Open Access Article
Open Access ArticlePreparation of pH-responsive asymmetric polysulfone ultrafiltration membranes with enhanced anti-fouling properties and performance by incorporating poly(2-ethyl-2-oxazoline) additive
Ruizhang Xu a,
Jiantao Wanga,
DanDan Chena,
Feng Yanga,
Jian Kang
a,
Jiantao Wanga,
DanDan Chena,
Feng Yanga,
Jian Kang *a,
Ming Xianga,
Lu Lib and
Xingyue Shengb
*a,
Ming Xianga,
Lu Lib and
Xingyue Shengb
aState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, People's Republic of China. E-mail: jiankang@scu.edu.cn; Fax: +86-028-85406578; Tel: +86-028-85405347
bChongqing Zhixiang Paving Technology Engineering Co., Ltd., Chongqing, 401336, China
First published on 11th December 2018
Abstract
Inspired by the special pH value-responsive and strong hydrophilic ability of poly(2-ethyl-2-oxazoline) (PEOX), in this study, asymmetric polysulfone (PSf) and PSf/PEOX ultrafiltration membranes were prepared by a phase separation method, wherein different dosages of PEOX (0–3 wt%) were incorporated into the PSf casting solution as polymeric additives. The effects of PEOX dosages on the phase separation kinetics, chemical properties, morphology, hydrophilicity, porosity and performances such as pure water flux (PWF), Bull Serum Albumin (BSA) rejection, pH value responsiveness and anti-fouling property were investigated in detail. The hydrophilicity, pure water flux, BSA rejection and anti-fouling property were improved significantly after the incorporation of PEOX. The PWF increased from 213.5 L m−2 h−1 to 419.8 L m−2 h−1 as the PEOX dosage increased from 0 to 1 wt%, meanwhile, the BSA rejection increased to more than 98.4%. Viscosity and effective diffusion coefficient of PSf/PEOX solution were studied to elucidate the role of PEOX in phase separation and morphology of the membrane. Results showed that the incorporation of PEOX leads to the phase separation of casting solution by making it more prone to instantaneous demixing, further determining the morphology and performances of the membrane. Interestingly, the resulting membranes showed pH value-responsive properties, whereby the water flux increased along with an increase in the pH value. This interesting feature of the membranes broadens their application potential in many specific cases. The related filtration mechanism has also been proposed.
1. Introduction
The phase separation process is a broadly utilized strategy for the preparation of asymmetric ultrafiltration membranes.1 Over the past few decades, such ultrafiltration membranes are widely used in many fields for the removal of impurities, toxic and harmful substances from water, and the manufacturing of industrial and medical instruments (e.g. fruit juice concentrator, and hemodialysis machine).2 Many scientists have carried out extensive research in this field.3,4The asymmetric ultrafiltration membrane formation method and mechanism have been widely reported.5–9 In lab-scale, a casting solution comprising of a polymer and a solvent (for instance, a solution of PSf in DMF) is cast uniformly onto a polyester film or a glass plate substrate using a hand-casting knife with a constant gap. After the casting solution is spread uniformly onto the substrate, the substrate is subsequently immersed into a non-solvent coagulation bath (generally, distilled water). Then, because of the low miscibility between the polymer phase and the non-solvent phase with the concurrent high miscibility between the solvent and the non-solvent, the thermodynamic instability leads to the occurrence of liquid–liquid phase separation. In this process, the solvent in the casting solution exchanges with the non-solvent across the interface between them; precipitation of polymer phase starts simultaneously. In detail, the high miscibility between the solvent and non-solvent causes a diffusional stream of the two liquids at some locations on the top surface and the sublayer of the polymer phase, and the invasion of the non-solvent into the polymer phase leads to the formation of nuclei of a polymer-poor phase (low miscibility results in the repulsion of polymer chains and water molecules). Upon continued diffusion, the nuclei grow until the polymer concentration in the non-solvent is too high and precipitation occurs, voids are formed and fixed in the process,10,11 and a porous asymmetric membrane with a dense top surface and a porous sublayer is obtained in this process.
The formation, the microstructure and the performance of the final ultrafiltration membrane are determined by the exchange rate between the solvent and the non-solvent, which can be controlled by changing many variables, such as the composition of polymer solution, coagulant temperature and additives.2,12,13 So, a membrane with satisfactory properties can be obtained by carefully controlling the phase separation conditions.14–16
Adding additives is a major method to modify the properties of membranes in phase separation process. Additives affect the structures and properties of membranes by enlarging or preventing the formation of macrovoids, adjusting the pore interconnectivity, and/or changing the hydrophilicity of membranes.6,17–21 In the early 1960s, research on additives was mainly concerned with small molecular additives, such as an organic salt.22 On one hand, the interaction forces between the poly-molecular chains are changed by the organic salt. On the other hand, during the phase separation process, organic salt can dissociate from the polymer phase and facilitate the formation of voids.13
Later on, it was found that the void structure of an ultrafiltration membrane could be altered to a large extent by changing the molecular weight and the dosage of additives. Thus, polymers were considered as the new kind of additives. Hydrophilic additives such as poly(ethylene glycol) (PEG) or poly(vinyl pyrrolidone) (PVP) are the two most commonly used polymer additives, which could play an important role in the process of phase separation.2,23–26 However, the influences of polymer additives on the morphologies and structures of the resulting membranes are different. The polymer additives can not only induce the formation of macrovoids,2,25 but can also act as macrovoid suppressors.6,20,21,24,26–28
Poly(2-ethyl-2-oxazoline) (PEOX) is a biodegradable (acceptable degradation rate in use29) and water-soluble polymer with pH- and temperature-responsive properties.30,31 In an aqueous solution, PEOX has a LCST (lower critical solution temperature). When the temperature is below its LCST, PEOX would hydrate with water through hydrogen bonding, which could result in the swelling of PEOX. So, when the temperature is low, PEOX has a relatively large swelling ratio. Besides, in an aqueous solution, when the pH value is low, the amide groups in PEOX are ionized, leading to the possible aggregation of the PEOX molecular chains via intermolecular hydrogen bonds. While the pH value increases, the hydrogen ions dissociate from the amide groups in PEOX, which can result in the breaking of hydrogen bonds. Shrunken PEOX molecular chains will start to expand and cause it to swell. That is to say, while the pH value is high, PEOX has a relatively large swelling ratio.31
PEOX shows high hydrophilicity because it can combine with water via plenty of hydrogen bonding, thus, water is the θ solvent for PEOX.32 The hydrophilic PEOX is thought to be much better than PEG or PVP because of its high hygroscopicity. Its N,N-dimethylacetamide (DMAc) group is considered as a broadly compatible polymeric solvent or a compatibility agent for many polymers.33 At the same time, PEOX is easy to dissolve in DMF.
Inspired by the interesting features of PEOX mentioned above, the addition of PEOX as an additive into the PSf solution might impart pH sensitive property to the permeation performance of the resulting PSf membranes, which has never been reported before and is of great importance in broadening the applications of such membranes in many areas. Moreover, the addition of PEOX is expected to improve the water permeability and practical performance of the PSf asymmetric ultrafiltration membrane. The high hydrophilicity of PEOX might not only increase the hydrophilicity of the resulting PSf ultrafiltration membrane but might also increase the compatibility between the PSf casting solution and the coagulation bath (thereby, increasing the thermodynamic instability of the casting solution in the phase separation process). On the one hand, a hydrophilic membrane surface can prevent the adsorption of foulants, suggesting a better anti-fouling property.34,35 On the other hand, the phase separation mechanism may be more prone to instantaneous demixing, which will induce more macrovoid structures and thereby result in better water permeability.36,37
Various combinations of PSf and PEOX dosages were adopted in this research in order to study the influence of PEOX on the phase separation mechanism and on the practical performance of the resulting ultrafiltration membranes.
2. Experimental section
2.1. Materials
PSf (Udel® P-3500, Solvay, China), average molecular weight of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, was used as the base polymer in the casting solution. N,N-Dimethylformamide (DMF) with analytical purity of 99.5%, purchased from Aladdin (China), was used as the solvent, and distilled water was used as the non-solvent. PEOX (purchased from Alfa Aesar, China) with an average molecular weight of 200
000 g mol−1, was used as the base polymer in the casting solution. N,N-Dimethylformamide (DMF) with analytical purity of 99.5%, purchased from Aladdin (China), was used as the solvent, and distilled water was used as the non-solvent. PEOX (purchased from Alfa Aesar, China) with an average molecular weight of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 was used as the additive. Bull Serum Albumin (BSA) used to perform rejection property and anti-fouling property experiments was purchased from Aladdin (China). Hydrochloric acid (AR) and sodium hydroxide (AR), purchased from Aladdin (China), were used to adjust the pH value of feed solutions.
000 g mol−1 was used as the additive. Bull Serum Albumin (BSA) used to perform rejection property and anti-fouling property experiments was purchased from Aladdin (China). Hydrochloric acid (AR) and sodium hydroxide (AR), purchased from Aladdin (China), were used to adjust the pH value of feed solutions.
2.2. Preparation of the membranes
Asymmetric PSf membranes were prepared by a phase separation method. Different amounts of PEOX were added into the PSf/DMF matrix (the weight ratio of PSf in the cast solution was constant at 18 wt%) and dissolved at room temperature with magnetic stirring for at least 24 h to ensure that all PSf and PEOX were dissolved in DMF. The prepared solutions were poured into injectors and then kept them sealed and undisturbed overnight to eliminate air bubbles in solutions.The casting solution was cast uniformly on a polyester film substrate by a hand-casting knife with a 250 μm knife gap. Then, the cast was immersed into a non-solvent (distilled water) bath immediately to complete the phase separation; the process needs to be done quickly because the moisture in the air can also lead to the phase separation process. After the casting solution was immersed into the non-solvent (distilled water) bath, an exchange between the solvent (DMF) and the non-solvent (distilled water) was induced. The coagulation bath temperature (CBT) of 25 °C was selected in our experiments.
When the phase separation process was done, the residual DMF in membranes was removed by transferring the membranes into another container with fresh distilled water, and then the membranes were kept in fridge at 4 °C and tested after 24 h. An overview of the compositions of different casting solutions is reported in Table 1. In this study, all the samples were named as M-X, where X corresponds to the amount of PEOX.
| Membranes | PEOX (wt%) | DMF (wt%) | PSf (wt%) |
|---|---|---|---|
| M-0 | 0 | 82 | 18 |
| M-0.5 | 0.5 | 81.5 | 18 |
| M-1 | 1.0 | 81 | 18 |
| M-2 | 2.0 | 80 | 18 |
| M-3 | 3.0 | 79 | 18 |
2.3. Characterization of the membranes
In this experiment, a drop of PSf solution, around 10 μL, was placed on a glass slide and covered with a hydrophilic coverslip as fast as possible to prevent any phase separation of PSf solution in air. The sample was moved to the optical microscope; the magnification was set at 50×. A drop of distilled water was added to the edge of the coverslip, and phase separation occurred when the water came into contact with the PSf solution. The forward velocity of the moving boundary was measured by the optical microscope; images were taken every 5 s. The formula for phase separation kinetics was as shown below:
| X = 2(Det)1/2 | (1) |
In this research, the kinetics of membrane formation was studied. The thermodynamic and rheological variation of the PSf solution affected by the addition of PEOX has been discussed.
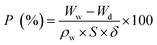 | (2) |
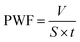 | (3) |
The rejection property of a membrane was tested with a 200 mg L−1 BSA solution (in 0.1 M phosphate-buffer solution (PBS), pH = 7.0), and the solution was fed at a transmembrane pressure of 0.25 MPa at 25 ± 0.1 °C. Distilled water was filtered through the membranes before the rejection testing, until the flux was stable. The BSA concentrations before and after testing were determined by an ultraviolet-visible spectrophotometer (TU-1900; Beijing Purkinje General Instrument Co., Ltd; China). The absorbance at the wavelength of 205 nm was read to estimate the BSA concentration. The rejection rates were calculated according to the following equation:
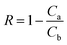 | (4) |
The anti-fouling property was quantitatively analyzed, and the total flux decline ratio (DRt) and flux recovery ratio (FRR) were calculated as follows:
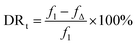 | (5) |
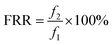 | (6) |
3. Results and discussions
3.1. Kinetic study
The membrane-forming process includes the shrinking and merging of the polymer phase. The morphology of the membranes is controlled and affected by the rate of the demixing process, which is divided into two forms: instantaneous demixing and delayed demixing.During instantaneous demixing process, due to the high exchange rate of solvent and non-solvent, precipitation of the polymer phase completes instantly which results in the formation of a thick membrane with macrovoids and loose top surface. Low concentration of the polymer phase in the sublayer gives the non-solvent enough time to form nuclei and grow, and the macrovoids are therefore formed.
In the process of delayed demixing, nucleation and precipitation occur slowly, and there is enough time for the polymer phase to shrink and merge. The shrinking and merging of the polymer phase lead to a high polymer concentration at the top surface and in the sublayer, resulting in a dense top surface and thin membranes. Besides, in the delayed demixing process, free growth of limited nuclei is prevented, as is the merging of nuclei. So, the number of nuclei is large; however, they are distributed in the polymer film and lead to a less porous structure with microvoids. Consequently, delayed demixing is the reverse of instantaneous demixing, often resulting in thinner and denser membranes.10,14,42
The addition of polymer additive into the PSf casting solution may change the phase separation rate. In order to reveal the effect of PEOX on the phase separation kinetics of PSf solution, the viscosities and effective diffusion coefficients of the PSf casting solutions were tested. The results are shown in Fig. 1. The results reveal that the addition of PEOX increases the viscosity of PSf solution. In general, the increasing viscosity of the casting solution always makes the phase separation kinetics more prone to delayed demixing. However, the effective diffusion coefficient test shows an opposite result. The addition of PEOX drastically increases the diffusion rate during phase separation. This may be due to the high hydrophilicity of PEOX, which can improve the thermodynamic instability of the casting solution. Thus, the exchange rate between the solvent and non-solvent can be accelerated efficiently during the phase separation process.36 The above results suggest that the phase separation process is more prone to instantaneous demixing.
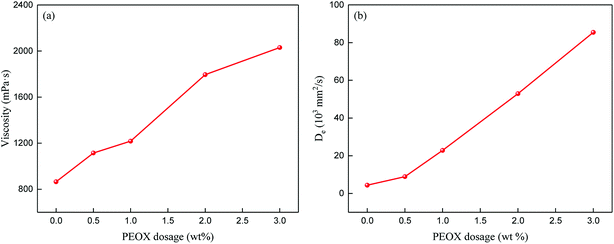 | ||
| Fig. 1 The viscosities and effective diffusion coefficients of PSf solutions with various PEOX dosages. (a) Viscosities of PEOX solutions; (b) effective diffusion coefficients of PEOX solutions. | ||
3.2. Characterization of the membranes
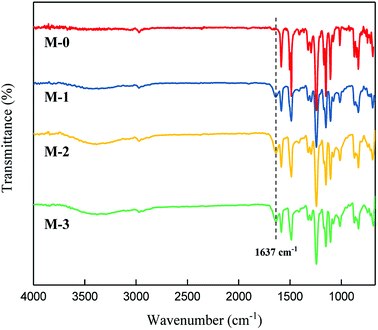
ATR-FTIR has been adopted to determine the chemical structures of the polysulfone membranes. The results are shown in Fig. 2. Bands at 1242 cm−1, 1487 cm−1 (aromatic ring breathing in the PSf chain43) and 1584 cm−1 are characteristics of the polysulfone chain.44 The identical absorption peaks of the primary amide groups (on the PEOX molecular chain) at 1637 cm−1 indicated that PEOX exists in all the PSf/PEOX membranes. The peak height of 1637 cm−1 increases along with the increased dosage of PEOX, indicating that more PEOX exists in the polymer matrix with the increase in PEOX dosage.3.3. Morphological study
The structure and morphology of the asymmetric PSf/PEOX membranes affected by various PEOX dosages were explored by an SEM method.SEM cross-sectional images of the PSf membranes with different PEOX dosages are depicted in Fig. 3. The incorporation of PEOX increases the thickness of the PSf membrane and induces the formation of macrovoids. The SEM images of the cross-section suggest that the presence of PEOX makes the phase separation process more prone to instantaneous demixing. The results are in agreement with the effective diffusion coefficient results in Fig. 1.
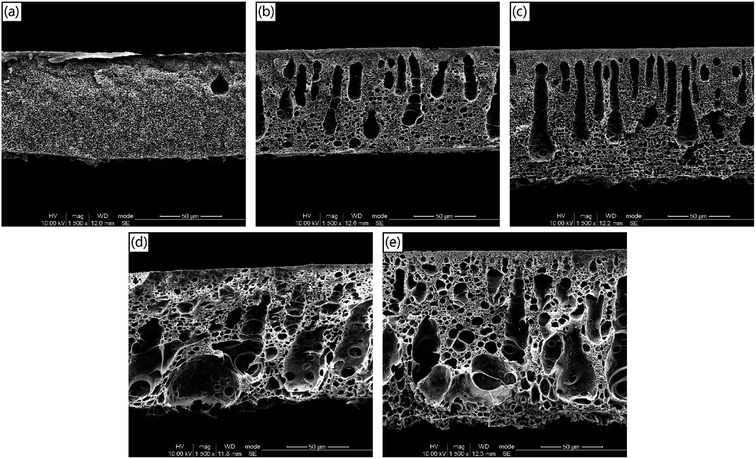 | ||
| Fig. 3 The cross-sectional morphologies of the PSf/PEOX membranes with different PEOX dosages. (a) M-0; (b) M-0.5; (c) M-1; (d) M-2; (e) M-3. | ||
Fig. 4 shows the SEM images of the top surfaces of different membranes prepared with different PEOX dosages. It reveals that in a unit area of the membrane surface, both the visible number and the size of pores decrease with the increasing PEOX dosage. When the PEOX dosage is 3.0 wt% (M-3), the pores are too small to be recognized.
 | ||
| Fig. 4 The top surface morphologies of the PSf/PEOX membranes with different PEOX dosages. (a) M-0; (b) M-0.5; (c) M-1; (d) M-2; (e) M-3. | ||
The top surface (skin layer) of a PSf membrane is formed by the imperfect merging of the PSf molecular chains.45,46 The addition of PEOX may decrease the pore size on the top surface because a more instantaneous demixing tendency suggests less inadequate merging time for the PSf phase. So, a better rejection rate is expected.
3.4. Porosity and contact angle (CA)
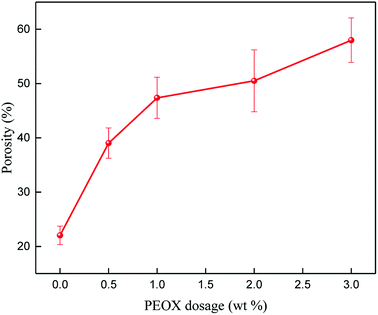
For an ultrafiltration membrane, its porosity and hydrophilicity are two crucial factors determining the membrane's permeability and rejection property. In the following sections, performances of PSf/PEOX membranes including porosity, contact angle, pure water flux (PWF) and rejection property were studied.Porosity of the membranes (shown in Fig. 5) increases gradually with the increasing PEOX dosage; the results are in agreement with the SEM images. High porosity means low hydraulic resistance of a membrane, suggesting high hydraulic permeability.
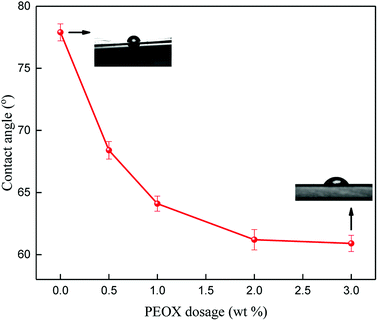
The contact angles (CA) of PSf/PEOX membranes with different PEOX dosages are exhibited in Fig. 6. It can be seen that the CA is around 78° for a pure PSf membrane, which gradually decreases with the increase in PEOX dosages to around 61° for a membrane with 3.0 wt% PEOX. This is mainly because the dispersion of hydrophilic PEOX in the membrane matrix leads to the decrease in CA.
3.5. Pure water flux (PWF) and rejection measurements
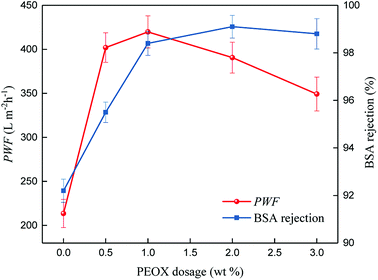
Pure water flux (PWF) and rejection property are the key practical performance parameters for any membrane.47 The PWF and the rejection property have a trade-off in almost all kinds of membranes; in general, a membrane with high PWF has a low rejection, and vice versa. For an ultrafiltration membrane, its PWF and rejection rates are determined by the number and size of pores on the top surface of the membrane, and the permeation mechanism is based on size exclusion.19,48 The effects of PEOX dosage on PWF and protein rejection are presented in Fig. 7.The PWF of a pure PSf membrane is 213.5 L m−2 h−1. When the dosage of PEOX increases, PWF increases accordingly. Particularly, the PWF can reach 419.8 L m−2 h−1 when the dosage of PEOX is 1.0 wt%, around 197% higher than that of the original PSf membrane. The BSA rejection by the resulting membranes also increases with the increase in PEOX dosage, and reaches more than 98.4% when the dosage of PEOX is 1.0 wt% and higher.
The results suggest that the practical performance of the resulting ultrafiltration membrane is drastically improved. According to the permeation mechanism, the rejection of BSA is mainly affected by the pores' size, and the rejection mechanism is affected by pore selection, i.e. smaller pores indicate better rejection.19
Moreover, the improvement in PWF is not disproportionate with the increase in CA. This correlation suggests that although the top surface pores become small, the increase in number of macrovoids and hydrogen bond interactions lead to the improvement of PWF. However, porosity of the top surface pore is hard to measure directly, but it can be tested indirectly via the flux and rejection measurements. With the continued increase in PEOX dosage, the PWF begins to decline, which may be because of the shrinkage of the top surface pores.
Commonly, the addition of hydrophilic additive, such as PEG, can double the water flux of the resulting membrane when the dosage of PEG is around 10 wt%, while the BSA rejection will decline.47 However, in our research, only a small dosage of PEOX (1.0 wt%) can double the water flux of the membrane and increase the BSA rejection.
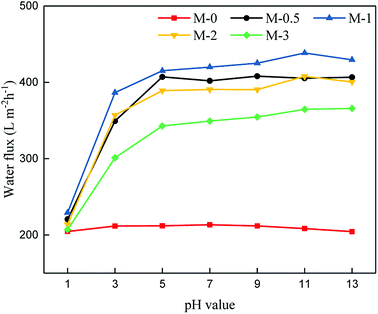
In addition, the pH value-responsive property of the resulting UF membrane was tested. The permeabilities of PSf/PEOX membranes were tested using feed solutions with various pH values ranging from 1.0–13.0. The fluxes of the membranes are shown in Fig. 8.
From Fig. 8, the pH value is observed to have little effect on the permeation property of the pure PSf UF membrane. Interestingly, the water fluxes of the PSf/PEOX UF membranes varies along with the change in pH value of the feed solution. When the pH value is 1.0, the PSf/PEOX UF membranes show a low water flux, which is similar to the water flux of a pure PSf UF membrane. As the pH value increases, the water fluxes of PSf/PEOX UF membranes improve significantly from around 200 L m−2 h−1 to 400 L m−2 h−1.
The change in water flux of the membrane may be attributed to the change in hydrogen bonding reactions between the PEOX chains and water. When the pH value is low, the amide groups in PEOX are ionized,30,31,49 and the PEOX molecular chains may aggregate via intermolecular hydrogen bonds. So, the hydrogen bonding between the PEOX chains and water becomes weak. The smaller surface pore size causes the PSf/PEOX UF membranes to have a similar water flux compared with that of the pure PSf UF membrane. When the pH value increases, the hydrogen bonding interaction between PEOX chains becomes weak, and that between PEOX chains and water becomes strong. As a result, the water fluxes of PSf/PEOX UF membranes increase because of the strong hydrogen bonding interactions between the membranes and water.31 The pH value-responsive property is found to be repeatable in our experiments.
This interesting pH-responsive behavior of the PSf/PEOX UF membrane has not been reported before, which broadens the application potential of the membrane in many specific cases.
3.6. Anti-fouling properties
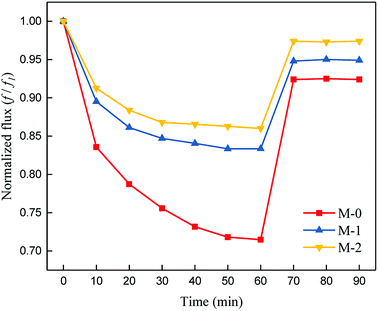
BSA was selected as the model foulant to simulate the protein foulants in this research; the anti-fouling results of the membranes were shown in Fig. 9. The DRt and FRR values were shown in Table 2. After 1 h of filtration, the flux of M-0 reduced to around 71% of the initial flux. However, the flux of PSf/PEOX membranes only reduced to around 84–87% of the initial flux. In addition, the FRR of a PSf/PEOX membrane is better than that of the neat PSf membrane.| M-0 | M-1 | M-2 | |
|---|---|---|---|
| DRt (%) | 28.5 ± 0.3 | 16.6 ± 0.5 | 14.0 ± 0.4 |
| FRR (%) | 92.4 | 94.8 | 97.4 |
Membrane fouling can be affected by back transport, permeation drag and chemical interactions between the membrane and the foulants.50 Since the test conditions for all membranes were the same, the different results mean that the interactions between the membranes and foulants became weak. The incorporation of PEOX makes the resulting PSf/PEOX UF membrane more hydrophilic than the pure PSf membrane. Therefore, the adsorption between the membrane and the foulants was reduced.
4. Conclusion
In this study, asymmetric polysulfone (PSf) and polysulfone/poly(2-ethyl-2-oxazoline) (PSf/PEOX) membranes were prepared by a phase separation method. PEOX was used at different dosages (0 wt%, 0.5 wt%, 1.0 wt%, 2.0 wt%, 3.0 wt%) as the polymeric additive in the casting solution. The effects of PEOX dosage on the phase separation kinetics, chemical properties, morphology, hydrophilicity, porosity and performances such as PWF, BSA rejection, pH value-responsiveness and anti-fouling property of the membranes were investigated in detail. The conclusions can be drawn as follows:Results of the viscosities and effective diffusion coefficients of PSf/PEOX solutions combined with the SEM morphology observations revealed that, after the addition of PEOX, the casting solution showed more thermodynamic instability, while the resulting membrane became thicker with more macrovoids, indicating that the incorporation of PEOX makes the phase separation process of the casting solution more prone to instantaneous demixing.
Hydrophilicity, pure water flux, BSA rejection and anti-fouling property of membranes have been improved significantly after the incorporation of PEOX. The PWF increases to 419.8 L m−2 h−1 when the dosage of PEOX is 1.0 wt% from the initial value of 213.5 L m−2 h−1 for the pure PSf membrane. BSA rejection by the resulting membranes increases with the increase in PEOX dosage, and reaches more than 98.4% when the dosage of PEOX is 1.0 wt% and higher.
The resulting PSf/PEOX UF membranes show an interesting pH value-responsive property, where the water flux changes along with the change in pH value of the feed solution. When the pH value of the feed solution is low, the water flux of the membrane declines; however, while the pH value of the feed solution increases, the water flux of the membrane improves significantly. This interesting feature of the membrane broadens the application potential of the membrane in many specific cases.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (NSFC 51503134, 51421061, 51702282), the State Key Laboratory of Polymer Materials Engineering (Grant No. SKLPME 2017-3-02) for the financial support.References
- K. Scott, Handbook of industrial membranes, Elsevier, 1995 Search PubMed.
- E. Saljoughi, M. Amirilargani and T. Mohammadi, Effect of PEG additive and coagulation bath temperature on the morphology, permeability and thermal/chemical stability of asymmetric CA membranes, Desalination, 2010, 262(1), 72–78 CrossRef CAS.
- P. Moradihamedani, N. A. Ibrahim, W. M. Z. W. Yunus and N. A. Yusof, Separation of CO2 from CH4 by pure PSF and PSF/PVP blend membranes: effects of type of nonsolvent, solvent, and PVP concentration, J. Appl. Polym. Sci., 2013, 130(2), 1139–1147 CrossRef CAS.
- V. Deimede, K. Nikitopoulou, A. Voege and J. Kallitsis, Study of polymer blend membranes composed of sulfonated polysulfone and PEO-grafted aromatic polyether with controllable morphology, Eur. Polym. J., 2015, 63, 113–122 CrossRef CAS.
- J. Wijmans, J. Baaij and C. Smolders, The mechanism of formation of microporous or skinned membranes produced by immersion precipitation, J. Membr. Sci., 1983, 14(3), 263–274 CrossRef CAS.
- R. Boom, I. Wienk, T. Van den Boomgaard and C. Smolders, Microstructures in phase inversion membranes. Part 2. The role of a polymeric additive, J. Membr. Sci., 1992, 73(2), 277–292 CrossRef CAS.
- C. Smolders, A. Reuvers, R. Boom and I. Wienk, Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids, J. Membr. Sci., 1992, 73(2), 259–275 CrossRef CAS.
- Q.-Z. Zheng, P. Wang and Y.-N. Yang, Rheological and thermodynamic variation in polysulfone solution by PEG introduction and its effect on kinetics of membrane formation via phase-inversion process, J. Membr. Sci., 2006, 279(1), 230–237 CrossRef CAS.
- Q.-Z. Zheng, P. Wang, Y.-N. Yang and D.-J. Cui, The relationship between porosity and kinetics parameter of membrane formation in PSF ultrafiltration membrane, J. Membr. Sci., 2006, 286(1), 7–11 CrossRef CAS.
- E. Saljoughi, M. Amirilargani and T. Mohammadi, Effect of poly(vinyl pyrrolidone) concentration and coagulation bath temperature on the morphology, permeability, and thermal stability of asymmetric cellulose acetate membranes, J. Appl. Polym. Sci., 2009, 111(5), 2537–2544 CrossRef CAS.
- J. Mulder, Basic principles of membrane technology, Springer Science & Business Media, 2012 Search PubMed.
- J. Xu, Y. Tang, Y. Wang, B. Shan, L. Yu and C. Gao, Effect of coagulation bath conditions on the morphology and performance of PSf membrane blended with a capsaicin-mimic copolymer, J. Membr. Sci., 2014, 455(4), 121–130 CrossRef CAS.
- A. K. Hołda and I. F. J. Vankelecom, Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—part A: influence of high molecular weight additives, J. Membr. Sci., 2014, 450(2), 512–521 CrossRef.
- E. Saljoughi, M. Sadrzadeh and T. Mohammadi, Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes, J. Membr. Sci., 2009, 326(2), 627–634 CrossRef CAS.
- A. Rahimpour and S. Madaeni, Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: preparation, morphology, performance and antifouling properties, J. Membr. Sci., 2007, 305(1), 299–312 CrossRef CAS.
- E. Saljoughi and T. Mohammadi, Cellulose acetate (CA)/polyvinylpyrrolidone (PVP) blend asymmetric membranes: preparation, morphology and performance, Desalination, 2009, 249(2), 850–854 CrossRef CAS.
- I. Wienk, R. Boom, M. Beerlage, A. Bulte, C. Smolders and H. Strathmann, Recent advances in the formation of phase inversion membranes made from amorphous or semi-crystalline polymers, J. Membr. Sci., 1996, 113(2), 361–371 CrossRef CAS.
- H.-T. Yeo, S.-T. Lee and M.-J. Han, Role of a polymer additive in casting solution in preparation of phase inversion polysulfone membranes, J. Chem. Eng. Jpn., 2000, 33(1), 180–184 CrossRef CAS.
- M.-J. Han and S.-T. Nam, Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane, J. Membr. Sci., 2002, 202(1), 55–61 CrossRef CAS.
- B. Jung, J. K. Yoon, B. Kim and H.-W. Rhee, Effect of molecular weight of polymeric additives on formation, permeation properties and hypochlorite treatment of asymmetric polyacrylonitrile membranes, J. Membr. Sci., 2004, 243(1), 45–57 CrossRef CAS.
- D. Mosqueda-Jimenez, R. Narbaitz, T. Matsuura, G. Chowdhury, G. Pleizier and J. Santerre, Influence of processing conditions on the properties of ultrafiltration membranes, J. Membr. Sci., 2004, 231(1), 209–224 CrossRef CAS.
- T. P. Hou, S. H. Dong and L. Y. Zheng, The study of mechanism of organic additives action in the polysulfone membrane casting solution, Desalination, 1991, 83(125), 343–360 CrossRef CAS.
- J.-H. Kim and K.-H. Lee, Effect of PEG additive on membrane formation by phase inversion, J. Membr. Sci., 1998, 138(2), 153–163 CrossRef CAS.
- J.-J. Shieh, T.-S. Chung, R. Wang, M. Srinivasan and D. R. Paul, Gas separation performance of poly(4-vinylpyridine)/polyetherimide composite hollow fibers, J. Membr. Sci., 2001, 182(1), 111–123 CrossRef CAS.
- A. Idris, N. M. Zain and M. Noordin, Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives, Desalination, 2007, 207(1–3), 324–339 CrossRef CAS.
- B. Chakrabarty, A. Ghoshal and M. Purkait, Effect of molecular weight of PEG on membrane morphology and transport properties, J. Membr. Sci., 2008, 309(1), 209–221 CrossRef CAS.
- Z.-L. Xu, T.-S. Chung, K.-C. Loh and B. C. Lim, Polymeric asymmetric membranes made from polyetherimide/polybenzimidazole/poly(ethylene glycol)(PEI/PBI/PEG) for oil–surfactant–water separation, J. Membr. Sci., 1999, 158(1), 41–53 CrossRef CAS.
- I.-C. Kim and K.-H. Lee, Effect of poly(ethylene glycol) 200 on the formation of a polyetherimide asymmetric membrane and its performance in aqueous solvent mixture permeation, J. Membr. Sci., 2004, 230(1), 183–188 CrossRef CAS.
- L. D. Villano, R. Kommedal, M. W. Fijten, U. S. Schubert, R. Hoogenboom and M. A. Kelland, A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly(2-alkyl-2-oxazoline)s, Energy Fuels, 2009, 23(7), 3665–3673 CrossRef.
- C.-H. Wang and G.-H. Hsiue, New amphiphilic poly(2-ethyl-2-oxazoline)/poly(L-lactide) triblock copolymers, Biomacromolecules, 2003, 4(6), 1487–1490 CrossRef CAS PubMed.
- C. H. Wang and G. H. Hsiue, Synthesis and characterization of temperature- and pH-sensitive hydrogels based on poly(2-ethyl-2-oxazoline) and poly(D,L-lactide), J. Polym. Sci., Part A: Polym. Chem., 2002, 40(8), 1112–1121 CrossRef CAS.
- P. Lin, C. Clash, E. M. Pearce, T. K. Kwei and M. A. Aponte, Solubility and miscibility of poly(ethyl oxazoline), J. Polym. Sci., Part A: Polym. Chem., 2010, 26(3), 603–619 CrossRef.
- K. Aoi and M. Okada, Polymerization of oxazolines, Prog. Polym. Sci., 1996, 21(1), 151–208 CrossRef CAS.
- H. Wu, J. Huang and Y. Liu, Polysulfone ultrafiltration membrane incorporated with Ag-SiO2 nanohybrid for effective fouling control, J. Water Health, 2017, 15(3), 341 CrossRef PubMed.
- I. Pinnau and B. D. Freeman, Membrane formation and modification, American Chemical Society, 2000 Search PubMed.
- D. M. Wang, F. C. Lin, T. T. Wu and J. Y. Lai, Formation mechanism of the macrovoids induced by surfactant additives, J. Membr. Sci., 1998, 142(2), 191–204 CrossRef CAS.
- C. A. Smolders, A. J. Reuvers, R. M. Boom and I. M. Wienk, Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids☆, J. Membr. Sci., 1992, 73(2), 259–275 CrossRef CAS.
- H. Strathmann, K. Kock, P. Amar and R. W. Baker, The formation mechanism of asymmetric membranes, Desalination, 1975, 16(2), 179–203 CrossRef CAS.
- S. K. Yong, K. H. Jin and Y. K. Un, Asymmetric membrane formation via immersion precipitation method. I. Kinetic effect, J. Membr. Sci., 1991, 60(2–3), 219–232 CrossRef.
- H. J. Kim, R. K. Tyagi, A. E. Fouda and K. Ionasson, The kinetic study for asymmetric membrane formation via phase-inversion process, J. Appl. Polym. Sci., 1996, 62(4), 621–629 CrossRef CAS.
- M. Sivakumar, D. R. Mohan and R. Rangarajan, Studies on cellulose acetate-polysulfone ultrafiltration membranes: II. Effect of additive concentration, J. Membr. Sci., 2006, 268(2), 208–219 CrossRef CAS.
- T. Mohammadi and E. Saljoughi, Effect of production conditions on morphology and permeability of asymmetric cellulose acetate membranes, Desalination, 2009, 243(1–3), 1–7 CrossRef CAS.
- J. Wang, R. Xu, F. Yang, J. Kang, Y. Cao and M. Xiang, Probing influences of support layer on the morphology of polyamide selective layer of thin film composite membrane, J. Membr. Sci., 2018, 556, 374–383 CrossRef CAS.
- K. Singh, P. G. Ingole, J. Chaudhari, H. Bhrambhatt, A. Bhattacharya and H. C. Bajaj, Resolution of racemic mixture of α-amino acid derivative through composite membrane, J. Membr. Sci., 2011, 378(1), 531–540 CrossRef CAS.
- R. Datta, S. Dechapanichkul, J. S. Kim, L. Y. Fang and H. Uehara, A generalized model for the transport of gases in porous, non-porous, and leaky membranes. I. Application to single gases, J. Membr. Sci., 1992, 75(3), 245–263 CrossRef CAS.
- P. Ingo and W. J. Koros, Gas-permeation properties of asymmetric polycarbonate, polyestercarbonate, and fluorinated polyimide membranes prepared by the generalized dry–wet phase inversion process, J. Appl. Polym. Sci., 2010, 46(7), 1195–1204 Search PubMed.
- Y. Ma, F. Shi, J. Ma, M. Wu, J. Zhang and C. Gao, Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes, Desalination, 2011, 272(1), 51–58 CrossRef CAS.
- R. W. Baker, Membrane Technology and Applications, Metallurgical Transactions A, 2nd edn, 2004, vol. 6 Search PubMed.
- Y. Zhao, Y. Zhou, D. Wang, Y. Gao, J. Li, S. Ma, L. Zhao, C. Zhang, Y. Liu and X. Li, pH-responsive polymeric micelles based on poly(2-ethyl-2-oxazoline)-poly(D, L-lactide) for tumor-targeting and controlled delivery of doxorubicin and P-glycoprotein inhibitor, Acta Biomater., 2015, 17, 182–192 CrossRef CAS.
- H. Hua, N. Li, L. Wu, H. Zhong, G. Wu, Z. Yuan, X. Lin and L. Tang, Anti-fouling ultrafiltration membrane prepared from polysulfone-graft-methyl acrylate copolymers by UV-induced grafting method, J. Environ. Sci., 2008, 20(5), 565–570 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |