Open Access Article
Open Access ArticleInteraction mechanism between TiO2 nanostructures and bovine leukemia virus proteins in photoluminescence-based immunosensors
Alla Tereshchenko*ab,
Valentyn Smyntynaa and
Arunas Ramanavicius *b
*b
aExperimental Physics Department, Faculty of Mathematics, Physics and Information Technologies, Odesa National I.I. Mechnikov University, Pastera 42, 65023, Odesa, Ukraine
bDepartment of Physical Chemistry, Institute of Chemistry, Faculty of Chemistry and Geosciences, Vilnius University, Naugarduko 24, LT-03225 Vilnius, Lithuania. E-mail: Arunas.Ramanavicius@chf.vu.lt; alla_teresc@onu.edu.ua
First published on 9th November 2018
Abstract
In this research a mechanism of interaction between a semiconducting TiO2 layer and bovine leukemia virus protein gp51, applied in the design of photoluminescence-based immunosensors, is proposed and discussed. Protein gp51 was adsorbed on the surface of a nanostructured TiO2 thin film, formed on glass substrates (TiO2/glass). A photoluminescence (PL) peak shift from 517 nm to 499 nm was observed after modification of the TiO2/glass by adsorbed gp51 (gp51/TiO2/glass). After incubation of the gp51/TiO2/glass in a solution containing anti-gp51, a new structure (anti-gp51/gp51/TiO2/glass) was formed and the PL peak shifted backwards from 499 nm to 516 nm. The above-mentioned PL shifts are attributed to the variations in the self-trapped exciton energy level, which were induced by the changes of electrostatic interaction between the adsorbed gp51 and the negatively charged TiO2 surface. The strength of the electric field affecting the photoluminescence centers, was determined from variations between the PL-spectra of TiO2/glass, gp51/TiO2/glass and anti-gp51/gp51/TiO2/glass. The principle of how these electric field variations are induced has been predicted. The highlighted origin of the changes in the photoluminescence spectra of TiO2 after its protein modification reveals an understanding of the interaction mechanism between TiO2 and proteins that is the key issue responsible for biosensor performance.
1. Introduction
Bovine leukemia is a lethal retroviral infection, which is induced by bovine leukemia virus (BLV). There is a significant risk that BLV can infect the other mammalians as in the case of other retroviruses' – such as, human immunodeficient virus (HIV). Therefore outbreaks of this infectious disease can significantly damage the ecosystem. For this reason the development of advanced bioanalytical systems for the determination of biomarkers of BLV-infection are required.1,2 Among many different immunosensors, the photoluminescence-based (PL) immunosensors seems to be the most promising for the improvement in diagnosis of virus induced diseases. In PL-immunosensors nanostructured ZnO or TiO2 can be used as very efficient photoluminescence transducers.3,4 TiO2 and its composites are extensively used as wide-band gap semiconductors with a unique combination of physical and chemical properties.5–7A good biocompatibility of TiO2 nanostructures, their applicability at physiological pHs in the range of ∼5.5–7.0, non-toxicity and excellent chemical stability have resulted in the extensive application of TiO2 in electrochemical,8–10 electrical11 and optical12–14 biosensors. Optical biosensors are increasingly studied class of biosensors because they allow to evaluate some inter-molecular interactions contactless15 and without any chemical/physical labels.3 The development of immunosensors, based on the optical detection methods, that can be applied for the determination of large variety of analytes in the complex biological samples7,12,14,15 is of great interest.
Nanostructured TiO2 is known as a material of intense photoluminescence at room temperature.16,17 The application of TiO2 photoluminescence properties in optical biosensors and immunosensors have been reported in the range of works. The changes in the photoluminescence spectra (shift of PL-maximum and the variation of PL-signal intensity) were exploited as analytical signals for the determination of target analyte.18,19 However the interaction mechanism of proteins with TiO2 and the origin of the changes in the photoluminescence spectra were not discussed, although the mechanism of the interaction between semiconductor and proteins is the key in solving many of problems, which are still arising during the development of TiO2-based immunosensors, such as an improvement of sensitivity and selectivity.3,13,14
This work is aiming to highlight the origin of the changes in the photoluminescence spectra of TiO2 resulted after the protein adsorption on its surface during the formation of biosensitive layer and after the interaction of biosensitive layer with the analyte. As it is proposed, the reason of the PL shifts observed after modification of TiO2/glass by gp51 proteins is an electrostatic interaction between adsorbed gp51 and negatively charged TiO2 surface. The strength of the electric field affecting photoluminescence centers, localized close to the surface of TiO2/glass, is determined from variations of the PL-spectra of TiO2/glass, gp51/TiO2/glass and anti-gp51/gp51/TiO2/glass. The principle of how these electric field variations are induced is discussed. The influence of several important parameters, including charge density of charged atoms/groups/domains in the specific binding biomolecules, effective coverage area by specific binding biomolecules and variation of the potential barrier are taken into account during the elaboration of the model.
2. Materials and methods
2.1. Formation and characterization of TiO2 samples
Nanostructured TiO2 layers containing TiO2 nanoparticles were formed on the glass substrates (TiO2/glass) by the deposition of colloidal suspension of TiO2 nanoparticles (Sigma Aldrich, 99.7%, particle size of 32 nm), dissolved in ethanol. Initial concentration of TiO2 nanoparticles was about 0.01 mg ml−1. The formed TiO2/glass structures were dried at room temperature and then annealed at 350 °C. Structural and surface characterization of the obtained samples showed that in TiO2/glass structures the TiO2 kept the anatase structure and TiO2 nanoparticles formed a high surface area porous structure, which was suitable for the formation of biosensitive layer. More detailed description of deposition and characterization procedures, which were applied for the characterization of nanostructured TiO2/glass layers is reported in earlier our researches.18,19Optical characterization of the nanostructured TiO2/glass layers was performed by photoluminescence measurements using 355 nm solid state laser as the excitation source. Optical setup is described in detail in previous our researches.19,20 The PL-spectra were recorded in the range of wavelength from 360 to 800 nm.
2.2. Fabrication and evaluation of an immunosensor
The immobilization of biological molecules was carried out by incubation in a solution containing leukemia virus proteins – gp51, similar to the immobilization procedure described in earlier works.18–20 Briefly: a solution of PBS containing gp51 antigens at a high concentration was directly immobilized on the surface of TiO2/glass structure. Then the sample was placed into a Petri plate for the incubation in a medium saturated with water vapor at 25 °C. After 10 min of incubation, the surface of the sample was washed with a PBS solution in order to remove not well adsorbed antigens from the surface of TiO2/glass structure. As a result, a layered structure of gp51/TiO2/glass was formed, which got affinity and selectivity for the one type of bio-molecules – antibodies against leukemia proteins gp51 (anti-gp51). To prevent a nonspecific interaction (i.e., binding of anti-gp51 antibodies directly to unmodified TiO2 surface), the surface of TiO2/glass was further treated with a solution of bovine serum albumin (BSA), which after the formation of gp51/BSA/TiO2/glass structure filled still free-remaining sites available for non-specific protein-adsorption standing. Thus it was expected that the selectivity of gp51/BSA/TiO2/glass structure has been improved in comparison to that of BSA-not modified gp51/TiO2/glass structure.3. Results and discussion
3.1. Interaction of gp51 proteins with TiO2 surface
Proteins consist of amino acids that might contain positively and/or negatively charged radicals that are determining the charge of different protein domains.21 A large quantity of negatively charged groups such as aldehyde (–CHO), hydroxyl (–OH), carboxyl (–COOH) and positively charged primary amine (–NH2) and some other groups, which are involved into the structure of amino acids, are responsible for the partial (δ+ and δ−) charges of particular protein domains. Therefore the proteins are characterized by electrostatic properties, and sometimes even significant electrostatic ‘asymmetry of protein molecule’ because the atoms and functional groups, which are forming the protein molecules are charged differently both in charge sign and in absolute charge value. Naturally, the charges in proteins at least partly are compensating each other, but the ternary structure of proteins is relatively rigid and the charged groups have only limited degree of freedom to move within the protein globule. Therefore, in some parts of the protein some uncompensated charge on the surface and inside of the protein still remains.21 The distribution of charged groups on the surface of the protein depends on the sequence of amino acids, which is pre-determined by the genome that was developed during millions or even billions of years lasting evolution of each protein. During the evolution, the genes which are promoting the synthesis of proteins whose structure the most efficiently matches their function, were selected for further generations.It should be taken into account that even if the structure of the most proteins is at some extent ‘rigid’ there is still some degree of flexibility because proteins in their polymeric structure contains a high number of σ-bonds, around which the rotation of protein-building segments is possible. Moreover, both secondary and tertiary structures of the protein are supported by a large number of hydrogen bonds but many of them are not very strong, therefore this factor adds additional degree of freedom for protein structure.22 The electrostatic interactions, which are based on Coulomb forces between the opposite charges, van der Waals forces and disulfide bonds also play an important role in the formation of both secondary and tertiary structures of protein, but they can be relatively easily disrupted by electrostatic-neutralization and/or displacement of some charged groups/domains, which are forming proteins.
TiO2 (in its allotropic form of anatase) is a semiconductor of n-type conductivity, usually with an ‘upward’ band bending of the energy levels when closing the surface of TiO2 (Fig. 1),4,6 which indicates the accumulation of a negative charge (bound at surface levels) on its surface.6,23 The adsorption of the most of molecules is known to introduce an additional charge on the solid state surface and it can change the existing surface energy levels or form the additional ones that are involved in the exchange of charges with the volume of a solid material.24 It should be taken into account, that in the open air, the oxygen adsorbates (Oads−) formed on the surface of TiO2 are resulting in an electron delocalization towards adsorbed oxygen what reduces the conductivity of TiO2 layer. This process is similar to that, which is well described for ZnO.25
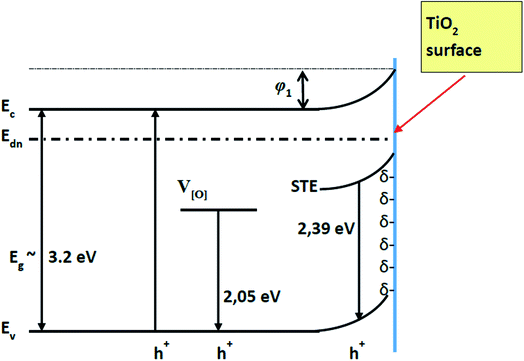 | ||
| Fig. 1 Energetic levels of pristine TiO2 (TiO2/glass structure). Ec, Ev – conduction and valence band of TiO2 respectively. Edn – electron demarcation level. | ||
Further analysis of the interaction between TiO2/glass and gp51 proteins is based on the evaluation of photoluminescence of TiO2 nanoparticles. The protein gp51 is specifically binding with antibodies against gp51 (anti-gp51) i.e. the target-analyte. The photoluminescence properties of the TiO2 nanoparticles have been previously investigated in some research papers.19,20 Moreover, optical effects, which are registered during gp51 protein adsorption on TiO2/glass, also where observed and even applied in the development of photoluminescence based immunosensor for the determination of gp51 antibodies.18,19 However in these publications no attention was paid to the nature of interaction between protein gp51 and semiconductor TiO2 and for the prediction of the mechanism of PL-based signal generation.
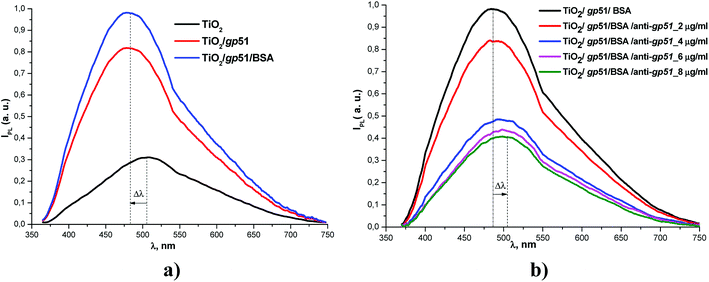
In a present research the success of immobilization of gp51 and the formation of gp51/TiO2/glass structure was similar to that reported in earlier our works.19,20 The immobilization of gp51 proteins on TiO2/glass leads to an increase in the intensity of the photoluminescence signal of TiO2/glass nanostructures and a UV-shift in the position of the maximum of the photoluminescence spectra (Fig. 2a).19 The application of BSA, which were used to block the free adsorption centers on the TiO2/glass surface, also leads to a slight increase in the photoluminescence intensity, but the spectrum shift was not observed.
Interaction between gp51/TiO2/glass and anti-gp51 leads to the inverse changes in the photoluminescence spectrum (Fig. 2b), i.e. a decrease in the integral intensity of the photoluminescence and the IR-shift of spectra. Therefore, the response of the immunosensor gp51/TiO2/glass to anti-gp51 can be estimated by two parameters: (i) the photoluminescence intensity and (ii) the position of the PL-maximum. The sensitivity of gp51/TiO2/glass based immunosensor towards anti-gp51 was in the range of 2–8 μg ml−1.19
3.2. Mechanism of interaction between TiO2/glass and gp51 proteins
A gp51 protein molecule has a molecular mass of 51 kDa. The characteristic geometric size of the gp51 molecules that are adsorbed on the TiO2 surface is about 6 nm in diameter.26 The authors, which have published a research on the formation of gp51 protein-based capsid of BLV, have constructed an image of the structure of the gp51 protein from the X-ray crystallography data and they have reported that this protein is extra-flexible, which provides very high functionality and the ability to associate and/or dissociate of mainly on gp51 protein based the capsid of BLV to/from the membrane of BLV infected cell.27 Therefore, it is expected that on the surface of TiO2 gp51 protein also forms a well-ordered monolayer. The formation of such layer was confirmed in previous our researches based on the application of spectroscopic ellipsometry.27–29The gp51 is not a redox-protein therefore the charge transfer between gp51 and TiO2/glass is not possible.30 But the gp51 protein, like many other proteins, contains a number of partially charged groups and domains, which in Fig. 3 and 4, are represented as partial charges “δ–” and “δ+”. These charges per charged atom or group are mostly lower in value than the total single-electron charge (1.6 × 10−19 coulombs). The presence of these partial charges suggests that the electrostatic influence of gp51 protein on the surface charge of TiO2/glass is coming from the side of partially uncompensated charges, which are localized in those parts of the gp51 protein that are located in close proximity to the surface of TiO2/glass and are mainly responsible for the adsorption of this protein on the TiO2/glass surface. The electrostatic Coulomb interaction, which takes place between charged groups in the gp51 protein and the negatively charged surface of the TiO2/glass, are the most strong at a distances ranging from several angstroms to few nanometers. Therefore, among the other interactions such as hydrogen bonds, disulfide bonds, van der Waals interaction, etc., which all also have significant role during the adsorption of proteins, the electrostatic interaction plays one of the most significant role during the adsorption of proteins onto electrically charged surfaces, such as TiO2. In addition, the local electric fields of charged domains of adsorbed proteins are electrostatically affecting the PL-centers of TiO2/glass and it causes the shift in the photoluminescence spectra of TiO2 nanoparticles. Therefore, the photoluminescence maximum of gp51/TiO2/glass, which is mainly caused by self-trapped excitons (STE), shifts from 517 to 499 nm (i.e., to 18 nm), which corresponds to ∼0.086 eV that is less than 0.1 eV, and it is one of the proofs of electrostatic interaction based physical adsorption of gp51.24
The splitting of the photoluminescence spectra into Gaussian curves at each stage of the experiment shows that after the adsorption of gp51 protein molecules on the TiO2 surface the energy value of excitation levels, which are responsible for the luminescence and associated with oxygen vacancies IV[O], almost does not change remaining at a value of 605 ± 2 nm. At the same time, the photoluminescence maximum caused by the recombination of STE31–33 shifts to short wavelengths, changing its position from 517 (ISTE1 = 2.39 eV) nm to 499 nm (ISTE2 = 2.48 eV). Since the involvement of the STE level in the process of radiative recombination is regulated by the surface, this indicates that STE level is located either on the surface plane or not very deeply within the surface layer of the TiO2. The displacement of the light emitting recombination peak indicates that the energy level of STE is complex and has its ‘basic’ and ‘excited’ states.31–33 The appearance of luminescence in the region of 499 nm indicates a radiative transition from the excited STE level. This indicates that the charge at the TiO2/gp51 boundary controls the energy level of the STE and shows that the electronic demarcation level practically coincides with the position of the STE level, i.e. is approximately 2.39 eV above the valence band. Therefore, the appearance of proteins on the TiO2 surface leads to a shift in the energy levels, including energy levels of the light emitting centers, relatively to the electron demarcation level Edn.
The blue-shift of the photoluminescence maximum by 18 nm as a result of adsorption of the gp51 protein, which corresponds to ΔESTE = STE2 − STE1 = 0.086 eV, also indicates that the initial value of the potential barrier (φ1) on the TiO2/glass surface has decreased by a value of 0.086 eV to φ2. Variation of the potential barrier means that the value of negative charge localized on the TiO2/glass surface has also changed, due to the charge–charge-based interaction with adsorbed protein gp51. Positively charged atoms and groups, which are provided by the gp51 protein, partially compensates the surface charge of TiO2/glass and reduces the energy of electrons localized at the surface levels, which are the most responsible for the generation of PL-signal. Taking into account the fact that the total negative charge predominates on the TiO2/glass surface, the positively charged parts of the gp51 protein electrostatically interact with the negatively charged TiO2/glass surface. As a result, a partial decrease of the surface charge reduces the electric field in the TiO2/glass surface region. Further interaction of gp51/TiO2/glass structure with analyte – anti-gp51, which is also a protein, leads to the inverse changes in the photoluminescence spectra, i.e., to UV-shift of the spectrum and decrease the photoluminescence intensity to the value that corresponds to the pure TiO2/glass. The latter effect is based on the formation of an immune complex between immobilized antigens gp51 and anti-gp51 antibodies, which are present in aliquot. The formation of this immune complex besides the van der Waals interaction and other interactions at a very high extent is based on the interaction between oppositely charged domains, functional groups and atoms in gp51 and anti-gp51 molecules (including the formation of number of hydrogen bounds, which can be estimated as specific kind of electrostatic interaction). It can be assumed that uncompensated charges (δ+ and δ) of both proteins (gp51 and anti-gp51) are involved in electrostatic interactions during the formation of immune complex. As a result, some of the charged groups of protein gp51 that were originally involved in the interaction between gp51 and TiO2/glass are at least partially compensated by the opposite charge of the anti-gp51 protein groups, thereby reducing the direct electrostatic effect from immobilized gp51 proteins to the charged surface of TiO2 and at the same time to PL-light emitting centers.
The effects described above have an effect on the shift of PL-maximum and on the decrease in the potential barrier on gp51/TiO2/glass interface due to the charge–charge interaction between TiO2/glass and gp51. The potential barrier at the interface between TiO2/glass and gp51 has a greater value in gp51/TiO2/glass structure in comparison with that in anti-gp51/gp51/TiO2/glass due to partial compensation (decrease in value) and/or delocalization of gp51 charges, which were initially involved into interaction between TiO2/glass and gp51 after formation of gp51/TiO2/glass structure.
The changes in the PL intensity from TiO2 after the protein adsorption can be caused by following reasons. An increase of the PL intensity after immobilization of gp51 proteins on the TiO2/glass surface could result from the fact that the oxygen, adsorbed on the surface of TiO2, forms a re-oxidized layer that is reduced by the atoms and groups in gp51 proteins. A decrease of the PL intensity after the interaction of biosensitive layer with the target analyte can be caused by both additional dispersion of light from the excitation source and exited PL signal generated from anti-gp51/gp51/TiO2/glass structure.
The distribution of charges in gp51/TiO2/glass structure can also be interpreted as a model based on an ‘imaginary capacitor’ (Fig. 3), formed as a result of the electrostatic interaction between oppositely charged protein gp51 layer and the TiO2/glass surface. The ‘imaginary capacitor’ is formed as a result of protein gp51 adsorption on TiO2/glass surface, after which the charges are distributed in energetically most favorable way, partially compensating each other. Consequently, the positive ‘imaginary capacitor plate’ is based on the positive charges, which are predominant in the protein gp51 surface area that after adsorption appears in close proximity to gp51/TiO2/glass interface and/or due to the negative electrostatic effect of TiO2/glass are induced/attracted closer to negatively charged TiO2/glass surface.
Charged atoms/groups/domains of gp51 that are localized in the close proximity to the TiO2/glass surface and they electrostatically affect the TiO2 emission centers and the energy value of the surface potential barrier. Thus the position of the energy levels of the TiO2/glass emission maximum depends on TiO2 surface modification stage (TiO2/glass or gp51/TiO2/glass) shifts from/backwards the initial position of the demarcation level. Fig. 3 represents an imaginary capacitor consisting of a negatively charged plate on the surface of TiO2/glass and an ‘imaginary positively charged plate’ formed in gp51 protein in close proximity to gp51/TiO2/glass interphase.
Hence, the interaction of gp51/TiO2/glass with anti-gp51 antibodies and the formation of gp51/anti-gp51-based immune complex leads to a ‘deformation’ and the reduction of charge ‘stored’ on ‘the positive imaginary capacitor plate’ (Fig. 4). This is mainly due to the redistribution and partial compensation of charges during the formation of the gp51/anti-gp51 immune complex, which in turn reduces the charge of ‘the imaginary capacitor plate’ based on gp51 (q2 < q1). This reduced charge can be interpreted as the reduction of the area of the same plate (S2) and/or the increase of the distance (d2) between the two imaginary capacitor plates based on gp51 and TiO2/glass. This effect is observed because some of the gp51 protein charges move from the gp51/TiO2/glass interface towards interacting anti-gp51 protein and are partially compensated by charge present in anti-gp51, whereby the imaginary positive gp51-based capacitor plate of the capacitor is reduced in imaginary surface area and/or correspondingly moving apart from the negative TiO2/glass plate. This effect leads to a decrease in the capacitance of this imaginary capacitor and the electric field induced by gp51 becomes reduced. Therefore, after the interaction of gp51/TiO2/glass with anti-gp51 antibodies and the formation of gp51/anti-gp51 complex, which is involved into anti-gp51/gp51/TiO2/glass structure, the electrostatic effect of gp51 initially adsorbed on TiO2/glass towards the TiO2 surface significantly decreases.
It should be taken into account that after the adsorption of gp51 proteins, the oppositely charged domains build up within a surface layers of both adsorbed-gp51 and TiO2/glass within the Debye-screening length of both materials. These oppositely charged electric layers forms a double-layered structure with obvious electric capacitance that is dependent on (i) the concentration of charged atoms/groups/domains, (ii) distance of these features from each other and dielectric constant (relative permittivity) of gp51 and space in between adsorbed gp51 and TiO2/glass. Any charge that is emerging within the Debye-screening length on the surfaces of both adsorbed-gp51 and TiO2/glass is affecting the capacitance of this imaginary capacitor. The variation of both (i) the number and (ii) the localization of charged parts depends on so called ‘Debye-screening length’ towards all directions of structures and influences the strength of electric field in gp51/TiO2/glass and anti-gp51/gp51/TiO2/glass structures. The relationships among the value of the Debye-screening length (LD) and the concentration of charged atoms/groups/domains of gp51 are provided in eqn (1) and/or (2):
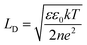 | (1) |
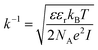 | (2) |
Debye-screening length for TiO2, calculated according to the eqn (1), while applying such values ε – permittivity in TiO2 (ε = 34),35 ε0 – dielectric constant (8.85 × 10−12 F m−1), n – concentration of electrons in TiO2 (n = 1019 sm−3), e – electron charge (1.6 × 10−19 Cb), k – Boltzmann's constant (1.38 × 10−23 J K−1) and T – absolute temperature (293 K) was estimated to be 15 nm. The estimated value of LD shows that within this distance from TiO2 surface the PL-centres are affected by electrostatic effects from adsorbed proteins gp51 and anti-gp51/gp51.
The measured PL-peak values determine how the concentration and distribution of charged atoms/groups/domains charge density affect the TiO2 surface potential and potential of energetic levels within Debye-screening length, which can be determined using eqn (1) and (2). This effect is similar to that observed on field effect transistors.36–38 The sensitivity of TiO2-based PL immunosensor is also influenced by the concentration and distribution of charged atoms/groups/domains (Debye-screening length) in protein gp51 or gp51/anti-gp51-based immune-complex.
4. Conclusions
The main aspects of the interaction mechanism between nanostructured TiO2/glass layer and bovine leukemia virus proteins gp51, during the formation of PL-based immunosensor, have been outlined. Bovine leukemia virus protein gp51, which was adsorbed on the surface of nanostructured TiO2/glass thin film, formed the biosensitive layer (gp51/TiO2/glass) that resulted in the TiO2 PL peak shift from 517 nm to 499 nm. The interaction gp51/TiO2/glass structure with specific antibodies against gp51 (anti-gp51) has shifted the PL peak backwards from 499 nm to 516 nm. These PL shifts are attributed to the variation of STE energy level, which was induced by changes of electrostatic interaction between adsorbed gp51 and negatively charged TiO2/glass surface. The displacement of the light emitting recombination peak confirms that the energy of STE level is complex and has its ground and excited states. The blue-shift of the photoluminescence maximum by 18 nm as a result of adsorption of the gp51 protein, which corresponds to ΔESTE = ISTE2 − ISTE1 = 0.086 eV, indicates that the initial value of the potential barrier on the TiO2/glass surface has decreased by 0.086 eV. The variation of the potential barrier means that the value of negative charge localized on the TiO2/glass surface has changed due to the charge–charge-based interaction with adsorbed protein gp51. Positively charged atoms and groups, provided by the gp51 protein, partially compensate the surface charge of TiO2 and reduce the energy of electrons localized at the surface levels, which are the most responsible for the generation of PL-signal.The charge–charge-based interaction in the double charged layers gp51/TiO2/glass can also be interpreted as a model based on ‘imaginary capacitor’, formed as a result of the electrostatic interaction between oppositely charged protein gp51 layer and the TiO2/glass surface. The positive charges of protein gp51, attracted to the negatively charged surface, form the positive ‘imaginary capacitor plate’ in the close proximity to gp51/TiO2/glass interface. The interaction of gp51/TiO2/glass with anti-gp51 antibodies and formation of gp51/anti-gp51-based immune complex leads to a deformation and reduction of charge in ‘the positive imaginary capacitor plate’, caused by redistribution and partial compensation of charges during the formation of the gp51/anti-gp51 immune complex. Debye-screening length, calculated for TiO2, is in the range of 15 nm, which shows that PL-centres within this distance can be electrostatically affected by adsorbed gp51 and anti-gp51/gp51 complex.
The highlighted origin of the changes in the photoluminescence spectra of TiO2 as a result of the formation of biosensitive layer and after its interaction with the analyte, bring us closer to an understanding of the interaction mechanism between TiO2 and proteins, that is the key in the solving of many issues related to an improvement of biosensor performance.
In our next work we are going to investigate more in detail some parts of here predicted interaction mechanism using other proteins adsorbed on the surface of TiO2/glass.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Ukrainian-Lithuanian Research project “Application of hybrid nanostructures which are based on TiO2 or ZnO and modified by biomolecules, in optoelectronic sensors” Lithuanian Research Council project No. P-LU-18-53.References
- A. Ramanaviciene and A. Ramanavicius, Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins, Biosens. Bioelectron., 2004, 20, 1076–1082 CrossRef CAS PubMed.
- B. Kurtinaitiene, D. Ambrozaite, V. Laurinavicius, A. Ramanaviciene and A. Ramanavicius, Amperometric immunosensor for diagnosis of BLV infection, Biosens. Bioelectron., 2008, 23, 1547–1554 CrossRef CAS PubMed.
- A. Tereshchenko, M. Bechelany, R. Viter, V. Khranovskyy, V. Smyntyna, N. Starodub and R. Yakimova, Optical biosensors based on ZnO nanostructures: advantages and perspectives, Sens. Actuators, B, 2016, 229, 664–677 CrossRef CAS.
- S. M. Gupta and M. Tripathi, A review of TiO2 nanoparticles, Chin. Sci. Bull., 2011, 56, 1639–1657 CrossRef CAS.
- Z. Bian, J. Zhu, Y. Huo, H. Li and Li Y. Lu, Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity Hexing, J. Am. Chem. Soc., 2007, 129(15), 4538–4539 CrossRef PubMed.
- H. Xu, P. Reunchan, S. Ouyang, H. Tong, N. Umezawa, T. Kako and J. Ye, Anatase TiO2 Single Crystals Exposed with High-Reactive {111} Facets Toward Efficient H2 Evolution, Chem. Mater., 2013, 25(3), 405–411 CrossRef CAS.
- J. Liu, D. Olds, R. Peng, L. Yu, G. S. Foo, Sh. Qian, J. Keum, B. S. Guiton, Z. Wu and K. Page, Quantitative Analysis of the Morphology of {101} and {001} Faceted Anatase TiO2 Nanocrystals and Its Implication on Photocatalytic Activity, Chem. Mater., 2017, 29(13), 5591–5604 CrossRef CAS.
- Y. Zhou, H. Wang, Y. Zhuo, Y. Chai and R. Yuan, Highly Efficient Electrochemiluminescent Silver Nanoclusters/Titanium Oxide Nanomaterials as a Signal Probe for Ferrocene-Driven Light Switch Bioanalysis, Anal. Chem., 2017, 89(6), 3732–3738 CrossRef CAS PubMed.
- Y. Li, S. Zhanga, H. Dai, Z. Hong and Y. Lin, An enzyme-free photoelectrochemical sensing of concanavalin A based on graphene-supported TiO2 mesocrystal, Sens. Actuators, B, 2016, 232, 226–233 CrossRef CAS.
- J. Qiu, S. Zhang and H. Zhao, Recent applications of TiO2 nanomaterials in chemical sensing in aqueous media, Sens. Actuators, B, 2011, 160(1), 875–890 CrossRef CAS.
- Y.-M. Chu, C.-C. Lin, H.-C. Chang, C. Li and C. Guo, TiO2, nanowire FET device: Encapsulation of biomolecules by electro polymerized pyrrole propylic acid, Biosens. Bioelectron., 2011, 26, 2334–2340 CrossRef CAS PubMed.
- K.-S. Mun, S. D. Alvarez, W.-Y. Choi and M. J. Sailor, A Stable, Label-free Optical Interferometric Biosensor Based on TiO2 Nanotube Arrays, ACS Nano, 2010, 4, 2070–2076 CrossRef CAS PubMed.
- S. Rajendran, D. Manoj, K. Raju, D. D. Dionysiou, M. Naushad, F. Gracia, L. Cornejo, M. A. Gracia-Pinilla and T. Ahamad, Influence of mesoporous defect induced mixed-valent NiO (Ni2+/Ni3+)-TiO2 nanocomposite for non-enzymatic glucose biosensors, Sens. Actuators, B, 2018, 264, 27–37 CrossRef CAS.
- T. Zheng, E. Feng, Z. Wang, X. Gong and Y. Tian, Mechanism of Surface-Enhanced Raman Scattering Based on 3D Graphene–TiO2 Nanocomposites and Application to Real-Time Monitoring of Telomerase Activity in Differentiation of Stem Cells, ACS Appl. Mater. Interfaces, 2017, 9(42), 36596–36605 CrossRef CAS PubMed.
- A. Tereshchenko, V. Fedorenko, V. Smyntyna, I. Konup, A. Konup, M. Eriksson, R. Yakimova, A. Ramanavicius, S. Balme and M. Bechelany, ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of Grapevine virus A-type proteins, Biosens. Bioelectron., 2017, 92, 763–769 CrossRef CAS PubMed.
- R. Plugaru, A. Cremades and J. Piqueras, The effect of annealing in different atmospheres on the luminescence of polycrystalline TiO2, J. Phys.: Condens. Matter, 2004, 16, S261–S268 CrossRef CAS PII: S0953-8984(04) 67008-1.
- J. Preclíková, P. Galář, F. Trojánek, S. Daniš, B. Rezek, I. Gregora, Y. Němcová and P. Malý, Nanocrystalline titanium dioxide films: Influence of ambient conditions on surface- and volume-related photoluminescence, J. Appl. Phys., 2010, 108, 113502 CrossRef.
- R. Viter, A. Tereshchenko, V. Smyntyna, N. Starodub, R. Yakimova, V. Khranovskyy and A. Ramanavicius, Toward development of optical biosensors based on photoluminescence of TiO2 nanoparticles for the detection of Salmonella, Sens. Actuators, B, 2017, 252, 95–102 CrossRef CAS.
- R. Viter, V. Smyntyna, N. Starodub, A. Tereshchenko, A. Kusevitch, I. Doycho, S. Geveluk, N. Slishik, J. Buk, J. Duchoslav, J. Lubchuk, I. Konup, A. Ubelis and J. Spigulis, Novel Immune TiO2 Photoluminescence Biosensors for Leucosis Detection, Procedia Eng., 2012, 47, 338–341 CrossRef CAS.
- R. Viter, N. Starodub, V. Smyntyna, A. Tereschenko, A. Kusevitch, J. Sitnik, J. Buk and J. Macak, Immune biosensor based on Silica Nanotube Hydrogels for rapid Biochemical Diagnostics of Bovine Retroviral Leukemia, Procedia Eng., 2011, 25, 948–951 CrossRef CAS.
- D. L. Nelson, M. M. Cox and A. L. Lehninger, Principles of Biochemistry, Worth Publishtrs Inc., New York, 2000 Search PubMed.
- V. Shewale, P. Joshi, S. Mukhopadhyay, M. Deshpande, R. Pandey, S. Hussain and S. Karna, First-principles study of nanoparticle-biomolecular interactions: anchoring of a (ZnO)12 cluster on nucleobases, J. Phys. Chem., 2011, 115, 10426–10430 CAS.
- M. C. Wu, A. Sapi, A. Avila, M. Szabó, J. Hiltunen, M. Huuhtanen, G. Tóth, Á. Kukovecz, Z. Kónya, R. Keiski, W.-F. Su, H. Jantunen and K. Kordás, Enhanced photocatalytic activity of TiO2 nanofibers and their flexible composite films: decomposition of organic dyes and efficient H2 generation from ethanol–water mixtures, Nano Res., 2011, 4(4), 360–369 CrossRef CAS.
- V. Smyntyna Electron and Molecular Phenomena on the Surface of Semiconductors, Nova Publishers, New York, 2013, p. 208 Search PubMed.
- S. G. Ansari, R. Wahab, Z. A. Ansari, Y. S. Kim, G. Khang, A. Al-Hajry and H. S. Shin, Effect of nanostructure on the urea sensing properties of sol–gel synthesized ZnO, Sens. Actuators, B, 2009, 137, 566–573 CrossRef CAS.
- G. Obal, F. Trajtenberg, F. Carrión, L. Tomé, N. Larrieux, X. Zhang, O. Pritsch and A. Buschiazzo, Conformational plasticity of a native retroviral capsid revealed by X-ray crystallography, Science, 2015, 5182, 1–7 Search PubMed.
- Z. Balevicius, I. Baleviciute, S. Tumenas, L. Tamosaitis, A. Stirke, A. Makaraviciute, A. Ramanaviciene and A. Ramanavicius, In situ study of ligand-receptor interaction by total internal reflection ellipsometry, Thin Solid Films, 2014, 571, 744–748 CrossRef CAS.
- I. Baleviciute, Z. Balevicius, A. Makaraviciute, A. Ramanaviciene and A. Ramanavicius, Study of Antibody/Antigen Binding Kinetics by Total Internal Reflection Ellipsometry, Biosens. Bioelectron., 2013, 39, 170–176 CrossRef CAS PubMed.
- Z. Balevicius, A. Makaraviciute, G. J. Babonas, S. Tumenas, V. Bukauskas, A. Ramanaviciene and A. Ramanavicius, Study of optical anisotropy in thin molecular layers by total internal reflection ellipsometry, Sens. Actuators, B, 2013, 181, 119–124 CrossRef CAS.
- T. Ogawa Biochemistry, Genetics and Molecular Biology: ‘Protein Engineering - Technology and Application’, 2013, ISBN 978-953-51-1138-2, DOI:10.5772/56265.
- M. C. Fravventura, L. D. A. Siebbeles and T. J. Savenije, Mechanisms of Photogeneration and Relaxation of Excitons and Mobile Carriers in Anatase TiO2, J. Phys. Chem. C, 2014, 118, 7337–7343 CrossRef CAS.
- I. Sildos, A. Suisalu, V. Kiisk, M. Schuisky, H. Mändar, T. Uustare and J. Aarik, Effect of Structure Development on Self-Trapped Exciton Emission of TiO2 Thin Films, Proc. SPIE, 2000, 4086, 427–430 CrossRef CAS.
- K. Wakabayashi, Y. Yamaguchi, T. Sekiya and S. Kurita, Time-resolved luminescence spectra in colorless anatase TiO2 single crystal, J. Lumin., 2005, 112, 50–53 CrossRef CAS.
- A. Ramanavicius, A. Finkelsteinas, H. Cesiulis and A. Ramanaviciene, Electrochemical impedance spectroscopy of polypyrrole based electrochemical immunosensor, Bioelectrochemistry, 2010, 7, 11–16 CrossRef PubMed.
- H. Tang, K. Prasad and R. Sanjinbs, Electrical and optical properties of TiO2 anatase thin films, J. Appl. Phys., 1994, 75(4), 2042–204 CrossRef CAS.
- Z. Li, Y. Chen, X. Li, T. I. Kamins, K. Nauka and R. S. Williams, Encoding Information in DNA: From Basic Structure to Nanoelectronics, Nano Lett., 2004, 4, 245–247 CrossRef CAS.
- E. Stern, R. Wagner, F. J. Sigworth, R. Breaker, T. M. Fahmy and M. A. Reed, Importance of the Debye Screening Length on Nanowire Field Effect Transistor Sensors, Nano Lett., 2007, 7(17), 3405–3409 CrossRef CAS PubMed.
- G. Zheng, F. Patolsky, Y. Cui, W. U. Wang and C. M. Lieber, Multiplexed electrical detection of cancer markers with nanowire sensor arrays, Nat. Biotechnol., 2005, 23, 1294–1301 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |