Open Access Article
Open Access ArticleEffects of sample pretreatment and particle size on the determination of nitrogen in soil by portable LIBS and potential use on robotic-borne remote Martian and agricultural soil analysis systems
Xiu T. Yana,
Karen M. Donaldson *a,
Christine M. Davidsonb,
Yichun Gaoc,
Hanling Wuc,
Andrew M. Houston
*a,
Christine M. Davidsonb,
Yichun Gaoc,
Hanling Wuc,
Andrew M. Houston b and
Aron Kisdid
b and
Aron Kisdid
aSpace Mechatronic Systems Technology Laboratory, Department of Design, Manufacture and Engineering Management, University of Strathclyde, Glasgow, UK. E-mail: Karen.donaldson@strath.ac.uk
bWestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK. E-mail: c.m.davidson@strath.ac.uk
cChina Academy of Launch Vehicle Technology, Beijing, China
dRAL Space, Science and Technology Facilities Council's (STFC), Rutherford Appleton Laboratory (RAL), UK. E-mail: aron.kisdi@stfc.ac.uk
First published on 31st October 2018
Abstract
Field determination of nitrogen in soil is of interest for both terrestrial and Martian applications. Improved management of soil nitrogen levels on Earth could benefit global food production, whilst the determination of soil nitrogen on Mars is required to assess the planet's future habitability. In this study, a mobile laser induced breakdown spectroscopy (LIBS) system with a 1064 nm Nd:YAG laser delivering 25 mJ per pulse was used to assess the effects of sample pretreatment on the measurement of nitrogen in soil. Although pelletisation was preferred, simply milling the sample to <100 mm particle size – which may be more feasible on a remote rover-based analytical platform – improved the spectra obtained. Ablation craters formed in targets prepared from different particle size fractions of the same commercially-available topsoil showed a clear trend in morphology, with smaller particles yielding more uniform craters with fewer fractures. The LIBS emission intensity at 746.83 nm followed a similar trend to results obtained for total nitrogen content in the soil particle size fractions by microanalysis (Perkin Elmer CHN Elemental Analyser) and was well-correlated (R2 = 0.94) with soil nitrate determined by ion chromatography (Dionex DX-100). Although correlations were less good when analysing field soil samples collected from central Scotland (R2 = 0.82 for comparison between LIBS and microanalysis) the study nevertheless demonstrates the potential of portable LIBS for measurement of soil nitrogen content.
1. Introduction
Laser induced breakdown spectroscopy (LIBS) is a form of atomic emission spectroscopy growing in popularity for elemental analysis of soil, sediment and related materials.1 The sample surface is ablated by a focused laser beam, creating a plasma plume, and the elemental composition of the sample is determined from the wavelengths and intensities of the atomic emissions from the plasma.2–7 Advantages of using LIBS for elemental analysis are that results are available in real time and that measurements can be performed directly on a solid sample. It is also widely reported that LIBS can be performed without sample preparation. Whilst this is true for some types of sample, the analysis of loosely aggregated heterogeneous material such as soil is facilitated by at least some degree of sample manipulation before exposure to the laser, and it has been highlighted in the literature8 that further research is needed in this area.In addition to applications for the determination of potentially toxic elements, LIBS can allow direct observation of key nutrients in soil.9 One element of particular interest because of its role as an essential element for plant growth is nitrogen. The ability to determine nitrogen directly, in soil, in the field, is challenging.10 However, it would be of enormous benefit to agriculture as it would allow more targeted application of fertilizers11–14 and more effective management of crop nitrogen utilisation.15,16
The in situ measurement of nitrogen in soils is also of interest in space science.17 It has been suggested that N2 was abundant in the early Martian atmosphere but was then depleted by hydrodynamic escape. Nitrates that were produced in the early history of Mars by photochemistry may still be present: indeed, it is estimated that the Martian surface could contain soil nitrates at average levels of 0.3% N (w/w). One of the key goals of research being conducted on Mars is to determine whether the planet has the ability to sustain microbial life, with the hope of creating a soil-based sustainable agriculture system for habitability in future missions. Nitrates are a fundamental source for nitrogen for terrestrial microorganisms. Therefore, the detection of soil nitrates is important to assess habitability in the Martian environment.18–30
The detection of nitrogen in soil using laboratory-based LIBS instruments has been reported by several authors.14,31,32 However, for agricultural and space science applications, it will be necessary to determine – and ideally map – the soil nitrogen distribution in situ using a field-portable instrument.
This is challenging because, as mentioned above 8 the quality of LIBS results obtained is enormously influenced by the chemical and physical properties of the sample, which in turn depend on how the sample was pretreated prior to analysis.33–40 Various methods of soil sample preparation have been developed to achieve the homogeneity required for LIBS analysis, including mechanical pressure,41 grinding and sieving42 and a combination of these.39,43–45 However, these potentially may be difficult to replicate in a field setting, especially using an autonomous rover analytical platform.
The aim of the current study is to gain a better understanding of how factors such as sample preparation and particle size are likely to affect the quality of nitrogen determination in soil using a prototype portable LIBS system designed for use with the four-wheel mobile AgriRover platform.46 Results were compared with those obtained by well-established laboratory techniques: a CHN Elemental Analyser (for total soil N), and ion chromatography and colorimetry (for soil nitrate concentrations).
2. Experimental
2.1 Soil sampling and sample preparation
Five surface soil samples were collected from agricultural land in central Scotland, UK. The field-moist soil was oven-dried at 40 °C for two days and sieved to a particle size of 1 mm. Test portions of one of the samples were then subjected to different pretreatment regimes before analysis by LIBS:(a) no further treatment.
(b) milled to <100 μm particle size.
(c) milled then pelletised.
(d) milled then frozen.
(e) milled then pelletised then frozen.
Milling was performed using a planetary ball mill. Pelletisation was carried out using a manual laboratory press: 7 tonnes of pressure was applied to 2 cm3 soil samples for 30 seconds using a 1/2′′ die. No binders were added. To produce frozen material, soil was placed in a freezer at a temperature of approximately −18 °C for two days.
To investigate the effects of soil particle size, a commercially processed topsoil was purchased from B&Q, Glasgow, UK. The soil was dried; ground in the ball mill; then passed through a set of stainless steel sieves with mesh sizes 1000, 500, 250, 125, 90, 63, 53 and 45 μm. Material in each particle size fraction (<45, 45–53, 53–63, 63–90, 90–125, 125–250, 250–500 and 500–1000 μm) was pelletised using the apparatus and procedure described above.
2.2 LIBS instrumentation and measurement procedure
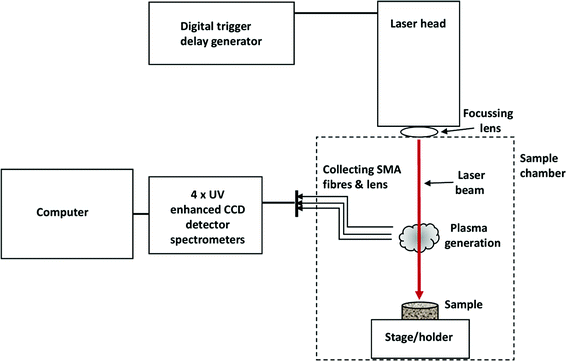
A mobile LIBS system, from StellarNet Inc, (Fig. 1) was used in a laboratory setting to measure the concentrations of nitrogen in soil samples. The system consisted of a laser, powered and controlled by a laser drive and control system, a soil sample holder, a sample chamber, and a spectrometer connected to a computer system for data collection and processing.A Nd:YAG laser operating at 1064 nm, with a beam diameter of 3 mm, pulse repetition rate of 1 Hz, nominal pulse width of 4 ns and energy of 25 mJ per pulse was used to sample the targeted soil. The laser pulse was guided and focused onto the soil surface by a focussing lens to form a high-temperature plasma for vaporization and atomization of the soil surface material. Plasma emissions were measured with fibre optic cables mounted at ∼45° to the soils surface and connected to four spectrometers, spanning the spectral range 400–800 nm. The spectrometers have an optical resolution of 4 cm−1 (equivalent to 1 nm with 25 μm slit). Background spectra were collected before the experiment and subtracted during data analysis.
Results obtained from LIBS can vary markedly across a single sample pellet.47 To obtain as representative a result as possible, the spectra obtained from 20 laser shots (five shots at each of four different locations across the target) were averaged. One to two preliminary shots per location were used to remove any dust adhering to the surface.
2.3 LIBS data analysis
The spectrum from the LIBS system contained complex information which described the elemental composition of the soil. A MATLAB script was written to remove the background spectrum and to highlight and identify peaks at wavelengths of interest.2.4 Investigation of ablation effects
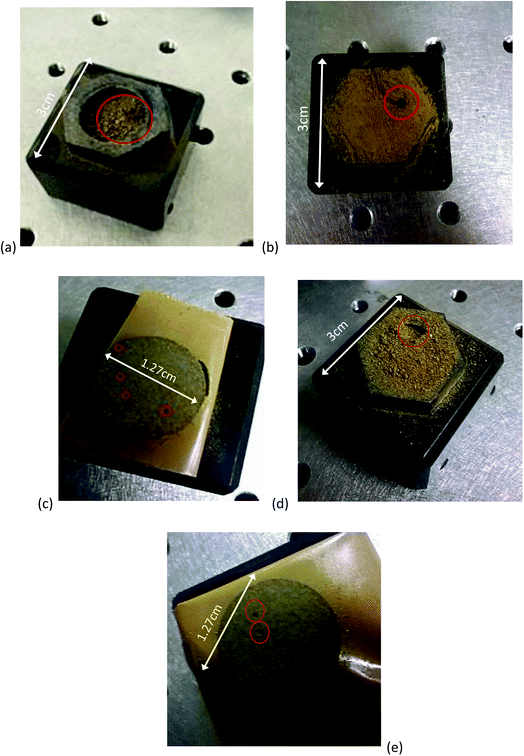
Soil (3 cm2) and pellets prepared from one of the agricultural soils following the different sample pretreatment procedures described in Section 2.1 were placed in a specimen holder and then positioned under the laser head. The ablation craters formed on samples subjected to different pretreatments were imaged and any differences noted. Pellets formed from different size fractions of the commercial topsoil were ablated and the ablation craters imaged using scanning electron microscopy (SEM). Samples were mounted using carbon tabs and coated with gold using a Polaron SC500A sputter coater, under argon. The scanning electron microscope used was a Stereoscan 90, from Cambridge instruments.2.5 Determination of soil nitrogen and nitrate
Total soil nitrogen content was determined using a Perkin Elmer 2400 CHN Elemental Analyser and manufacturer's recommended conditions. Samples (0.01 g) were analysed in duplicate.Soil nitrate determination was carried out by either ion chromatography (IC) or using a colorimetric method. For IC, 1 g test portions were shaken in 5 mL deionized water for 4 hours, filtered, and the filtrate collected for analysis. Extracts were analysed in triplicate (giving RSD values < 3.2%) using a Dionex DX-100 instrument with an AS22 column and 5.5 M sodium bicarbonate mobile phase. In the colorimetric method, 8 g test portions were stirred in 25 mL deionized water for 10 minutes, filtered, and 5 mL of filtrate reacted with a chromogenic reagent to give a pink colour. The nitrate content was determined at λmax = 530 nm using an S.I. Photonics Model 420 spectro-photometer.
3 Results and discussion
3.1 Effect of sample pre-treatment
The ablation craters formed in soil pretreated in different ways were notably different (Fig. 2). The crater formed in the soil that had only been dried and sieved was relatively large and poorly defined compared to that of the other pretreatments. The raw spectrum obtained was extremely noisy and it was observed that, on contact with the laser, substantial amounts of sample material were ejected leading to contamination of the sample chamber. This highlights that simply scooping soil into a specimen holder – whether in the terrestrial or Martian context – is likely to lead to measurements with high analytical uncertainty that are prone to cross-contamination between test portions. Milling to <100 μm gave a better-defined crater and reduced spectral noise. When the soil was frozen its texture changed, with particles adhering together more readily, perhaps due to the presence of a small amount of residual moisture. Regardless of whether the soil had previously been frozen, samples that had been pressed into pellets – as expected – gave the smallest and most well-defined ablation craters and the least spectral noise. The incorporation of suitable apparatus to compress a soil sample is technically feasible on an agricultural rover. However, payload weight restrictions may preclude this option for Martian deployment.As shown in Fig. 3, the relative intensities of the nitrogen spectral lines differed between samples pretreated in different ways. In particular, the 746.83 nm line increased in prominence following pelletisation. This wavelength has been used previously for quantification of nitrogen by laboratory-based LIBS, using internal calibration with spiked soil14 and sand.32
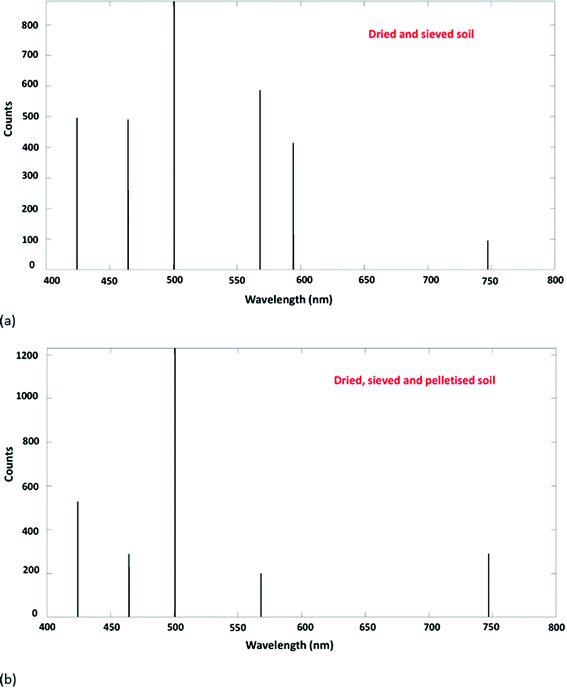 | ||
| Fig. 3 LIBS spectra obtained from (a) dried and sieved soil and (b) dried, sieved and pelletised soil. | ||
This investigation suggests that, although pelletisation would be preferred, even milling a soil sample prior to analysis improves the spectral quality and is therefore to be recommended.
3.2 Effect of soil particle size
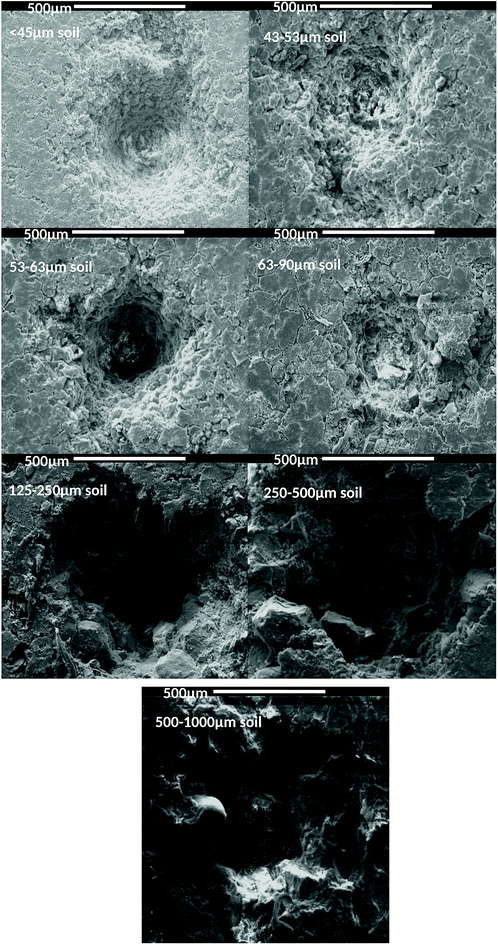
When the ablation craters produced in pellets of the commercial topsoil were imaged by SEM, the crater diameter appeared generally to increase with average soil particle size (Fig. 4).There was also a clear change in crater morphology. Pellets prepared with particles in the ranges 125–250, 250–500 and 500–1000 μm had a more jagged profile, relative to the more uniform aspect of those prepared with particles in the <45 and 45–53 μm size fractions. Larger particles also led to an increase in the number and size of fractures emanating from the ablated crater.
The general trends observed agree with expectations from literature. In a study of plant materials, de Carvalho et al. found that after 20 consecutive laser pulses there were notable changes in the crater morphology for targets formed from different particle size fractions of the bulk sample. For the largest particle size (mean diameter 121 μm) the ablation crater was non-reproducible and disordered. Smaller particle sizes (mean diameter ≤32 μm) produced a crater hole without indication of fractures.39 Capitelli et al. found that ablation craters on sand and sludge differed greatly. They too related this to the form of the sample, including its preparation and texture, as well as its thermal properties, all of which may affect the interaction between the laser beam and the sample surface, hence in turn the LIBS performance.48
3.3 Comparison between LIBS and elemental analysis for total nitrogen determination
The total nitrogen content was determined in the different particle size fractions of the commercial topsoil using the CHN Analyser. Results varied between 1.02 and 0.32% – similar to for example the range (0.1–1.2%) recently reported for a suite of Canadian agricultural soils49 – and general decreased with increasing particle size (Fig. 5). The trend in nitrogen concentration correlated well with the total carbon content determined by microanalysis (R2 = 0.905) and the organic matter content estimated by loss-on-ignition (R2 = 0.904) suggesting that the majority of nitrogen present was, as expected,50 associated with soil organic matter. The LIBS emission intensities at 746.83 nm followed a remarkably similar trend, indicating that LIBS provided a reliable estimate of the relative soil nitrogen content in the pellets and that – although the sample chamber was not evacuated or flushed with inert gas – the presence of atmospheric nitrogen did not give rise to significant interference.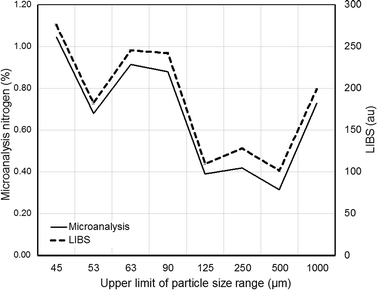 | ||
| Fig. 5 Comparison between response from LIBS system at 746.83 nm and nitrogen concentration determined by microanalysis for pellets containing soil particles in different size ranges. | ||
Comparison between total nitrogen content obtained by microanalysis and LIBS emission intensity at 746.83 nm for the five agricultural soil samples is shows in Fig. 6. A reasonably strong correlation (R2 = 0.82) was observed. Previous workers have shown that it is possible to calibrate the LIBS response by spiking sand32 or soil14 with extraneous nitrogen compounds. Although stand-alone quantification of nitrogen by portable LIBS was beyond the scope of the current study, Fig. 6 indicates that the approach can at least discriminate between intrinsic nitrogen levels in real environmental samples.
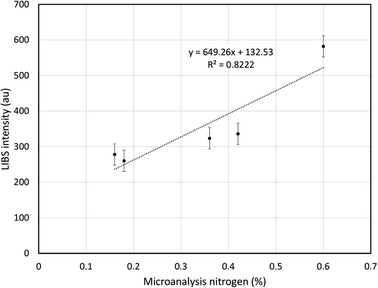 | ||
| Fig. 6 Relationship between LIBS emission intensity at 746.83 nm and nitrogen concentration determined by microanalysis in five agricultural soils. | ||
3.4 Comparison between LIBS, ion chromatography and colorimetry for the determination of nitrate
Since a key objective of Mars LIBS deployment is the search for soil nitrates, comparisons were also made between nitrogen determination by field portable LIBS and nitrate concentration determination by established laboratory techniques.Ion chromatography indicated that 58–179 mg kg−1 of the nitrogen present in the commercial topsoil was in the form of nitrate (<2% of total soil nitrogen content). This is as expected: nitrate typically constitutes a small fraction of total soil nitrogen in terrestrial systems because the species is soluble and readily lost to the hydrosphere through leaching. The chromatography and LIBS results were found to be highly correlated for the different particle size fractions (Fig. 7) demonstrating that portable LIBS has the sensitivity to differentiate soil nitrate concentrations.
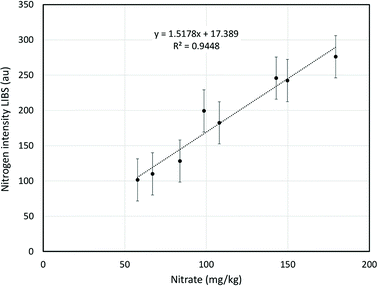 | ||
| Fig. 7 Comparison between response from LIBS system at 746.83 nm and nitrate concentration determined by ion chromatography for pellets containing soil particles in different size ranges. | ||
Finally, the nitrate levels in four of the agricultural soil samples were determined by colorimetry and results compared with LIBS. Broadly similar trends were obtained (Fig. 8) but the correlation between the results of the two techniques (R2 = 0.596) was much poorer than when different size fractions of a single soil were analysed (Fig. 7). Given that the agricultural soils were collected from adjoining fields and likely to be geochemically similar, further work is needed to study the applicability of LIBS for nitrogen determination in soils that differ markedly in matrix composition (mineral and organic matter content).
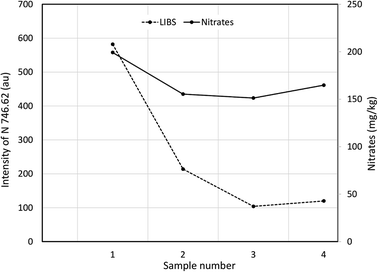 | ||
| Fig. 8 Comparison between response from LIBS system at 746.83 nm and nitrate concentration determined by colorimetry for four agricultural soils. | ||
4 Conclusions
In this study the effect of sample preparation and particle size on the feasibility of using a portable LIBS system to measure the concentrations of nitrogen in field soil samples has been evaluated. Spectral quality was improved when the samples were pelletised, but even milling to reduce particle size and increase sample homogeneity was beneficial. When benchmarked against well-established chemical analysis techniques, it was found that the relative nitrogen concentrations determined by LIBS followed similar trends to quantitative results for total soil N content obtained by microanalysis (using a CHN Elemental Analyser) and for soil nitrate content obtained using ion chromatography and colorimetry.Further work is required to develop suitable calibration models for stand-alone nitrogen determination, to establish the limit of detection of the method, and to evaluate the stability and repeatability of field measurement with the LIBS instrument mounted on the AgriRover platform. For terrestrial applications it would be useful to investigate soils with different geochemical characteristics, and to assess whether any scope exists for LIBS-based nitrogen speciation analysis (since it is not the total, but the phytoavailable nutrient content that affects crop yield). This can be investigated by spiking samples with different forms of nitrogen e.g. nitrate, ammonium, and organic species, prior to LIBS analysis. It would also be of interest to carry out LIBS on soil that had been treated to more closely simulate samples likely to be encountered in a Martian setting e.g. by removal of organic matter.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored in part by the UK Space Agency.References
- O. T. Butler, W. R. L. Cairns, C. M. Davidson and R. E. Mertz, Atomic spectrometry update – a review of advances in environmental analysis, J. Anal. At. Spectrom., 2018, 33, 8–56 RSC.
- D. W. Hahn and N. Omenetto, Laser-Induced Breakdown Spectroscopy (LIBS), Part I: Review of Basic Diagnostics and Plasma-Particle Interactions: Still-Challenging Issues Within the Analytical Plasma Community, Appl. Spectrosc., 2010, 64, 335A–336A CrossRef CAS PubMed.
- D. W. Hahn and N. Omenetto, Laser-Induced Breakdown Spectroscopy (LIBS), Part II: Review of Instrumental and Methodological Approaches to Material Analysis and Applications to Different Fields, Appl. Spectrosc., 2012, 66, 347–419 CrossRef CAS PubMed.
- F. Anabitarte, A. Cobo and J. M. Lopez-Higuera, , Laser-Induced Breakdown Spectroscopy: Fundamentals, Applications, and Challenges, ISRN Spectrosc., 2012, 2012, 285240 Search PubMed.
- T. Hussain and M. A. Gondal, Laser induced breakdown spectroscopy (LIBS) as a rapid tool for material analysis, J. Phys.: Conf. Ser., 2013, 439, 1–12 Search PubMed.
- L. Radziemski and D. Cremers, A brief history of laser-induced breakdown spectroscopy: From the concept of atoms to LIBS 2012, Spectrochim. Acta, Part B, 2013, 87, 3–10 CrossRef CAS.
- C. Pasquini, J. Cortez, L. M. C. Silva and F. B. Gonzaga, Laser Induced Breakdown Spectroscopy, J. Braz. Chem. Soc., 2017, 18, 463–512 CrossRef.
- S. C. Jantzi, V. Motto-Ros, F. Trichard, Y. Markushin and N. Melikechi, Sample treatment and preparation for laser induced breakdown spectrometry, Spectrochim. Acta, Part B, 2016, 115, 52–63 CrossRef CAS.
- J. Y. Peng, F. Liu, F. Zhou, K. L. Song, C. Zhang, L. H. Ye and Y. He, Challenging applications of multi-element analysis by lased-induced breakdown spectroscopy in agriculture: a review, TrAC, Trends Anal. Chem., 2016, 85, 260–272 CrossRef CAS.
- J. S. Sinfield, D. Fagerman and O. Colic, Evaluation of sensing technologies for on-the-go detection of macro-nutrients in cultivated soils, Comput. Electron. Agric., 2010, 70, 1–18 CrossRef.
- M. Z. Martin, N. Labbé, N. André and R. Harris, High resolution applications of laser-induced breakdown spectroscopy for environmental and forensic applications, Spectrochim. Acta, Part B, 2007, 62, 1426–1432 CrossRef.
- N. Yang, Elemental Analysis of Soils Using Laser-Induced Breakdown Spectroscopy (LIBS), Master of science thesis, The University of Tennessee, Knoxville, August 2009.
- L. C. Trevizan, D. Santos Jr., R. E. Samad and N. D. Vieira Jr., Evaluation of laser induced breakdown spectroscopy for the determination of macronutrients in plant material, Spectrochim. Acta, Part B, 2008, 63, 1151–1158 CrossRef.
- D. M. Dong, C. J. Zhao, W. G. Zheng, X. D. Zhao and L. Z. Jiao, Spectral Characterization of Nitrogen in Farmland Soil by Laser-Induced Breakdown Spectroscopy, Spectrosc. Lett., 2013, 46, 421–426 CrossRef CAS.
- W. Pierce, S. M. Christian, M. J. Myers and J. D. Myers, Field-testing for environmental pollutants using briefcase sized portable LIBS system, LIBS_2004, 3rd International Conference on Laser Induced Plasma Spectroscopy and Applications, Malaga, Spain, 2004 Search PubMed.
- L. K. Sharma and S. K. Bali, A Review of Methods to Improve Nitrogen Use Efficiency in Agriculture, Sustainability, 2018, 10(1), 51 Search PubMed.
- A. K. Knight, N. L. Scherbarth, D. A. Cremers and M. J. Ferris, Characterization of Laser-Induced Breakdown Spectroscopy (LIBS) for Application to Space Exploration, Appl. Spectrosc., 2000, 54, 331–340 CrossRef CAS.
- A. Cousin, O. Forni, S. Maurice, O. Gasnault and C. Fabre, Laser induced breakdown spectroscopy library for the Martian environment, Spectrochim. Acta, Part B, 2011, 66, 805–814 CrossRef CAS.
- C. S. Boxe, K. P. Hand, K. H. Nealson, Y. L. Yung and A. Saiz-Lopez, An active nitrogen cycle on Mars sufficient to support a subsurface biosphere, Int. J. Astrobiol., 2012, 11, 109–115 CrossRef CAS.
- E. Iniguez, R. Navarro-Gonzalez, J. de la Rosa, F. Ureña-Núñez, P. Coll, F. Raulin and C. P. McKay, On the oxidation ability of the NASA Mars-1 soil simulant during the thermal volatilization step: Implications for the search of organics on Mars, Geophys. Res. Lett., 2009, 36, L21205 CrossRef.
- J. P. Grotzinger, J. Crisp, A. R. Vasavada, R. C. Anderson, C. J. Baker, R. Barry, D. F. Blake, P. Conrad, K. S. Edgett, B. Ferdowski, R. Gellert, J. B. Gilbert, M. Golombek, J. Gomez-Elvira, D. M. Hassler, L. Jangura, M. Litvak, P. Mahaffy, J. Maki, M. Meyer, M. C. Malin, I. Mitrofanov, J. J. Simmonds, D. Vaniman, R. V. Welch and R. C. Wiens, Mars Science Laboratory Mission and Science Investigation, Space Sci. Rev., 2012, 170, 5–56 CrossRef.
- N. L. Lanza, R. C. Wiens, S. M. Clegg, A. M. Ollila, S. D. Humphries, H. E. Newsom and J. E. Barefield, Calibrating the ChemCam laser-induced breakdown spectroscopy instrument for carbonate minerals on Mars, Appl. Opt., 2010, 49, C211–C217 CrossRef CAS.
- R. Navarro-Gonzalez, J. Stern, C. Freissinet, H. Franz, C. McKay, P. Coll, B. Sutter, D. Archer, A. McAdam, M. Cabane, D. Ming, D. Glavin, J. Eigenbrode, L. Leshin, M. Wong, S. Atreya, J. Wray, A. Steele, A. Buch, and B. prates, Search for nitrates on Mars by the Sample Analysis at Mars (SAM) Instrument, Geophys. Res. Abst., 2014, 16, pp. EGU2014-14820 Search PubMed.
- R. L. Mancinelli, The search for Nitrogen compounds in the surface of Mars, Adv. Space Res., 1996, 18, 241–248 CrossRef CAS.
- R. L. Mancinelli and A. Banin, Where is the nitrogen on Mars?, Int. J. Astrobiol., 2003, 217–225 CrossRef CAS.
- R. C. Wiens, S. Maurice, B. Barraclough and M. Saccoccio, et al., The ChemCam Instrument Suite on the Mars Science Laboratory (MSL) Rover: Body Unit and Combined System Tests, Space Sci. Rev., 2012, 170, 167–227 CrossRef.
- R. Gaudiuso, M. Dell'Aglio, O. De Pascale, G. S. Senesi and A. De Giacomo, Laser Induced Breakdown Spectroscopy for Elemental Analysis in Environmental, Cultural Heritage and Space Applications: A Review of Methods and Results, Sensors, 2010, 10, 7434–7468 CrossRef CAS PubMed.
- S. M. Clegg, R. Wiens, A. K. Misra and S. K. Sharma, Planetary Geochemical Investigations Using Raman and Laser-Induced Breakdown Spectroscopy, Appl. Spectrosc., 2014, 68, 925–936 CrossRef CAS PubMed.
- S. Maurice, R. C. Wiens, M. Saccoccio, B. Barraclough and O. Gasnault, et al., The ChemCam Instrument Suite on the Mars Science Laboratory (MSL) Rover: Science Objectives and Mast Unit Description, Space Sci. Rev., 2012, 170, 95–166 CrossRef CAS.
- S. Silverstone, M. Nelson, A. Alling and J. P. Allen, Soil and crop management experiments in the Laboratory Biosphere: An analogue system for the Mars on Earth facility, Adv. Space Res., 2005, 35, 1544–1551 CrossRef CAS PubMed.
- M. Z. Martin, M. A. Mayes, K. R. Heal, D. J. Brice and S. D. Wullschleger, Investigation of laser-induced breakdown spectroscopy and multivariate analysis for differentiating inorganic and organic C in a variety of soils, Spectrochim. Acta, Part B, 2013, 87, 100–107 CrossRef CAS.
- R. D. Harris, D. A. Cremers, M. H. Ebinger and B. K. Bluhm, Determination of Nitrogen in Sand Using Laser-Induced Breakdown Spectroscopy, Appl. Spectrosc., 2004, 58(No. 7), 770–775 CrossRef CAS PubMed.
- B. Bousquet, J.-B. Sirven and L. Canioni, Towards quantitative laser-induced breakdown spectroscopy analysis of soil samples, Spectrochim. Acta, Part B, 2007, 62, 1582–1589 CrossRef.
- V. S. Burakov, S. N. Raikov, N. V. Tarasenko, M. V. Belkov and V. V. Kiris, Development of a Laser-Induced Breakdown Spectroscopy method for soil and ecological analysis (Review)”, J. Appl. Spectrosc., 2010, 77, 595–608 CrossRef CAS.
- P. M. Mukhono, K. H. Angeyo, A. Dehayem-Kamadjeu and K. A. Kaduki, Laser induced breakdown spectroscopy and characterization of environmental matrices utilizing multivariate chemometrics, Spectrochim. Acta, Part B, 2013, 87, 81–85 CrossRef CAS.
- R. S. Harmon, F. C. De Lucia, A. W. Miziolek and K. L. McNesby, Laser-induced breakdown spectroscopy (LIBS) – an emerging field-portable sensor technology for real-time, in situ geochemical and environmental analysis, Geochem.: Explor., Environ., Anal., 2005, 5, 21–28 CrossRef CAS.
- R. S. Harmon, R. E. Russo and R. R. Hark, Applications of laser-induced breakdown spectroscopy for geochemical and environmental analysis: A comprehensive review, Spectrochim. Acta, Part B, 2013, 87, 11–26 CrossRef CAS.
- I. Rauschenbach, V. Lazic, S. G. Pavlov, H. W. Hübers and E. K. Jessberger, Laser induced breakdown spectroscopy on soils and rocks: Influence of the sample temperature, moisture and roughness, Spectrochim. Acta, Part B, 2008, 63, 1205–1215 CrossRef.
- G. G. A. de Carvalho, D. Santos Jr., M. d. S. Gomes, L. C. Nunes, M. B. B. Guerra and F. J. Krug, Influence of particle size distribution on the analysis of pellets of plant materials by laser-induced breakdown spectroscopy, Spectrochim. Acta, Part B, 2015, 105, 130–135 CrossRef CAS.
- V. Lazic, I. Rauschenbach, S. Jovicevic, E. K. Jessberger, R. Fantoni and M. Di Fino, Laser induced breakdown spectroscopy of soils, rocks and ice at subzero temperatures in simulated martian conditions, Spectrochim. Acta, Part B, 2007, 62, 1546–1556 CrossRef.
- G. Nicolodelli, B. S. Marangoni, J. S. Cabral, P. R. Villas-Boas, G. S. Senesi, C. Hilario dos Santos, R. A. Romano, A. Segnini, Y. Lucas, C. R. Montes and D. M. P. Milori, Quantification of total carbon in soil using laser-induced breakdown spectroscopy: a method to correct interference lines, Appl. Opt., 2014, 53(10), 2170–2176 CrossRef CAS PubMed.
- N. Yang, N. Eash, J. Lee, M. Z. Martin, Y. S. Zhang and F. R. Walker, Multivariate analysis of laser-induced breakdown spectroscopy spectra of soil samples, Soil Sci., 2010, 135(9), 447–452 CrossRef.
- A. Segnini, A. P. Xavier, P. L. Otaviani-Jr, E. C. Ferreira, A. M. Watanabe, M. A. Speranca, G. Nicolodelli, P. Riberiro, V. Boas, P. P. A. Oliveria and D. M. B. P. Milori, Physical and Chemical Matrix effects in soil Carbon Quantification using Laser-induced breakdown Spectroscopy, Am. J. Anal. Chem., 2014, 5, 722–729 CrossRef CAS.
- S. C. Jantzi and J. R. Almirall, Characterization and forensic analysis of soil samples using laser-induced breakdown spectroscopy (LIBS), Anal. Bioanal. Chem., 2011, 400, 3341–3351 CrossRef CAS PubMed.
- V. K. Unnikrishnan, R. Nayak, K. Aithal, V. B. Kartha, C. Santhosh, G. P. Gupta and B. M. Suri, Analysis of trace elements in complex matrices (soil) by Laser Induced Breakdown Spectroscopy (LIBS), Anal. Methods, 2013, 5, 2194 RSC.
- X. T. Yan, M. A. Post, A. Bianco, C. Niu, R. Palazzetti, Y. L. C. Melville, A. Kisdi and W. Tubby, The AgriRover: A Mechatronic Platform from Space Robotics for Precision Farming, Reinventing Mechatronics, Proceedings of MECHATRONICS 2018, ed. X.-T. Yan, D. Bradley and P. Moore, University of Strathclyde, Glasgow, UK, 2018, ISBN: 978-1-909522-37-4 (e-Book) Search PubMed.
- K. Li, W. Zhou, Q. Shen, Z. Ren and B. Peng, Laser ablation assisted spark induced breakdownspectroscopy on soil samples, J. Anal. At. Spectrom., 2010, 25, 1475–1481 RSC.
- F. Capitelli, F. Colao, M. R. Provenzano, R. Fantoni, G. Brunetti and N. Senesi, Determination of heavy metals in soils by Laser Induced Breakdown Spectroscopy, Geoderma, 2002, 106, 45–62 CrossRef CAS.
- L. Zhang, X. Yang, C. Drury, M. Chantigny, E. Gregorich, J. miller, S. Bittman, D. Reynolds and J. Yang, Infrared spectroscopy prediction of organic carbon and total nitrogen in soil and particulate organic matter from diverse Canadian agricultural regions, Can. J. Soil Sci., 2018, 98, 77–90 CAS.
- M. Cresser, K. Killham and A. Edwards, Soil Chemistry and its Applications, Cambridge University Press, 1993 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |