubMed.
- I. Moreels, Y. Justo, B. De Geyter, K. Haustraete, J. C. Martins and Z. Hens, ACS Nano, 2011, 5, 2004 CrossRef CAS PubMed.
- Y. Du, Z. Yin, J. Zhu, X. Huang, X. J. Wu, Z. Zeng, Q. Yan and H. Zhang, Nat. Commun., 2012, 3, 1177 CrossRef PubMed.
- F. M. van der Kooij, K. Kassapidou and H. N. W. Lekkerkerker, Nature, 2000, 406, 868 CrossRef CAS PubMed.
- J. E. Kim, T. H. Han, S. H. Lee, J. Y. Kim, C. W. Ahn, J. M. Yun and S. O. Kim, Angew. Chem., Int. Ed., 2011, 50, 3043 CrossRef CAS PubMed.
- Z. Xu and C. Gao, Nat. Commun., 2011, 2, 571 CrossRef PubMed.
- A. R. Koltonow, C. Luo, J. Luo and J. Huang, ACS Omega, 2017, 2, 8005 CrossRef CAS.
- Y. Wang, Y.-H. Liu, Y. Zhang, P. J. Kowalski, H. W. Rohrs and W. E. Buhro, Inorg. Chem., 2013, 52, 2933 CrossRef CAS PubMed.
- Z. Sun, M. Xiao, S. Wang, D. Han, S. Song, G. Chen and Y. Meng, J. Mater. Chem. A, 2014, 2, 9289 Search PubMed.
- L. Shen, H. Wang, S. Liu, Z. Bai, S. Zhang, X. Zhang and C. Zhang, J. Am. Chem. Soc., 2018, 140, 7878 CrossRef CAS PubMed.
- R. E. Davis and H. F. Nakshbendi, J. Am. Chem. Soc., 1962, 84, 2085 CrossRef CAS.
- E. Tae Kim, W. Jin Chung, J. Lim, P. Johe, R. S. Glass, J. Pyun and K. Char, Polym. Chem., 2014, 5, 3617 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: In the ESI section are the optical images of the S–OA platelet derivatives precipitated from the elemental sulfur in oleyl amine solutions, additional XRD, TEM and AFM data as well FT-IR spectroscopy characterization. See DOI: 10.1039/c8ra08325h |
| This journal is © The Royal Society of Chemistry 2018 |