Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radical and electrical exfoliation
Xin Wang ab and
Long Zhang
ab and
Long Zhang *a
*a
aJilin Provincial Engineering Laboratory for the Complex Utilization of Petro-resources and Biomass, School of Chemical Engineering, Changchun University of Technology, Changchun, Jilin 130012, P. R. China. E-mail: zhanglongzhl@163.com; Tel: +8618686672766
bSchool of Petrochemical Technology, Jilin Institute of Chemical Technology, Jilin 132022, P. R. China
First published on 5th December 2018
Abstract
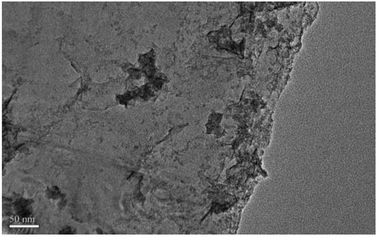
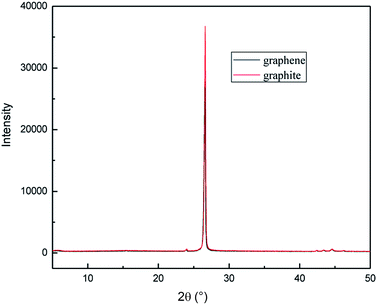
A novel, simple and efficient method, involving the combination of hydroxyl radicals and electrical exfoliation of graphite for the green production of high-quality graphene from graphite, was developed for the first time. The process parameters were optimized by single factor experiments; the optimal conditions were 4.0 g graphite dosage, sodium chloride solution with concentration of 5.0% (w/v), applied current strength of 10 mA, and air flow rate of 1.0 L h−1 for 3 h. Under the optimized conditions, graphite was successfully exfoliated to graphene. SEM and TEM results revealed that the graphene product has the characteristic features of a thin-layer graphene sheet. XRD results showed that the graphene products still maintained the structure of the carbon atoms or molecules. FT-IR and Raman results indicated that the products have the characteristic peaks and absorption peaks of graphene. AFM imaging results reveal that the layer number of the graphene product obtained by this method is about 3, while the graphene products from the individual hydroxyl radical oxidation and electrical exfoliation processes were 50 and 133 layers, respectively, under the same experimental conditions. The good quality of the graphene product can be attributed to the synergistic effect between the strong oxidation of the hydroxyl radicals and electrical exfoliation. The proposed method has the advantages of simple operation, mild preparation conditions, non-utilization of aggressive reagents, recycling of the reaction medium, etc. Thus, this method could serve as a green and efficient alternative for the production of graphene and its derivatives in industry.
1. Introduction
In 2004, graphene was first reported by the British scientists K. S. Novoselov and A. K. Geim et al.1 Unlike the thicker graphite, graphene is a two-dimensional planar material with a hexagonal lattice-like structure formed by a single-layer of carbon atoms through the sp2 hybrid orbital. In graphene, each carbon atom has an unbonded electron, which can move freely at high speed (one-third of the speed of light) in the crystal, so graphene has good electrical conductivity.1 At the same time, graphene also has many other excellent properties, such as a large theoretical specific surface area (∼2630 m2 g−1),2 excellent light transmission, (∼97.7%),3 and high Young's modulus (∼1.0 TPa).4 The special two-dimensional structure of graphene and its various excellent properties endow it with broad application prospects in many fields. Thus far, the most popular methods for the preparation of graphene include micro-mechanical stripping,5–9 chemical vapor deposition10–13 and oxidation dispersion reduction method.14–16 The above preparation methods often use aggressive reagents and are restricted by the relatively high energy consumption, complex operation, environmental pollution, occasionally lower yield and poor product quality. The liquid phase exfoliation method has been extensively studied because it is easy to operate and can obtain higher quality graphene product.17,18 However, the use of excess organic solvents often leads to environmental problems and high production expenditure. Therefore, it is necessary to develop a novel green production method to resolve the problems mentioned above.The hydroxyl radical is the most common and most important of the free radicals. Its oxidation–reduction potential is 2.8 V, which is only lower than that of F.19 The hydroxyl radical can react with many organic molecules due to its strong oxidizing capacity. It can initiate and transfer chain reactions and oxidize and decompose organic matter into low toxicity or non-toxic small molecules. The common ways to generate hydroxyl radicals include the Fenton reaction,19,20 the Haber–Weiss reaction21 and electrochemical methods.22–24 These hydroxyl radical generation methods have the disadvantages of high energy consumption and complex production procedures; hence, they are not suitable for mass applications. At present, the hydroxyl radical is mainly used in the field of sewage treatment, sterilization and preservation.20–23 Wei et al.24 have used ˙OH produced by the Fenton reagent to achieve good chemical modification of carbon nanotubes. Feng et al.25 have introduced ˙OH (produced by UV light irradiation) into the synthesis process for zeolites, and it was found that ˙OH can significantly accelerates the nucleation of zeolite, thus accelerating its crystallization process. However, there have been no reports on the application of hydroxyl radicals for the exfoliation of graphite to prepare graphene. Therefore, in the present study, for the first time, we used hydroxyl radical exfoliation for the facile and green production of high-quality graphene from graphite.
To achieve these goals, we designed a new apparatus for the production of ˙OH and exfoliation of graphite. The effect of the process parameters (such as exfoliation time, sodium chloride solution concentration, graphite dosage, applied current strength and air flow rate) on the production of graphene were investigated systematically. Hydroxyl radical oxidation and electrical exfoliation were also performed individually for comparison. The experimental results show that graphite has been successfully exfoliated into graphene by this method. The layer number of graphene product was determined to be about 3, while that of the graphene product from the individually performed hydroxyl radical oxidation experiment and electrical exfoliation experiment were 50 and 133, respectively, under the same experimental conditions.
2. Experimental
2.1 Materials and instruments
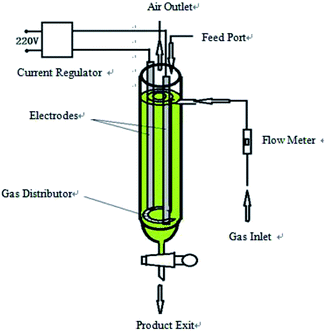
Flake graphite (0.5 mm) was purchased from Sinopharm Chemical reagent Co. (Shanghai, China). Sodium chloride (AR) and ethanol (AR) were purchased from Sinopharm Chemical reagent Co. (Shanghai, China).The hydroxyl radical production and graphite exfoliation apparatus was designed and manufactured by our laboratory; the detailed device diagram was shown in Fig. 1. The analytical balance (TG328A) was purchased from Balance instrument factory (Shanghai, China). The pumping equipment was purchased from Guohua Electric Co. (Shanghai, China). The vacuum drying oven was purchased from Anteing Electronic Instrument Factory (Shanghai, China).
2.2 Experimental devices
The device is a high efficiency hydroxyl radical generation apparatus. The apparatus consists of a current regulator, electrode (graphite or stainless steel) system, gas inlet and distributor, flow meter, feed port, air outlet and product exit. The reaction liquid (water and electrolyte) and graphite were added from the feed port. The air flow rate was adjusted by a flow meter. The air and graphite were evenly distributed in the reaction liquid under the effect of an air distributor; the intensity of the applied current was adjusted by the current regulator. When an electric current was applied, water decomposed and oxidized to produce ˙OH in the reactor with graphite as the catalyst, which subsequently reacted with the graphite to produce graphene.After completion of exfoliation, the obtained graphene was discharged out the reactor from the product exit, filtered and dried to get the product. The diagram of the apparatus is shown in Fig. 1.
2.3 Preparation procedures
Graphite powders were loaded in the hydroxyl radical apparatus containing a volume of sodium chloride solution according to the experimental design. The applied current strength in the process was adjusted by a variable resistor having 50–500 Ω. The exfoliation experiments were started when the electric current was applied, water was decomposed and oxidized to produce ˙OH in the reactor with graphite as the catalyst, which subsequently reacted with graphite to produce graphene. The flake graphite added to the reaction liquid also served as a catalyst. This resulted in the simultaneous generation of the hydroxyl radical and the reaction between the hydroxyl radical and the graphite in solution. After the designated exfoliation time, the solution was filtered, and the solid was dried and ground to powder for characterization, and the filtrate was reused for the next experimental run.We also investigated the effects of different preparation parameters on the quality of the graphene products. Five process factors (exfoliation time, sodium chloride solution concentration, graphite dosage, applied current strength and air flow rate) were defined and adjusted in the exfoliation process.
2.4 Characterization
3. Results and discussion
3.1 Optimization of process parameters
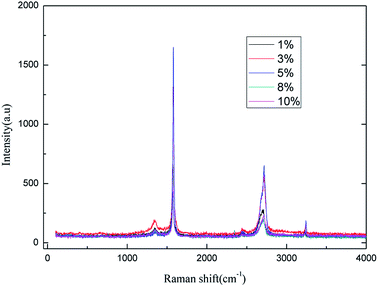
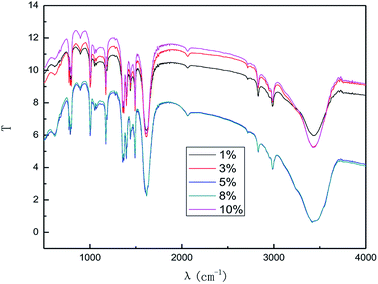
It can be easily seen from Fig. 5 that the intensity of the characteristic peak for graphene increased with the increase in electrolyte concentration up to a maximum at 5% (w/v), and then decreased. This phenomenon can be attributed to the increase in conductivity of the solution when a higher electrolyte concentration is used. Moreover, good conductivity leads to good ˙OH formation and electrical exfoliation effect. Furthermore, the hydroxyl radicals generated from water decrease due to less water at higher electrolyte concentration, which leads to the intensity of the characteristic peak of graphene to decrease. Hence, when the electrolyte concentration is less than 5.0% (w/v), the increase in the conductivity dominated, and when the concentration exceeds 5.0% (w/v), the decrease in hydroxyl radical content was more dominant. Literature 29–33 reports also indicate the optimal electrolyte concentration for the production of hydroxyl radicals in different electrolyte systems using different production methods. Li et al.31 used a highly efficient generation reactor to produce hydroxyl radicals, and showed that the optimum electrolyte concentration was in the range of 3.0–6.0% (w/v). Therefore, it is reasonable to expect that an optimal concentration of electrolyte may exist. Moreover, the higher concentration of sodium chloride solution results in stronger corrosion of the electrodes. As can be inferred from the results, 5.0% (w/v) electrolyte concentration was found to be optimal for the investigation.
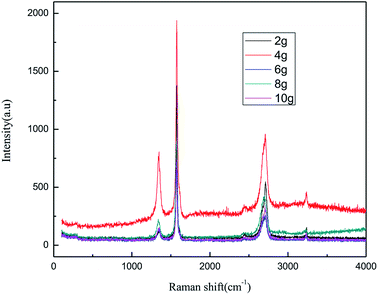
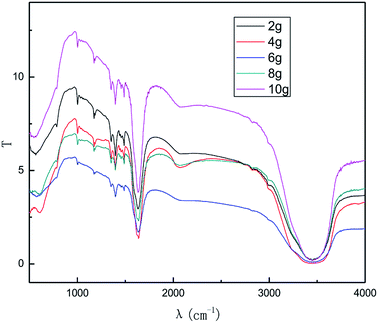
As seen from Fig. 7 and 8, the intensity of the characteristic peak of graphene first increased with the increase in graphite dosage, achieving a maximum at 4.0 g, and then decreased. This is because higher dosage of graphite leads to better catalytic activity, which improved the intensity of the characteristic peak of graphene. However, higher graphite dosage induced more graphene aggregation and uneven distribution, resulting in the decline in the intensity of the characteristic peak of graphene. It can be seen from the results that a suitable graphite dosage is 4.0 g.
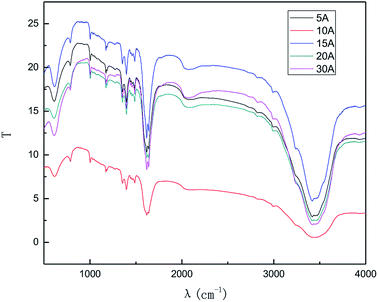
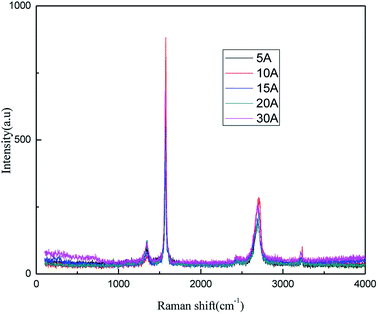
The results from Fig. 9 and 10 indicate that the intensity of the characteristic peak of graphene initially enhanced with the increase in the applied current strength, reaching a maximum at 10 mA, and then decreased. This may be attributed to the combined effects of hydroxyl radical exfoliation and electrical exfoliation. Over a certain range, a relatively higher applied current strength, indicating higher power energy input, can result in higher hydroxyl radical production and better electrical exfoliation effect. However, when the current is too high, the cathode and anode will exhibit the side effects of hydrogen and oxygen precipitation.34–36 The reactions are shown below ((1) and (2)). The bipolar side effects lead to a decrease in hydroxyl radical production, current efficiency and the effect of electrical exfoliation.
| 2H2O − 4e → O2↑ + 4H+ | (1) |
| 2H+ + 2e → H2↑ | (2) |
In order to ensure an improved preparation process, 10 mA was selected as the optimal applied current strength.
In summary, the optimal exfoliation conditions for graphene were as follows: electrolyte concentration, 5.0% (w/v); graphite dosage. 4.0 g; exfoliation time, 3 h; applied current strength, 10 mA; air flow rate 1.0 L h−1. At these conditions, the layer number of the graphene was 3.
3.2 Mechanism discussion
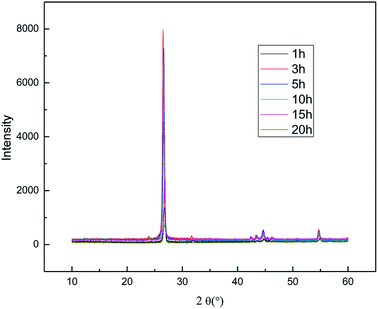
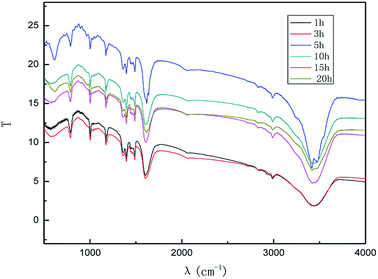
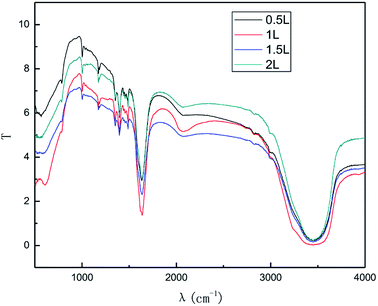
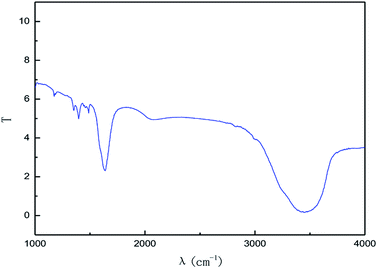
The effect of the hydroxyl radical on the graphene structure was studied by FT-IR spectroscopy. The results are shown in Fig. 16. It can be seen that the main absorption peaks were at 1045 cm−1, 1264 cm−1, 1512 cm−1, 1620 cm−1 and 3400 cm−1. The peak at 1045 cm−1 was caused by the C–OH vibration.42 The peaks at about 1264 cm−1 and 1512 cm−1 were caused by the C–O–C vibration39,43 and the stretching of C–O bond, respectively.44,45 The absorption peak at 1620 cm−1 is attributed to the sp2 structure of the graphite crystal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration peak.40 The broad and strong absorption peak in the fingerprint region at around 3000–3700 cm−1 is attributed to the OH stretching vibration peaks.39,41,42 These results further indicate that the preparation method does not change the carbon-based structure and introduces hydroxyl radicals into graphene at the same time. The FT-IR spectroscopy results are in good agreement with those of XRD and TEM.
C stretching vibration peak.40 The broad and strong absorption peak in the fingerprint region at around 3000–3700 cm−1 is attributed to the OH stretching vibration peaks.39,41,42 These results further indicate that the preparation method does not change the carbon-based structure and introduces hydroxyl radicals into graphene at the same time. The FT-IR spectroscopy results are in good agreement with those of XRD and TEM.
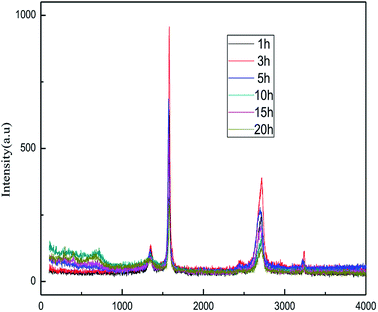
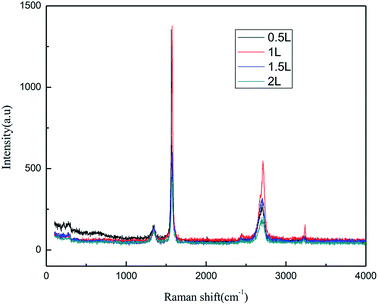
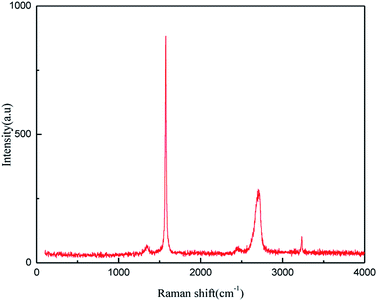
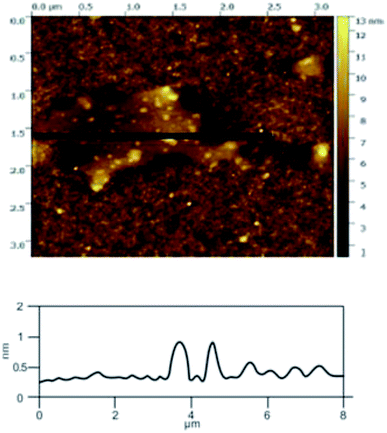
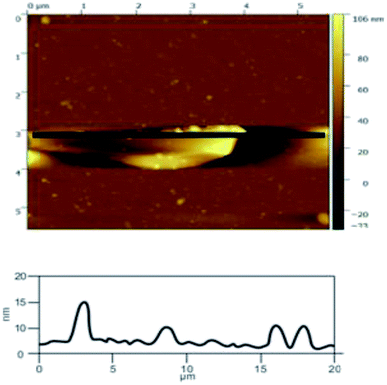
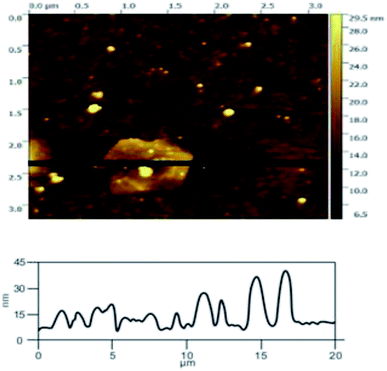
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the graphite layer. In addition, the intensity ratio of the G band to the D band also represents the sp2/sp3 carbon atom ratio.47,48 As can be seen from Fig. 17, the intensity of the G band is far stronger than that of the D band, indicating that the carbon skeleton structure has not changed, which is in good agreement with the FT-IR spectral analysis. The D′ (2D) and G′ modes belong to the sum and frequency of the disordered Raman modes and are Raman allowed in the presence of intact graphite crystals and defects; hence, they have strong Raman signals. Graphene has a low degree of graphitization. Therefore, the D′ (2D) and G′ modes are usually very weak and wide. The second-order Raman peak is not considered here.42 The graphene absorption peak at 2720 cm−1 moves slightly at different layers. Femri et al.49 studied the change in the 2D peak position with the number of layers of graphene and used the double resonance model to explain this phenomenon. In order to further study the number of layers in the graphene products, AFM test was performed.
C double bonds in the graphite layer. In addition, the intensity ratio of the G band to the D band also represents the sp2/sp3 carbon atom ratio.47,48 As can be seen from Fig. 17, the intensity of the G band is far stronger than that of the D band, indicating that the carbon skeleton structure has not changed, which is in good agreement with the FT-IR spectral analysis. The D′ (2D) and G′ modes belong to the sum and frequency of the disordered Raman modes and are Raman allowed in the presence of intact graphite crystals and defects; hence, they have strong Raman signals. Graphene has a low degree of graphitization. Therefore, the D′ (2D) and G′ modes are usually very weak and wide. The second-order Raman peak is not considered here.42 The graphene absorption peak at 2720 cm−1 moves slightly at different layers. Femri et al.49 studied the change in the 2D peak position with the number of layers of graphene and used the double resonance model to explain this phenomenon. In order to further study the number of layers in the graphene products, AFM test was performed.
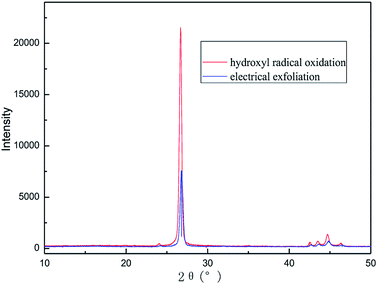 | ||
| Fig. 19 XRD patterns of samples obtained by hydroxyl radical oxidation and electrical exfoliation experiments, respectively. | ||
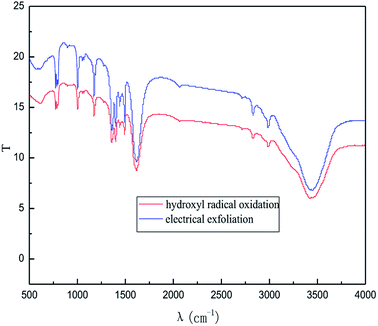 | ||
| Fig. 20 FI-IR patterns of samples obtained by hydroxyl radical oxidation and electrical exfoliation experiments, respectively. | ||
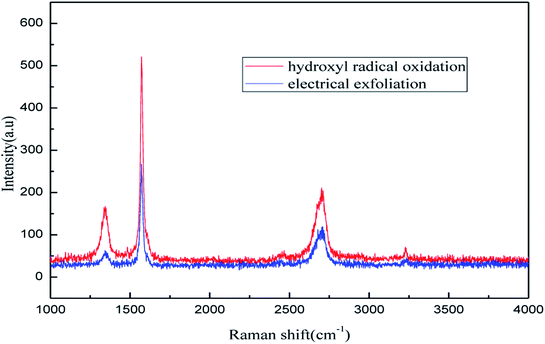 | ||
| Fig. 21 Raman patterns of samples obtained by hydroxyl radical oxidation and electrical exfoliation experiments, respectively. | ||
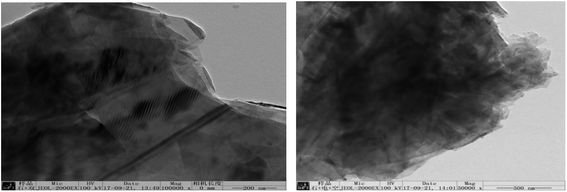 | ||
| Fig. 22 TEM images of samples obtained by hydroxyl radical oxidation and electrical exfoliation experiments, respectively. | ||
3.3 Yield of the production
The graphene dispersion has good Lambert–Beer behavior, and the concentration of the graphene dispersion has a good linear relationship with the ultraviolet absorbance. A/I = αC, where A/I is the absorbance value of the unit cuvette length, C is the concentration of the graphene dispersion, and α is the extinction coefficient of the graphene dispersion. It is reported in the literature50 that the extinction coefficient (α) of the graphene dispersion at λ = 660 nm is 3620 L−1 g−1 m−1.The experiment was performed under the previously determined optimal conditions, with a graphite dosage of 4 g, and 1 L of reaction liquid. The reaction solution after the exfoliation experiment was diluted 1000 times and then subjected to ultraviolet detection at 660 nm. The concentration of the graphene dispersion was measured to be 3.102 g L−1, and the yield of the product was calculated as 3.102 g L−1/(4 g/1 L) × 100% = 77.5%.
4. Conclusion
In this study, we investigated a new method by combining hydroxyl radical oxidation and electrical exfoliation to produce graphene from graphite. The effect of the exfoliation conditions on the quality of the graphene products was investigated by single factor experiments. The optimal conditions were obtained as 4.0 g of graphite, sodium chloride solution of concentration 5.0% (w/v), applied current strength of 10 mA, and air flow rate of 1.0 L h−1 for 3 h. Under optimized conditions, the number of layers of graphene product is about 3, while the products from the individually performed hydroxyl radical oxidation experiment and electrical exfoliation experiment were 50 and 133 layers under the same experimental conditions, respectively. The good quality of the graphene product can be attributed to the synergistic effects of strong oxidation of the hydroxyl radicals and electrical exfoliation. The method has the advantages of simple processing, mild conditions (atmospheric pressure and room temperature), non-utilization of aggressive reagents, recyclability of the reaction medium, etc. In general, the new method could be a green and potential method for the production of graphene and graphene derivatives in industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Materials Institute and Advanced Materials Research Institute for their test support in the process of Characterization.References
- K. S. Novoselov, A. K. Geim and S. V. Morozov, et a1., Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. D. Stoller, S. J. Park and Y. W. Zhu, et a1., Graphene-based ultracapacitors, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- R. R. Nair, P. Blake and A. N. Grigorenko, et a1., Fine structure constant defines visual transparency of grapheme, Science, 2008, 320, 1308 CrossRef CAS PubMed.
- C. Lee, X. Wei and J. W. Kysar, et a1., Measurement of the elastic properties and intrinsic strength of monolayer graphene, Sience, 2008, 321, 385–388 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Electric field effect in atomically thin carbon films, Science, 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- J. C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, T. J. Booth and S. Roth, The structure of suspended graphene sheets, Nature, 2007, 446(7131), 60–63 CrossRef CAS PubMed.
- J. C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, D. Obergfell, S. Roth, C. Girit and A. Zettl, On the roughness of single-and bi-layer graphene membranes, Solid State Commun., 2007, 143(1–2), 101–109 CrossRef CAS.
- A. Fasolino, J. H. Los and M. I. Katsnelson, Intrinsic ripples in grapheme, Nat. Mater., 2007, 6(11), 858–861 CrossRef CAS PubMed.
- C. Knieke, A. Berger and M. Voigt, et a1., Scalable production of graphene sheets by mechanical delamination, Carbon, 2010, 48(11), 3196–3204 CrossRef CAS.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Large-scale pattern growth of graphene films for stretchable transparent electrodes, Nature, 2009, 457(7230), 706–710 CrossRef CAS.
- A. Reina, X. T. Jia, J. Ho, D. Nezich, H. B. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition, Nano Lett., 2009, 9(1), 30–35 CrossRef CAS PubMed.
- X. S. Li, W. W. Cai, J. H. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Large-area synthesis of high-quality and uniform graphene films on copper foils, Science, 2009, 324(5932), 1312–1314 CrossRef CAS PubMed.
- X. S. Li, W. W. Cai, L. Colombo and R. S. Ruoff, Evolution of graphene growth on Ni and Cu by carbon isotope labeling, Nano Lett., 2009, 9(12), 4268–4272 CrossRef CAS PubMed.
- C. A. Amarnath, C. E. Hong, N. H. Kim, A. Reina, X. T. Jia, J. Ho, D. Nezich, H. B. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Efficient synthesis of graphene sheets using pyrrole as a reducing agent, Carbon, 2011, 49(11), 3497–3502 CrossRef CAS.
- Y. Shao, J. Wang and M. Engelhard, et a1., Facile and controllable electrochemical reduction of graphene oxide and its applications, J. Mater. Chem., 2010, 20(4), 743–748 RSC.
- H. Guo, X. Wang and Q. Qian, et a1., A Green Approach to the Synthesis of graphene Nanosheets, ACS Nano, 2009, 3(9), 2653–2659 CrossRef CAS PubMed.
- Y. Hemandez, V. Nicolosi and M. Lotya, et al., High-yield production of graphene by liquid-phase exfolimion of graphite, Nat. Nanotechnol., 2008, 3(9), 563–568 CrossRef.
- X. L. Li, G. Y. Zhang, X. D. Bai, X. M. Sun, X. R. Wang, E. Wang and H. J. Dai, Highly conducting graphene sheets and langmuir-blodgett films, Nat. Nanotechnol., 2008, 3(9), 538–542 CrossRef CAS PubMed.
- N. Azbar, T. Yonar and K. Gestioglu, Comparison of various advanced Oxidation processes and chemit·af treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent, Chemosphere, 2004, 55(1), 35–43 CrossRef CAS PubMed.
- X. J. Ma and Y. Q. Cong, et a1., Electrochemical induced hydroxyl radical degradation of phenolic pollutants in water, Journal of Chemical Industry and Engineering, 2008, 59(1), 60–63 CAS.
- D. S. Sun and R. Hu, Application of hydroxyl radical oxidation in drinking water treatment, Journal of Qinghai Environment, 2004, 14(3), 110–112 Search PubMed.
- H. F. Sun and J. Q. Du, Application of Hydroxyl Radical Active Oxygen in High Concentration Organic Wastewater Treatment, Modern Chemical Industry, 2010, 30(1), 102–105 Search PubMed.
- X. Bao, Study on Food Purification Technology and Equipment Based on Hydroxyl Radicals, Zhejiang University, Zhejiang, 2016 Search PubMed.
- W. Li, Chemical Modification of Carbon Nanotubes with Hydroxyl Radical, East China Normal University, ShangHai, 2005 Search PubMed.
- G. Feng, P. Cheng and W. Yan, et a1., Accelerated crystallization of zeolites via hydroxyl free radicals, Science, 2016, 351(6278), 1188–1191 CrossRef CAS PubMed.
- K. Parvez, Z.-S. Wu, R. Li, X. Liu, R. Graf, X. Feng and K. Mullen, J. Am. Chem. Soc., 2014, 136, 6083 CrossRef CAS PubMed.
- K. H. Park, D. Lee, J. Kim, J. Song, Y. M. Lee, H.-T. Kim and J.-K. Park, Nano Lett., 2014, 14, 4306 CrossRef CAS PubMed.
- K. H. Park, B. H. Kim, S. H. Song, J. Kwon, J. Kong, B. S. Kong, K. Kang and S. Jeon, Nano Lett., 2012, 12, 2871 CrossRef CAS PubMed.
- B. Halliwell, M. A. Murcia and S. Chirico, et a1., Free radicals and antioxidants in food and in vivo:what they do and how they work, Crit. Rev. Food Sci. Nutr., 1995, 35, 7–20 CrossRef CAS PubMed.
- J. Lee, N. Koo and D. B. MiIl, Reactive oxygen species, aging, and antioxidative nutraceuticals, Compr. Rev. Food Sci. Food Saf., 2004, 3, 21–33 CrossRef CAS.
- L. Chen, et al., Hydroxyl radical generating device and its using method, CN, 104496003 A, China, 2015.
- M. Zhang, Hydroxyl radicals react with inorganic oxyacids, Dalian Maritime University, Dalian, 2015 Search PubMed.
- M. Jiang, Study on the Influence of Electrochemical Detection of Hydroxyl Free Radicals and Generations, Xi'an University of Architecture and Technology, Xi'an, 2015 Search PubMed.
- M. N. Schuchmann and C. von Sonntag, Hydroxyl Radical-Induced Oxidation of 2-Methyl-2-propanol In Oxygenated Aqueous Solution. A Product and Pulse Radiolysis Study, J. Phys. Chem., 1979, 83(7), 780–784 CrossRef CAS.
- J. M. Chen, W. W. Pan and C. L. Liu, Research Progress on the Mechanism and Detection of Hydroxyl Radicals in Electrochemical System, J. Zhejiang Univ. Technol., 2008, 36(4), 416–422 CAS.
- F. Sicilio and R. E. Florin, et al., Kinetics of the Hydroxyl Radica in Aqueous Solution, J. Phys. Chem., 1966, 70(1), 47–52 CrossRef CAS.
- D. C. Marcano and D. V. Kosynkin, et al., Improved synthesis of graphene oxide, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. Wang and Z. D. Han, The combustion behavior of polyacrylate ester graphite oxide composites, Polym. Adv. Technol., 2006, 17(4), 335–340 CrossRef CAS.
- W. S. Ma, et al., Preparation and Characterization of Graphene, J. Chem. Eng. Chin. Univ., 2010, 24(4), 719–722 CAS.
- K. Bissessur and S. F. Scully, Intercalation of solid polymer electrolytes into graphite oxide, Solid State Ionics, 2007, 178(11–12), 877–882 CrossRef.
- S. Hummers and R. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef.
- Y. H. Yang, H. J. Sun and T. Peng, Preparation and Characterization of Graphene by Redox, Chin. J. Inorg. Chem., 2010, 26(11), 2083–2090 CAS.
- H. He, J. Klinowski and A. Lcrf, et a1., A new structural model for graphite oxide, Chem. Phys. Lett., 1998, 287(2), 53–56 CrossRef CAS.
- X. X. Ma, Enzymatic Hydrolysis of Natural Cellulose, Beijing Chemical University, MSC, Beijing, 2010 Search PubMed.
- L. L. Wang, G. T. Han and Y. M. Zhang, Comparative study of composition, structure and properties of Apocynum venetum fibers under different pretreatments, Carbohydr. Polym., 2007, 69, 391–397 CrossRef CAS.
- C. A. Ferrari and J. Robertson, Raman Spectroscopy in Carbons: from Nanotubes to Diamond, Chemical Industry Press, Beijing, 2007, p. 193 Search PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61(20), 14095–14107 CrossRef CAS.
- C. Gomez-Navarro, R. T. Weitz and A. M. Bittner, et al., Electronic transport properties of individual chemically reduced graphene oxide sheets, Nano Lett., 2007, 7(11), 3499–3503 CrossRef CAS PubMed.
- A. C. Femri, J. C. Meyer and V. Scardaci, et al., Phys. Rev. Lett., 2006, 97(18), 7401 Search PubMed.
- U. Khan, A. O'Neil and M. Lotyal, et al., High-Concentration Solvent Exfoliation of Graphene, Small, 2010, 6(7), 864–871 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |