Open Access Article
Open Access ArticleCholesterol-sensing role of phenylalanine in the interaction of human islet amyloid polypeptide with lipid bilayers†
Ruijie Hao,
Yang Li,
Liping Guan,
Tong Lu,
Feihong Meng,
Chunyu Wang and
Fei Li *
*
State Key Laboratory of Supramolecular Structure and Materials, Jilin University, 2699 Qianjin Avenue, Changchun 130012, P. R. China. E-mail: feili@jlu.edu.cn
First published on 5th December 2018
Abstract
The interactions between hIAPP and the pancreatic β-cells are associated with β-cell death in type II diabetes. Cholesterol modulates hIAPP-membrane interaction and hIAPP aggregation. The molecular mechanism underlying this is not well understood. Here we explore the cholesterol-sensing role of F15 in the interactions of hIAPP and hIAPP1–19 with various compositions of lipids, including DOPC, DPPC and DOPC/DPPC using NMR, CD, ThT fluorescence and dye leakage assays. We show that both hIAPP and hIAPP1–19 are more potent in the disruption to the membranes with cholesterol than they are in the disruption to the membranes without cholesterol. A substitution of F15 by leucine affects the binding and disruption of the peptides to the membranes slightly in the absence of cholesterol, but decreases the activities largely in the presence of cholesterol. F15 also plays a role in accelerating fibrillar assembly of hIAPP, but the function is independent of cholesterol in nature. The promotion of cholesterol to the disruptive potency of hIAPP is more effective in the membrane with raft-like domains than in the membrane with a dispersed distribution of cholesterol. Our results suggest that F15 plays a key role in the cholesterol-sensing binding and disruption of hIAPP to the PC membranes and the distribution of cholesterol in the membranes has an influence on the disruptive activity of hIAPP.
Introduction
Human islet amyloid polypeptide (hIAPP), or amylin, is a 37 amino-acid residue peptide hormone co-secreted with insulin from β-cells of the pancreatic islets. The amyloid deposit of hIAPP in the β-cells causes mass loss and dysfunction of the β-cells, a symptom generally observed in type II diabetes (T2D).1,2 A close link between the occurrence of hIAPP amyloid and the concomitant β-cell death implies that the interaction between hIAPP and cellular membranes could be a significant factor in the pathogenic mechanism.Cholesterol (Chol) is a key component for phase behavior of cellular membranes and participates in the formation of micro-domains or lipid rafts in membranes. Such micro-domains seem to be involved in a range of biological processes, from cytoskeleton organization, apoptosis, cell adhesion, intracellular flow of proteins, uptake of pathogens (viruses, parasites, bacteria and their toxins) to signal transduction.3 Several lines of evidence have shown a link between Chol and neurodegenerative diseases.4,5 Chol regulates amyloid-β processing and promotes amyloidogenesis in the brain. An abnormal rise in Chol is a risk factor of Alzheimer's disease6 and Parkinson's disease.7 There is also evidence that Chol is involved in the lipotoxicity of islets underlying the β-cell dysfunction in T2D.8 A recent study demonstrates that an elevation in islet Chol promotes IAPP aggregation and islet amyloid formation in mice, worsening β-cell function and glucose homeostasis.9 Studies on model membranes show that the effects of Chol on the interactions between IAPP and membranes and on the amyloidogenesis of IAPP change with the property of membrane.10,11
Quite a number of studies have shown that the residues in the N-terminal region of hIAPP play a key role in the interaction of hIAPP with membranes12,13 and are involved in the early stages of oligomerization of the peptide.14 Therefore, extensive studies have focused on hIAPP1–19, a fragment of hIAPP including the 1–19 amino-acid residues. Previous studies have reported that hIAPP1–19 is damaging to model membranes composed of pure anionic lipids or mixtures of anionic lipids with zwitterionic lipids, although the peptide is weakly fibrillogenic both in solution and in membrane environment.12,13,15 Our recent study demonstrated that the interaction of hIAPP1–19 with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) bilayer is promoted by Chol and the residue phenylalanine at position 15 (F15) plays an important role in the Chol-promoting peptide-membrane interaction.16 However, whether the Chol-sensing role of F15 establishes for the full length hIAPP peptide is a question to be elucidated. If it establishes indeed, then, how the distribution of Chol in membrane affects the Chol-sensing role of F15 in the interaction between hIAPP and membrane also needs to be clarified. To find out the answers of these questions, we built up the model membranes using DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), DPPC and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of DOPC and DPPC and modified the full length hIAPP and hIAPP1–19 by F15L substitution in this work.
2 mixture of DOPC and DPPC and modified the full length hIAPP and hIAPP1–19 by F15L substitution in this work.
Both DOPC and DPPC comprise the zwitterionic headgroups, but they are different in the property of the hydrophobic tails. DPPC has two saturated fatty acyl chains, which makes the lipid capable of packing in ordered array in artificial lipid layers and easier to interact with Chol by van der Waals force.17 The mixture of DPPC with even relatively small amount of Chol, e.g., 5 mol%, can result in phase separation, or the coexistence of the liquid-disordered (ld) domain enriched in phospholipids and the liquid-ordered (lo) domain enriched in Chol at a higher temperature or the coexistence of ripple phase (Pβ) and lo at a lower temperature.18–20 On the contrary, DOPC contains two cis-unsaturated fatty acyl chains. Chol increases vertical order and compactness of DOPC bilayer.21–23 Phase separation can form only when the molar ratio of Chol/DOPC is increased to 0.75.24 The hybrid membrane built up by DPPC, DOPC and Chol is used frequently as a model membrane in the study of the interaction of amyloid peptide with phospholipid membrane.11,25 The ternary mixture is divided into ordered and disordered area, and the size of the ordered region depends on the fraction of lo.26
By building up different compositions of lipid vesicles and modifying the hIAPP peptides with F15L substitution, we examined the binding and disruption of the hIAPP peptides (hIAPP, hIAPPF15L, hIAPP1–19 and hIAPP1–19/F15L) to the PC membranes in the absence and presence of Chol using various spectroscopic methods. We also monitored the fibrillar assemblies of these peptides in bulk solution and at the PC membranes. Our results demonstrated that not only the N-terminal segment hIAPP1–19 but also the full length hIAPP are sensitive to Chol in the interactions with the membranes composed of DOPC, DPPC and DOPC/DPPC, and F15 plays a significant role therein. F15 also plays a significant role in accelerating fibrillar assembly of hIAPP, but the activity is independent of Chol in nature. The disruptive potency of hIAPP is promoted by Chol more efficiently for the membrane with raft-like domains than the membrane with a dispersed distribution of Chol.
Results
Membrane damage by hIAPP peptides
The disruptive potencies of the hIAPP peptides to the PC membranes were probed firstly by the leakage assays of the calcein encapsulated LUVs. The addition of either hIAPP or hIAPPF15L in the LUV suspended solutions led to an increase in dye release with time. However, the dye leakages induced by hIAPP showed a more rapid increase in the presence of Chol than that in the absence of Chol, whereas the dye leakages induced by hIAPPF15L were not considerably different in the absence and presence of Chol in the entire period of incubation (Fig. 1, panels (A–C)). This indicates that F15 plays a key role in the Chol-sensing disruption of hIAPP to the PC membranes. Furthermore, the results in Fig. 1A–C showed that the differences between the maximal leakages of the Chol-containing and Chol-free LUVs induced by hIAPP are varied with the compositions of lipids from ca. 15–17% for DPPC and DOPC/DPPC systems to ca. 11% for DOPC systems. This may be correlated with the distributions of Chol in these membranes.Compared with the full length hIAPP, hIAPP1–19 was much less potent in disrupting the membranes. The leakage percentages reached the plateau in much longer time (several days), even though the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio used in the hIAPP1–19/LUV systems (1
L ratio used in the hIAPP1–19/LUV systems (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) was much higher than that used in the hIAPP/LUV systems (1
20) was much higher than that used in the hIAPP/LUV systems (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Nevertheless, the leaking results of hIAPP1–19/LUV systems (Fig. 1, panels (D–F)) were coincident qualitatively with those of hIAPP/LUV systems. The dye release induced by hIAPP1–19 increased with the addition of Chol, while the dye release induced by hIAPP1–19/F15L was less (for DPPC system) or not (for other two lipid systems) affected by the presence of Chol. Moreover, an observable difference between the leakages of the Chol-containing and Chol-free LUVs appeared earlier for DPPC and DOPC/DPPC systems (at the 1st and 2nd day of incubation) than DOPC systems (at the 4th day of incubation).
200). Nevertheless, the leaking results of hIAPP1–19/LUV systems (Fig. 1, panels (D–F)) were coincident qualitatively with those of hIAPP/LUV systems. The dye release induced by hIAPP1–19 increased with the addition of Chol, while the dye release induced by hIAPP1–19/F15L was less (for DPPC system) or not (for other two lipid systems) affected by the presence of Chol. Moreover, an observable difference between the leakages of the Chol-containing and Chol-free LUVs appeared earlier for DPPC and DOPC/DPPC systems (at the 1st and 2nd day of incubation) than DOPC systems (at the 4th day of incubation).
Assemblies of hIAPP peptides at the membranes
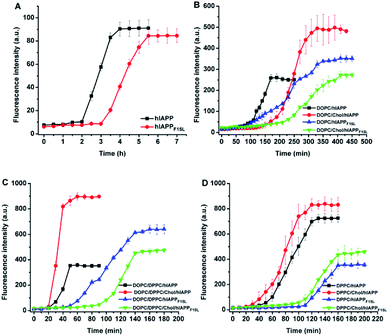
The assemblies of hIAPP and hIAPPF15L in solution and at the membranes were monitored by ThT fluorescence assays (Fig. 2). A 25 mM phosphate buffer solution with 50 mM NaCl was used in the ThT fluorescence assays, replacing a 10 mM Tris–HCl buffer solution with 100 mM NaCl used in leaking assays. The changes in buffer solution and salt concentration have no effect on ThT fluorescence results (Fig. S1†). Compared with the results of ThT assays of the peptides obtained in the absence of membrane, the intensity of the ThT fluorescence increased dramatically in the presence of the PC membranes. The lag time in the fibrillation kinetics of the peptides was also shortened by the compositions of lipids except for DOPC/20% Chol which displayed little effect. This indicates that the fibrillar assemblies of both peptides were promoted by the membranes. However, whatever impact the environments exerted, the fibrillar assembly of hIAPPF15L was always slower (with longer lag time) than that of hIAPP (with shorter lag time) both in solution and membrane environments. This suggests that the decrease in the fibrillation rate of F15L variant relative to that of the wild type peptide arises from the decrease in peptide–peptide interaction in nature, even though the extent of the decrease may vary with environment. In addition, it was noted that the fibrillation of hIAPP is either inhibited by Chol (e.g., at DOPC membrane, Fig. 2B)27 or promoted by Chol (e.g., at DOPC/DPPC membrane, Fig. 2C),11 depending on the composition of membrane, whereas the disruptive potency of hIAPP was always enhanced by Chol in all these membrane systems, as observed in leakage assays. This indicates that the regulation of Chol to the oligomerization/fibrillation of hIAPP is not a direct cause leading to the increase in the disruptive potency of the peptide.The structural transitions from initial state (random coil or α-helix dominant structure) to β-sheet state were observed in the CD spectra of the two peptides after certain period of incubation either in the absence and in the presence of lipid membranes (Fig. 3, also see Fig. S2, S3, Tables S1 and S2 in ESI†). Upon mixture with DOPC LUVs, both hIAPP and hIAPPF15L showed slower structural transition to β-sheet in the presence of Chol than in the absence of Chol. Slower structural transition in the presence of Chol than in the absence of Chol was also observed for hIAPPF15L mixed with DOPC/DPPC LUVs, while the structural transition was slightly accelerated by Chol for hIAPP incorporated with DOPC/DPPC LUVs. Either hIAPP or hIAPPF15L, when mixed with DPPC LUVs in the absence and presence of Chol, underwent the structural transition at a similar rate. Moreover, regardless of the effects of Chol, the structural transitions of hIAPP were faster than those of hIAPPF15L for all these compositions of lipids. The effects of both Chol and F15L mutation on the rates of β-sheet formation observed in the CD spectra were basically consistent with those on the rates of fibrillar assemblies observed in ThT fluorescence assays.
It was noted that the differences between the CD spectra recorded in the absence and presence of Chol are more evident for hIAPP than those recorded for hIAPPF15L for all these compositions of lipids (Fig. 3). The secondary structure data showed that the initial structures of the wild type peptide formed in the absence of Chol are largely different from those formed in the presence of Chol, while the initial structures of the F15L variant formed in the absence and presence of Chol are similar (Table S2 in ESI†). Considering the results of the leaking assays that the dye releases induced by the wild type peptide are largely different in the absence of Chol from those in presence of Chol, while the dye releases induced by the variant are very similar in the absence and presence of Chol, we infer that the initial assemblies of the peptides formed at various compositions of membrane may play a pivotal role in the membrane disruption.
We also monitored the morphologies of the oligomers and fibrils of the two peptides formed in phosphate buffer at pH 7.4 by TEM (Fig. S4 in ESI†). The oligomers of hIAPP and hIAPPF15L observed after 30 min incubation were globular and amorphous, respectively. After 5 h incubation, the fibrils alone were observed in the TEM image of hIAPP, while the coexistence of fibrils and a large amount of amorphous aggregates was observed in the TEM image of hIAPPF15L.
Binding of hIAPP peptides to the PC membranes
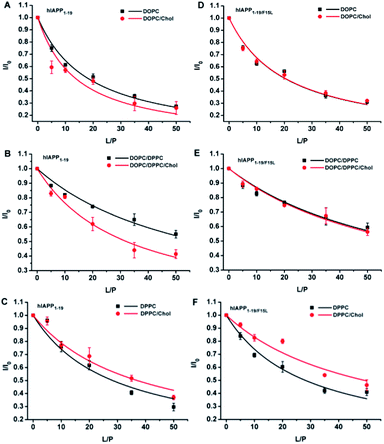
As hIAPP1–19 has a very weak fibrogenic preference and is similar to the full-length peptide in membrane disruption behavior, we determined the binding affinities of hIAPP1–19 and hIAPP1–19/F15L for the PC membranes using 1H-NMR measurements. Rapid structural transition and aggregation of the full-length peptide at these types of membranes, especially at the membrane of DOPC/DPPC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, limit the application of the method. The 1H-NMR spectra of the peptides incorporated with LUVs were recorded at a series of L
2, limit the application of the method. The 1H-NMR spectra of the peptides incorporated with LUVs were recorded at a series of L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios (Fig. 4, S5–S8 in ESI†) and the intensities of the 1H-NMR signals in the selected regions were measured at various L
P ratios (Fig. 4, S5–S8 in ESI†) and the intensities of the 1H-NMR signals in the selected regions were measured at various L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. The dissociation constants (Kd) of the peptides binding to the membranes with and without Chol were obtained by fitting the data according to eqn (1) (Fig. 5 and Table 1).
P. The dissociation constants (Kd) of the peptides binding to the membranes with and without Chol were obtained by fitting the data according to eqn (1) (Fig. 5 and Table 1).
| Lipid | Kda (mM) | |
|---|---|---|
| hIAPP1–19 | hIAPP1–19/F15L | |
| a The data were obtained from the signals in the regions of 7.20–7.40 ppm (aromatic protons of F15), 6.95–7.03 ppm (H18-Hδ) and 3.96–4.03 ppm (V17-Hα) for hIAPP1–19; 6.95–7.03 ppm (H18-Hδ), 3.96–4.03 ppm (V17-Hα) and 2.97–3.02 ppm (K1-Hε) for hIAPP1–19/F15L, see Fig. 4 and S5–S8 in ESI. | ||
| DOPC | 5.34 ± 0.33 | 6.00 ± 0.54 |
| DOPC/20% Chol | 3.98 ± 0.65 | 6.04 ± 0.44 |
DOPC/DPPC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
17.41 ± 1.10 | 19.84 ± 1.56 |
DOPC/DPPC/Chol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.49 ± 0.66 | 19.12 ± 1.00 |
| DPPC | 8.33 ± 1.09 | 8.28 ± 0.60 |
| DPPC/20% Chol | 10.96 ± 1.09 | 14.59 ± 1.60 |
The Kd value of hIAPP1–19 binding with DOPC/20% Chol LUVs was smaller than that of the peptide binding with bare DOPC LUVs, while the Kd values of hIAPP1–19/F15L were almost the same for the two compositions of lipids. Similar results were also obtained for the two peptides binding with DOPC/DPPC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and DOPC/DPPC/Chol 1
2 and DOPC/DPPC/Chol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LUVs. In contrast, the Kd values of both hIAPP1–19 and hIAPP1–19/F15L for DPPC LUVs were larger in the presence of 20% Chol than those in the absence of Chol. These results indicate that the presence of Chol favors the binding of hIAPP1–19 to DOPC-containing LUVs, but disfavors the binding to DPPC LUVs. Nevertheless, the comparison between the Kd values of the two peptides in the same composition of lipids revealed that the binding affinities of hIAPP1–19 are similar to those of hIAPP1–19/F15L in the absence of Chol, but higher (with smaller Kd) than those of hIAPP1–19/F15L in the presence of Chol. This indicates that the substitution of F15L decreases the binding affinity of peptide for the membranes in the presence of Chol, but has little effect on the binding affinity in the absence of Chol. Obviously, F15 plays an important role in the Chol-sensing binding of hIAPP1–19 to the membranes. The interaction of hIAPP1–19 with Chol may be mediated by F15. A specific interaction of hIAPP1–19 with Chol in DPPC could partially balance the disadvantage in binding. This could explain why the presence of Chol in DPPC LUVs induces a smaller increase in Kd of hIAPP1–19 than that of hIAPP1–19/F15L relative to the Kd values obtained in the absence of Chol.
1 LUVs. In contrast, the Kd values of both hIAPP1–19 and hIAPP1–19/F15L for DPPC LUVs were larger in the presence of 20% Chol than those in the absence of Chol. These results indicate that the presence of Chol favors the binding of hIAPP1–19 to DOPC-containing LUVs, but disfavors the binding to DPPC LUVs. Nevertheless, the comparison between the Kd values of the two peptides in the same composition of lipids revealed that the binding affinities of hIAPP1–19 are similar to those of hIAPP1–19/F15L in the absence of Chol, but higher (with smaller Kd) than those of hIAPP1–19/F15L in the presence of Chol. This indicates that the substitution of F15L decreases the binding affinity of peptide for the membranes in the presence of Chol, but has little effect on the binding affinity in the absence of Chol. Obviously, F15 plays an important role in the Chol-sensing binding of hIAPP1–19 to the membranes. The interaction of hIAPP1–19 with Chol may be mediated by F15. A specific interaction of hIAPP1–19 with Chol in DPPC could partially balance the disadvantage in binding. This could explain why the presence of Chol in DPPC LUVs induces a smaller increase in Kd of hIAPP1–19 than that of hIAPP1–19/F15L relative to the Kd values obtained in the absence of Chol.
With the increase in L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, only the decrease in intensity was observed, while the change in chemical shift was not observed for all signals in 1H-NMR spectra. This indicates that the binding of the peptides with these PC membranes is very weak. This may be attributed to the lack of anionic component needed for a stronger electrostatic interaction between peptide and membrane. The Kd data obtained by 1
P, only the decrease in intensity was observed, while the change in chemical shift was not observed for all signals in 1H-NMR spectra. This indicates that the binding of the peptides with these PC membranes is very weak. This may be attributed to the lack of anionic component needed for a stronger electrostatic interaction between peptide and membrane. The Kd data obtained by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode are in the mM order of magnitude, also showing a very weak binding. This means that the estimation of Kd by the simple binding mode is reasonable for the peptide-lipid systems used in this study, even though the method, to a large extent, is an approximation for the complex peptide-membrane systems.
1 binding mode are in the mM order of magnitude, also showing a very weak binding. This means that the estimation of Kd by the simple binding mode is reasonable for the peptide-lipid systems used in this study, even though the method, to a large extent, is an approximation for the complex peptide-membrane systems.
Discussion
The results above demonstrate that F15 is significant for hIAPP in the fibril formation, membrane binding, and membrane disruption. It is interesting that the promotion role of F15 to the fibril formation of hIAPP is effective at the membranes both with and without Chol, while the promotion roles of F15 to the membrane binding and membrane disruption are effective only at the membranes with Chol. This suggests that the promotion role of F15 in fibrillar assembly is independent of Chol in nature, while the promotion roles of F15 in membrane binding and membrane damage may be associated with specific interaction of the peptide with Chol.It is noted that the hIAPP-induced dye release occurs earlier than the fibril formation or even the structural transition to β-sheet for all these peptide/LUV systems. This confirms that the oligomers instead of fibrils are the disruptive species of the peptides. In addition, the disruptive potency of hIAPP is always enhanced by Chol for all the lipid systems, while the fibrillation of the peptide is either accelerated or restricted by Chol, depending on the composition of lipids. This implies that the oligomeric species formed off the fibrillation pathway may be responsible for the disruptive activity of hIAPP at these PC membranes. The interfering of F15L mutation to the peptide oligomerization/fibrillation could not be a direct cause leading to the decrease in the Chol-sensing activity in membrane disruption. Otherwise, the difference between the leaking results of the two peptides should also be observed for the membranes without Chol. This is not true obviously.
The model membranes used in this study contain different fractions of saturated and unsaturated components, which leads to different distributions of Chol in the membranes. Chol is prone to clustering to form raft-like domains in the lipid membranes with saturated acyl chains, e.g., DPPC/20% Chol and DOPC/DPPC/Chol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 membrane. Therefore, a phase separation of the micro-area enriched in Chol from that enriched in lipids occurs. A curvature strain resulting from different membrane thicknesses between the Chol-rich and Chol-poor domains may exist, which renders the domain boundaries more vulnerable to membrane disrupting agents.11,28 In contrast, Chol distributes in the membrane with unsaturated acyl chains, e.g., DOPC membrane, more dispersedly and no phase separation occurs at the concentration used in this study. The leaking results of both hIAPP and hIAPP1–19 reveal that DOPC/DPPC and DPPC LUVs are more effectively promoted by Chol in dye release than DOPC LUVs. This suggests that the disruptive potency of hIAPP/hIAPP1–19 is promoted by Chol more efficiently in the membranes with raft-like domains than in the membrane without the domains. The recognition of hIAPP/hIAPP1–19 to Chol mediated by F15 may play a significant role therein. The recognition of hIAPP/hIAPP1–19 to Chol in the raft-like domains could facilitate the aggregation of the peptides at the Chol-rich area or the boundary region of the domains. The interaction of hIAPP/hIAPP1–19 with aggregated Chol may also facilitate the formation of damaging oligomers more efficiently than the interaction with dispersedly distributed Chol in the membrane (Fig. 6).
1 membrane. Therefore, a phase separation of the micro-area enriched in Chol from that enriched in lipids occurs. A curvature strain resulting from different membrane thicknesses between the Chol-rich and Chol-poor domains may exist, which renders the domain boundaries more vulnerable to membrane disrupting agents.11,28 In contrast, Chol distributes in the membrane with unsaturated acyl chains, e.g., DOPC membrane, more dispersedly and no phase separation occurs at the concentration used in this study. The leaking results of both hIAPP and hIAPP1–19 reveal that DOPC/DPPC and DPPC LUVs are more effectively promoted by Chol in dye release than DOPC LUVs. This suggests that the disruptive potency of hIAPP/hIAPP1–19 is promoted by Chol more efficiently in the membranes with raft-like domains than in the membrane without the domains. The recognition of hIAPP/hIAPP1–19 to Chol mediated by F15 may play a significant role therein. The recognition of hIAPP/hIAPP1–19 to Chol in the raft-like domains could facilitate the aggregation of the peptides at the Chol-rich area or the boundary region of the domains. The interaction of hIAPP/hIAPP1–19 with aggregated Chol may also facilitate the formation of damaging oligomers more efficiently than the interaction with dispersedly distributed Chol in the membrane (Fig. 6).
 | ||
| Fig. 6 Schematic mode of mechanism proposed for the interactions between hIAPP/hIAPPF15L and the PC membranes in the absence and presence of Chol. | ||
The Kd data show that hIAPP1–19 binds to the membranes with a higher affinity than hIAPP1–19/F15L does in the presence of Chol. A higher level of dye release induced by hIAPP1–19 than by hIAPP1–19/F15L for the membranes with Chol was also observed for these compositions of lipids. However, a stronger binding of hIAPP1–19 to DPPC LUVs (smaller Kd) than to DPPC/20% Chol (a larger Kd) is related to a less efficient disruption of the former than the latter by the peptide. This indicates that a positive correlation of disruptive efficiency with binding affinity only establishes for the peptide (hIAPP1–19 or hIAPP) at the membrane with the same composition of lipids, but is not necessarily true when the comparison is done between the membranes with different compositions of lipids.
Conclusions
The presence of Chol in the membranes composed of DOPC, DPPC and DOPC/DPPC enhances the susceptibility of the membranes to hIAPP/hIAPP1–19. The unfavorable effect of Chol on the stability of the membranes is mainly attributed to a prone interaction of the hIAPP peptides with Chol. The existence of raft-like domains enlargers the susceptibility of the membranes to the peptides, likely by increasing curvature strain of the membranes or promoting the formation of more damaging oligomers of the peptides. The aromatic residue phenylalanine at position 15 plays a pivotal role in the specific interaction of the peptides with Chol. Without the aromatic ring, hIAPP loses the Chol-sensing potency in membrane disruption partially or even totally whether the raft-like domains form or not.Experimental
Materials
The peptides KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY (hIAPP), KCNTATCATQRLANFLVHS (hIAPP1–19), and the F15L variants of the peptides (hIAPPF15L and hIAPP1–19/F15L) were synthesized by Shanghai Sci. Pep. Biol. Technol. Co., Ltd. (Shanghai, China). The cysteines at positions 2 and 7 were oxidized to form a disulfide bond and the C-termini were amidated in the peptides. The purity of the peptides was assessed by high performance liquid chromatography and mass spectroscopy, and the peptide power with a purity greater than 95% was obtained. Phospholipids DOPC and DPPC were obtained from Avanti Polar Lipid, Inc. (Alabaster, AL). Other chemical agents were purchased from Sigma-Aldrich (St. Louis, MO).Pre-treatment of peptide
The synthesized peptide was dissolved in 1,1,1,3,3,3-hexafluoro-2-isopropanol (HFIP) solution at a concentration of 1 mg mL−1 and sonicated in a water bath for 1 hour at a temperature of 30 °C to disrupt the pre-aggregated assembly. After sonication, the peptide was lyophilized overnight.Preparation of large unilamellar lipid vesicles (LUVs)
Certain quantities of phospholipids (DOPC, DPPC and Chol) were dissolved in chloroform/methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) co-solvent separately and mixed with desired ratios. The mixtures were evaporated by a stream of nitrogen, and then kept under vacuum overnight. The dried lipid film was hydrated either with phosphate buffer or with 1 mL Tris–HCl buffer containing 70 mM calcein (NaOH of 5 M was then added dropwise until the solution turned clear). After 1 h incubation at ∼30 °C (for DOPC and DOPC/Chol) or at ∼55 °C (for DPPC-containing lipids), the solution was freeze-thawed 15 times and extruded 10 cycles through polycarbonate filter of 0.1 μm. The calcein encapsulated LUVs were further dialyzed through a membrane with a cut-off of 1000 Da in buffer for three days to eliminate the non-encapsulated calcein.
1 v/v) co-solvent separately and mixed with desired ratios. The mixtures were evaporated by a stream of nitrogen, and then kept under vacuum overnight. The dried lipid film was hydrated either with phosphate buffer or with 1 mL Tris–HCl buffer containing 70 mM calcein (NaOH of 5 M was then added dropwise until the solution turned clear). After 1 h incubation at ∼30 °C (for DOPC and DOPC/Chol) or at ∼55 °C (for DPPC-containing lipids), the solution was freeze-thawed 15 times and extruded 10 cycles through polycarbonate filter of 0.1 μm. The calcein encapsulated LUVs were further dialyzed through a membrane with a cut-off of 1000 Da in buffer for three days to eliminate the non-encapsulated calcein.
NMR spectroscopy
1H-NMR spectra were performed on a Bruker Avance 600 spectrometer (Bruker Bio-Spin, Fällanden, Switzerland) at 25 °C. In the 1H-NMR experiments, the LUVs suspended in 25 mM phosphate buffer containing 50 mM NaCl and 10% D2O at pH 7.4 were used. The peptide concentration was fixed to 300 μM (for DOPC and DOPC/DPPC LUV systems) or 60 μM (for DPPC LUV systems), changing lipid concentrations to obtain the molar ratios of lipid-to-peptide (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) from 0 to 50. The spectra were collected with scans of 256 (for DOPC and DOPC/DPPC LUV systems) or 512 (for DPPC LUV systems) and a relaxation delay of 3 s. DSS (2,2-dimethyl-2-silapentane-5-sulfonate-d6) was used as an internal standard. The 1H-NMR intensities of peptide were measured at various L
P) from 0 to 50. The spectra were collected with scans of 256 (for DOPC and DOPC/DPPC LUV systems) or 512 (for DPPC LUV systems) and a relaxation delay of 3 s. DSS (2,2-dimethyl-2-silapentane-5-sulfonate-d6) was used as an internal standard. The 1H-NMR intensities of peptide were measured at various L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and the dissociation constants (Kd) of peptide binding to LUVs were estimated by eqn (1) derived from a simple bimolecular binding equilibrium:10,29
P and the dissociation constants (Kd) of peptide binding to LUVs were estimated by eqn (1) derived from a simple bimolecular binding equilibrium:10,29
 | (1) |
Circular dichroism (CD) spectroscopy
CD measurements were carried out using a PMS-450 spectropolarimeter (Biologic, France) at room temperature using a quartz cuvette with 0.5 mm path length under a constant flow of N2. Peptide was dissolved in 25 mM phosphate buffer at pH 7.4 and the CD spectra were recorded in the absence and presence of LUVs at room temperature. The concentration of peptide was 15 μM and the total concentration of lipids was 3 mM. The CD experiments were performed in a scan range from 190 nm to 260 nm at a scan rate of 1 nm in an interval of 10 s. Three independent experiments were collected and averaged. The blank spectra were subtracted. The contents of secondary structure components of peptide were calculated by CDPro software package using program CONTIN/LL.Membrane leakage assays
The membrane leakage assays were performed on a fluorescence spectrophotometer RF-5301 PC (Shimadzu, Japan) at room temperature using an excitation wavelength of 495 nm and an emission wavelength from 530 nm to 650 nm. After peptide was mixed with LUVs in 10 mM Tris–HCl buffer containing 100 mM NaCl at pH 7.4, fluorescence intensities of calcein released from LUVs were measured at different incubation time and analysed using eqn (2):30–32
 | (2) |
Thioflavin-T fluorescence assays
The Thioflavin-T fluorescence assays were carried out on a fluorescence spectrophotometer RF-5301 PC (Shimadzu, Japan) at room temperature using an excitation wavelength of 440 nm and an emission wavelength from 450 nm to 600 nm. A slit of 3 nm was used in the scanning process. Peptide was dissolved in 25 mM phosphate buffer (50 mM NaCl, pH 7.4) containing 20 μM ThT and the fluorescence assays were performed in the absence and presence of LUVs at room temperature. The concentration of peptide was 15 μM and the total concentration of lipids was 3 mM.Transmission electron microscopy (TEM)
A peptide solution of 15 μM was diluted to 3 μM after incubation for 30 min or 5 h. A 5 μL of the solution was applied on a 300-mesh Formvar/carbon coated copper grid. After waiting for 10 min, the sample was washed twice with 10 mL phosphate buffer. The sample was air-dried overnight. TEM images were observed under a transmission electron microscope (JEM-2100F, JEOL Co., Ltd, Japan) operating at an accelerating voltage of 200 kV.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 21673099).References
- A. Abedini and A. M. Schmidt, FEBS Lett., 2013, 587, 1119–1127 CrossRef CAS PubMed.
- F. M. Ashcroft and P. Rosman, Cell, 2012, 148, 1160–1171 CrossRef CAS PubMed.
- J. Fantini, N. Garmy, R. Mahfoud and N. Yahi, Expert Rev. Mol. Med., 2002, 4, 1–22 Search PubMed.
- J. P Liu, Y. Tang, S. Zhou, B. H. Toh, C. Mclean and H. Li, Mol. Cell. Neurosci., 2010, 43, 33–42 CrossRef PubMed.
- J. Fantini and N. Yahi, Expert Rev. Mol. Med., 2010, 12, e27 CrossRef PubMed.
- I. J. Martins, T. Berger, M. J. Sharman, G. Verdile, S. J. Fuller and R. N. Martins, J. Neurochem., 2009, 111, 1275–1308 CrossRef CAS.
- L. Mascitelli, F. Pezzetta and M. R. Goldstein, Neurology, 2008, 70, 1972–1979 CrossRef.
- L. R. Brunham, J. K. Kruit, M. R. Hayden and C. B. Verchere, Curr. Diabetes Rep., 2010, 10, 55–60 CrossRef CAS PubMed.
- N. Wijesekara, A. Kaur, C. Westwell-Roper, D. Nackiewicz, G. Soukhatcheva, M. R. Hayden and C. B. Verchere, Diabetologia, 2016, 59, 1242–1246 CrossRef CAS PubMed.
- L. Caillon, L. Duma, O. Lequin and L. Khemtemourian, Mol. Membr. Biol., 2014, 31, 239–249 CrossRef CAS PubMed.
- M. F. Sciacca, F. Lolicato, G. Di Mauro, D. Milardi, L. D'Urso, C. Satriano, A. Ramamoorthy and C. La Rosa, Biophys. J., 2016, 111, 140–151 CrossRef CAS PubMed.
- L. Khemtémourian, M. F. M. Engel, R. M. J. Liskamp, J. W. M. Höppener and J. A. Killian, Biochim. Biophys. Acta, 2010, 1798, 1805–1811 CrossRef PubMed.
- J. R. Brender, E. L. Lee, M. A. Cavitt, A. Gafni, D. G. Steel and A. Ramamoorthy, J. Am. Chem. Soc., 2008, 130, 6424–6429 CrossRef CAS PubMed.
- Y. Mazor, S. Gilead, I. Benhar and E. Gazit, J. Mol. Biol., 2002, 322, 1013–1024 CrossRef CAS.
- D. L. Heyl, J. M. Osborne, S. Pamarthy, S. Samisetti, A. W. Gray, A. Jayaprakash, S. Konda, D. J. Brown, S. R. Miller, R. Eizadkhah and M. C. Milletti, Int. J. Pept. Res. Ther., 2010, 16, 43–54 CrossRef CAS.
- Y. Li, L. Guan, T. Lu, H. Li, Z. Li and F. Li, RSC Adv., 2016, 6, 96837–96846 RSC.
- J. Fantini and F. J. Barrantes, Biochim. Biophys. Acta, 2009, 1788, 2345–2361 CrossRef CAS PubMed.
- T. P. W. Mcmullen, R. N. A. H. Lewis and R. N. Mcelhaney, Biochemistry, 1993, 32, 516–522 CrossRef CAS PubMed.
- T. P. W. Mcmullen and R. N. Mcelhaney, Biochim. Biophys. Acta, 1995, 1234, 90–98 CrossRef.
- M. Eeman and M. Deleu, Biotechnol., Agron., Soc. Environ., 2010, 14, 719–736 Search PubMed.
- E. Drolle, N. Kučerka, M. I. Hoopes, Y. Choi, J. Katsaras, M. Karttunen and Z. Leonenko, Biochim. Biophys. Acta, Biomembr., 2013, 1828, 2247–2254 CrossRef CAS PubMed.
- M. Alwarawrah, J. Dai and J. Huang, J. Phys. Chem. B, 2010, 114, 7516–7523 CrossRef CAS PubMed.
- M. Jurak, J. Phys. Chem. B, 2013, 117, 3496–3502 CrossRef CAS.
- M. Jurak and E. Chibowski, RSC Adv., 2015, 5, 66628–66635 RSC.
- K. Weise, D. Radovan, A. Gohlke, N. Opitz and R. Winter, ChemBioChem, 2010, 11, 1280–1290 CrossRef CAS.
- R. F. M. de Almeida, L. M. S. Loura, A. Fedorov and M. Prieto, J. Mol. Biol., 2005, 346, 1109–1120 CrossRef CAS.
- L. Caillon, O. Lequin and L. Khemtemourian, Biochim. Biophys. Acta, Biomembr., 2013, 1828, 2091–2098 CrossRef CAS PubMed.
- P. E. S Smith, J. R. Brender and A. Ramamoorthy, J. Am. Chem. Soc., 2009, 131, 4470–4478 CrossRef PubMed.
- C. M. Pfefferkorn and J. C. Lee, J. Phys. Chem. B, 2010, 114, 4615–4622 CrossRef CAS PubMed.
- D. Milardi, M. F. M. Sciacca, M. Pappalardo, D. M. Grasso and C. L. Rosa, Eur. Biophys. J., 2011, 40, 1–12 CrossRef CAS PubMed.
- M. F. M. Engel, L. Khemtémourian, C. C. Kleijer, H. J. D. Meeldijk, J. Jacobs, A. J. Verkleij, B. de Kruijff, J. A. Killian and J. W. M. Höppener, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6033–6038 CrossRef CAS PubMed.
- C. A. D. Carufel, N. Quittot, P. T. Nguyen and S. Bourgault, Angew. Chem., Int. Ed., 2015, 54, 14383–14387 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07310d |
| This journal is © The Royal Society of Chemistry 2018 |