Open Access Article
Open Access ArticleAmino-modified molecular sieves for adsorptive removal of H2S from natural gas
Jiaojing Zhang ab,
Hua Song
ab,
Hua Song *ab,
Yanguang Chenab,
Tianzhen Haoc,
Feng Liab,
Dandan Yuanab,
Xueqin Wangab,
Liang Zhao
*ab,
Yanguang Chenab,
Tianzhen Haoc,
Feng Liab,
Dandan Yuanab,
Xueqin Wangab,
Liang Zhao d and
Jinsen Gao*d
d and
Jinsen Gao*d
aProvincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, 163318 Daqing, China
bKey Laboratory of Enhanced Oil & Gas Recovery of Education Ministry, College of Petroleum Engineering, Northeast Petroleum University, 163318 Daqing, China
cHebei Jingzhi Technology Co., LTD, 061000 Cangzhou, China
dState Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing), 102249 Beijing, China. E-mail: jsgao@cup.edu.cn
First published on 20th November 2018
Abstract
Amine-modified MCM-41 adsorbents (APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41) were prepared and characterized by XRD, N2 adsorption–desorption, FT-IR, TEM, SEM and TG-DTA. The performance of each adsorbent in a fixed adsorption bed for H2S removal was measured using a mixture of oxygen, nitrogen and hydrogen sulfide gases. It was found that the specific surface area decreased and the topography changed significantly after the use of each modified adsorbent. Nevertheless, all amine-modified MCM-41 adsorbents retained mesoporous silica of MCM-41. The H2S removal rate and saturated H2S capacity of APTMS/MCM-41 improved from 32.3% to 54.2% and 119.5 to 134.4 mg g−1, respectively, compared with that of MCM-41, and it showed the best performance among all adsorbents. APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 were regenerated by maintaining at 423, 523 and 373 K in nitrogen for 3 h, respectively, and thus possessed high regenerability.
Introduction
Hydrogen sulfide (H2S) is frequently found in natural gas and other industrial hydrocarbon feedstocks. Since H2S is one of the most noxious industrial gases to the atmosphere, the H2S content of natural gas before use must be minimized to ensure no harm to human life and daily living.1 Nowadays, alcohol amine solution is commonly used in industrial H2S capture, and this method actually removes H2S gas through a chemical reaction.2 It utilizes the reaction between alcohol amines and H2S to capture H2S and the reverse reaction to regenerate the absorbent; this is the most efficient method for H2S removal.3 However, the disadvantages of this method for industrial applications include high energy consumption, large equipment volume, and significant equipment corrosion.4 More researchers are investigating a more effective desulphurizing method. Solid adsorbents such as zeolites, activated carbon and molecular sieves have received much attention because the operations are simpler and less energy-consuming.5 Research in the last few years has been focused on metal oxide-based adsorbents for efficient H2S removal.6–8 Many transition metals such as iron, copper and magnesium have been studied to load in carriers to improve the desulfurization capability of adsorbents.9 However, the regeneration of adsorbents depends on high temperature, which restricts the application of solid adsorbents. Compared to existing absorption technologies based on amine solutions, amine-containing solid adsorbents are remarkable with less corrosiveness, amine loss and energy demand during the regeneration.10,11 Among these solid supports, mesostructured MCM-41 is commonly used because of its high surface area, tuneable pore diameter and large pore volume.12–17In the present study, APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 were prepared, and their adsorptive desulfurization performances toward a simulated low-H2S gas were studied. By comparing the loading with that of other amines, we directly attached certain amines to the carrier surfaces, but a chemical reaction occurred between some amines and the carrier to form a more stable material.
Experimental
Preparation of APTMS/MCM-41
Quantitatively calcined MCM-41 (all-silicon, Nankai University Catalyst Factory) was suspended in 30 mL of toluene (>99.5%, Nanjing Chemical Reagent Co., LTD) in a round-bottomed flask, into which 0.6 mL of (3-aminopropyl)trimethoxysilane (APTMS; >97%, Aladdin Reagent (Shanghai) Co., LTD) was cautiously added dropwise. After refluxing for 8 h, the resulting mixed solution was centrifuged to separate the target product from the solvent. The target product was then filtrated carefully first with ethanol and then distilled water; finally, it was dried at room temperature for 12 h.Preparation of PEI/MCM-41
Quantitative polyethylenimine (PEI; >99%, Aladdin Reagent (Shanghai)) was completely dissolved in isopropyl alcohol (99.7%, Fu Chen (Tianjin) Chemical Reagent Factory). Then, 0.4 g of MCM-41 was added to the mixed solution, and the solution was stirred with a magnetic stirrer for some time. The resulting solution was filtered in a drying oven at 100 °C and dried for 4 h.Preparation of AAPTS/MCM-41
Quantitatively calcined MCM-41 was suspended in 30 mL of toluene in a round-bottomed flask, into which 5 mL of N-ethyl-γ-amino propyl trimethoxy silane (AAPTS; 99.7%, Aladdin Shanghai) was cautiously added dropwise under a nitrogen atmosphere. After refluxing for 6 h, the resulting solution was centrifuged to separate the target product from the solvent. The product was filtrated carefully with distilled water and then dried at 100 °C for 12 h.Characterization of adsorbent
The low-angle XRD patterns of all adsorbents were recorded on a Netherlands Panalytical diffractometer at 40 kV and 30 mA using monochromatized Cu-Kα radiation. The patterns were measured in the range of 2θ = 0.5–10°.Physicochemical properties of the adsorbent and support were characterized on a NOVA2000e device (Quantachrome Instruments, USA). Each adsorbent was outgassed at 180 °C up to the vacuum pressure of 6 mmHg. The liquid nitrogen adsorption isotherms were monitored at −196 °C. Using the equation at a relative pressure (p/p0) of 0.05–0.35, the Brunauer–Emmett–Teller (BET) surface area of each adsorbent was estimated from the adsorption isotherm. Total pore volume (Vtotal) was computed from the nitrogen volume at p/p0 = 0.99.
The adsorbents were analyzed on a Bruker Tensor 27 Fourier transform infrared spectroscopy (FT-IR) spectrometer at room temperature. In advance, the adsorbents were mixed with dried KBr powder and tableted.
Morphology was analyzed on a JEOL 100S transmission electron microscope (TEM) was obtained on a JEOL 2010 microscope. For both TEM analyses, each adsorbent was prepared by adding a reduced sample (1 mg) to methanol (5 mL) and sonicated for 10 min. Several drops of suspension were added dropwise onto a hollow copper grid coated with a self-made carbon film.
Scanning electron microscopy (SEM) images were recorded on SIGMA SEM (Carl Zeiss AG, Germany) in vacuum at an accelerating voltage from 100 V to 30 kV to enlarge the sample by 2000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times.
000 times.
The thermogravimetric-differential thermal analysis (TG-DTA) was recorded using a Perkin-Elmer Diamond thermal analyzer under atmospheric conditions and a temperature rate of 10 °C min−1.
Desulfurization performance tests
Adsorbent activity measurements were conducted in a fixed-bed reactor. The fixed-bed reactor, which consisted of a quartz glass tube with 10 mm internal diameter and 300 mm length, was placed vertically in a clamshell furnace. The prepared adsorbent (0.2 g) was loaded into the center region of the reactor. The upper and lower sides of the adsorbent were filled with quartz sand. The model fuel gas (H2S, N2 and O2 mixture; N2 as balance gas; the H2S concentration was controlled at about 20 mg L−1) from a feed tank was passed through a reducing valve and entered into the reactor at a flow rate of 20 mL min−1. Any unadsorbed H2S gas in the effluent gas was dissolved by (the solvent of zinc acetate, cadmium acetate and acetic acid) in the absorption bottle, and the H2S content in (the solvent of zinc acetate, cadmium acetate and acetic acid) was determined using iodometry at intervals of 5 min. The saturated H2S capacity and H2S removal rate of the absorbent were calculated using the H2S breakthrough curves.The saturated H2S capacity is defined as the amount of adsorbed H2S per gram of adsorbent when the H2S concentrations in the effluent and in the initial gas are the same. It can be calculated as follows:
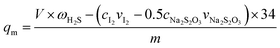 | (1) |
The H2S removal rate (η) can be expressed as follows:
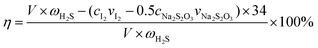 | (2) |
Results and discussion
Low-angle XRD patterns of the four adsorbents are shown in Fig. 1. All adsorbents have one characteristic peak at 2θ = 2.2°, which is ascribed to the (100) plane; the three weaker peaks correspond to the reflections of planes (110) at 3.8°, (200) at 4.4° and (210) at 5.7°, which are characteristics of the hexagonal mesoporous structure (Fig. 1).18,19 The peak corresponding to the (100) plane could be considered as a feature of the MCM-41 mesostructure, indicating that the hexagonal mesoporous structure of MCM-41 was preserved after being loaded with APTMS, PEI or AAPTS. However, the (100) plane of the modified MCM-41 adsorbents was weaker compared with that of bare MCM-41, and even the peaks of other planes disappeared. As reported, the amine coating outside MCM-41 crystals did not significantly affect the diffraction intensity of MCM-41.20,21 It is suggested that fast pore filling during amine modification is due to the smaller molecule size of amine. As more molecules entered pores during modification, structures collapsed rapidly and thereby reduced the diffraction intensity.22–24Fig. 2 shows the N2 adsorption–desorption isotherms and Barrett–Joyner–Halenda (BJH) pore size distributions for the four adsorbents and Table 1 lists their textural data. The isotherms belong to type IV according to the International Union of Pure and Applied Chemistry (IUPAC) classification, and they never varied after the loading of amine (APTMS, PEI or AAPTS); this indicated that the organized network of MCM-41 was preserved after loading, which was in accordance with the XRD results (Fig. 1).25,26 The H1 type hysteresis loop at p/p0 > 0.9 was due to N2 condensation and evaporation with interparticles.27,28 The nitrogen adsorption linearly increased at low relative pressures due to monolayer adsorption before the steep nitrogen uptake in the mesopores. Accordingly, micropore filling occurred at a low relative pressure, whereas sharp pore condensation was observed at a high pressure in the extended multilayer zone. The steep gas uptake was due to capillary condensation of nitrogen in the mesopores, implying that MCM-41 had a narrow pore size distribution. These results are consistent with the XRD patterns.
 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherms and BJH pore size distribution curves of MCM-41, APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41. | ||
| Adsorbents | Surface area/(m2 g−1) | Pore diameter/nm | Pore volume/(mL g−1) |
|---|---|---|---|
| MCM-41 | 1095.2 | 3.64 | 1.22 |
| APTMS/MCM-41 | 873.7 | 2.89 | 0.64 |
| PEI/MCM-41 | 882.2 | 2.80 | 0.70 |
| AAPTS/MCM-41 | 823.9 | 2.80 | 0.60 |
The BJH pore sizes of MCM-41 adsorbents (Fig. 2) were in the range of 2–6 nm, indicating that each adsorbent has excellent mesoporous ordering and a narrow pore size distribution. Bare MCM-41 exhibited a large specific surface area of 1095.2 m2 g−1 and a pore volume up to 1.22 mL g−1. The loading of APTMS, PEI or AAPTS changed the Brunauer–Emmett–Teller (BET) specific surface area, pore diameter and pore volume. The specific surface area and pore diameter significantly decreased with further increase in amine content. The external surface was saturated and more amines entered the mesopores of MCM-41, which caused pore blockage and therefore decreased the surface area.25 These results were evidenced by the decrease in pore size with loading amine content. The specific surface area of PEI/MCM-41 was larger than those of APTMS/MCM-41 and AAPTS/MCM-41. This finding was possibly because PEI having a larger molecular size could not easily enter the inner pores of MCM-41 to be loaded on the internal surface of MCM-41; therefore, the loss in the surface area was less serious. The specific surface area of AAPTS/MCM-41 was the smallest possibly because the molecular size of AAPTS was smaller than that of PEI; therefore, it could easily enter the inner pores of MCM-41 as compared to PEI. In addition, the molecular size was larger than that of APTMS; therefore, the effect of AAPTS on the pore volume and surface area was more significant.
The FT-IR spectra of the four adsorbents are shown in Fig. 3. The symmetrical stretching mode near 791 cm−1 and the asymmetric mode near 1076 cm−1 are related to the framework of silicon for the vibration of Si–O–Si. The adsorption bands at 3429 and 1632 cm−1 observed in all spectra are ascribed to the –OH stretching of silanol groups.26,27 NH2-MCM-41 shows a typical peak at 2939 cm−1 corresponding to the asymmetric vibration of –CH2 in the propyl chain of the silylating agent.28 The peaks of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 at 1532, 1463 and 1469 cm−1 (Fig. 3) correspond to the stretching vibration of C–N in the amine, which is specific to APTMS, PEI and AAPTS.29 These results further confirm the successful immobilization of APTMS, PEI or AAPTS onto the surface of MCM-41.
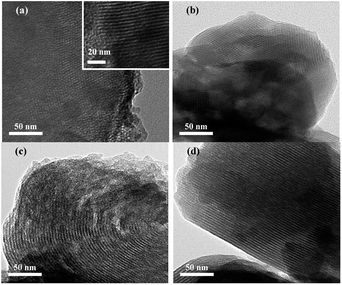
The TEM image of MCM-41 shows the very rational and orderly channels on surface (Fig. 4a), revealing a uniform ordered hexagonal structure and a distinctive feature with pore diameter of ∼4 nm. APTMS, PEI and AAPTS particles are uniformly distributed in the corresponding adsorbent (Fig. 4b–d) and thus are highly dispersed. The APTMS, PEI and AAPTS particles appear either on the external surface walls of the pores or inside the pores, indicating strong interactions between organic amine and MCM-41.
The SEM images of the four adsorbents show various particle shapes and sizes (Fig. 5). The micrographs show that the coupling agent APTMS, PEI or AAPTS influences the microstructure of MCM-41 to a greater extent. The SEM images show that the surface of MCM-41 is relatively smooth with different sizes and relatively sparse channels (Fig. 5a). However, the SEM images of APTMS/MCM-41 show greater changes on the surfaces. The APTMS/MCM-41 particles are worm-like. The particles are well ordered and arranged in the best way for amine loading (Fig. 5b).30 PEI distributes unevenly on MCM-41 pores and clogs parts when it is loaded on MCM-41 (Fig. 5c). The active constituent in AAPTS/MCM-41 distributes much more evenly than in PEI/MCM-41, indicating that the existence of AAPTS is propitious to the dispersion of the active component. The surface channel distribution of modified MCM-41 is dense; the holes are filled with the active component, and pore formation is completed during loading although the surface area decreases with smaller pore volumes. These results are consistent with BET data.
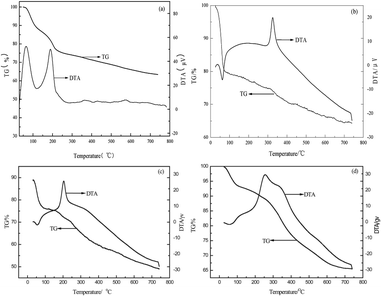
The TG-DTA curves of the four adsorbents are shown in Fig. 6. MCM-41 displays one stage of weight loss with an endothermic peak within 50–200 °C (22.3%), whereas APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 show one stage of weight loss at endothermic peaks within the ranges of 25–70 °C (19.2%), 30–93 °C (23.7%), 25–85 °C (5.5%), respectively. These findings can be ascribed mainly to water elimination from the surface and pores. Other exothermic peaks at 326, 204 and 256 °C are owing to –NH2 decomposition of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41, respectively. In addition, the different features from the TG/DTA curves of the four adsorbents, which show a greater mass loss for pure MCM-41 than that for modified MCM-41 adsorbents, may indicate interaction between the incorporated amine component and the surfactant molecules. Thus, the stability of MCM-41 is improved.31 The decomposition temperature of –NH2 decreases in the order APTMS/MCM-41 > AAPTS/MCM-41 > PEI/MCM-41. This shows that APTMS/MCM-41 has the highest stability. This result is consistent with the order of the molecular size of loaded molecules, which indicates that smaller molecules are more stable.
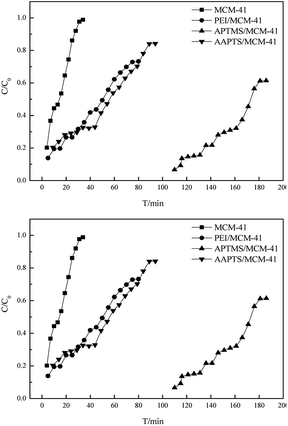
All adsorbents were evaluated at room temperature (298.15 K) and gas speed of 20 mL min−1 in a fixed-bed reactor (Fig. 7 and Table 2). The H2S removal performances of the adsorbents improved significantly after amine modification, which proves that the presence of organic amine is critical in H2S removal. The saturated H2S capacities decreased in the order APTMS/MCM-41 > AAPTS/MCM-41 > PEI/MCM-41 > MCM-41, with 134.4, 46.6, 40.2 and 14.9 mg g−1, respectively. For APTMS/MCM-41, the desulfurization performance with saturated adsorption at 186 min was higher than that of all the modified adsorbents. The saturated H2S capacity and H2S removal rate maximized to 134.38 mg g−1 and 54.19%, respectively, at the APTMS loading of 1 g. The higher saturated H2S capacity and H2S removal obtained by APTMS/MCM-41 could be explained as follows: the molecular size of the loaded molecule increased in the order of APTMS < AAPTS < PEI (Fig. 8). APTMS with small size could easily enter the inner pores of MCM-41 and react with –OH on the inner pore surface of MCM-41. As a result, APTMS/MCM-41 possessed many active sites compared to PEI/MCM-41 and AAPTS/MCM-41. In addition, as compared to the results for AAPTS/MCM-41, the FT-IR spectra of APTMS/MCM-41 showed a greater stretching vibration peak for C–N in the amine around 1532 cm−1 (Fig. 3). This may clearly indicate that APTMS/MCM-41 has high alkalinity, which can lead to more active sites.32 In conclusion, saturated H2S capacity and H2S removal obtained by APTMS/MCM-41 were much higher than those of PEI/MCM-41 and AAPTS/MCM-41. As compared to PEI, AAPTS possessed a much smaller molecular size; however, the saturated H2S capacity and H2S removal rate were just slightly higher than that of PEI/MCM-41. This can be explained by the much lower surface area of AAPTS/MCM-41 as compared to that of PEI/MCM-41 (Table 1).
| Adsorbents | Saturated adsorption time/min | Saturated H2S capacity/(mg g−1) | H2S removal rate/% |
|---|---|---|---|
| MCM−41 | 34 | 14.9 | 21.9 |
| APTMS/MCM−41 | 186 | 134.4 | 54.2 |
| PEI/MCM−41 | 80 | 40.2 | 42.1 |
| AAPTS/MCM−41 | 94 | 46.6 | 44.8 |
Regeneration experiments in a fixed-bed reactor showed that regeneration of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 was achieved by maintaining at 423, 523 and 373 K in nitrogen for 3 h, respectively. The breakthrough curves of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 after regeneration time of 3 h are shown in Fig. 9 and Table 3. The saturated H2S capacities after regeneration time of 3 h decreased in the order APTMS/MCM-41 > AAPTS/MCM-41 > PEI/MCM-41, with the calculated values of 103.6, 32.5 and 27.3 mg g−1, respectively. The recovered saturated H2S capacities of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 after regeneration time of 3 h were still much higher than that of MCM-41, indicating high regenerability of the amine-modified MCM-41 adsorbents.
 | ||
| Fig. 9 Breakthrough curves of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 adsorbents after regeneration time of 3 h. | ||
| Adsorbents | Saturated adsorption time/min | Saturated H2S capacity/(mg g−1) | H2S removal rate/% |
|---|---|---|---|
| a AAPTS/MCM-41 adsorbents after regeneration time of 3 h. | |||
| APTMS/MCM-41 | 164 | 103.6 | 51.2 |
| PEI/MCM-41 | 70 | 27.3 | 36.7 |
| AAPTS/MCM-41 | 86 | 32.5 | 38.8 |
The adsorption mechanism of desulfurization can be described by the following steps:33–35 (1) H2S molecules diffuse to the external surface of the adsorbent from the bulk of gas; (2) H2S molecules diffuse from the external surface to the inside of the pores; (3) a chemical adsorptive desulfurization reaction occurs and results in the formation of a sulfur-containing organic compound; (4) complete sulfidation of the active phase and the absorbent leads to adsorption saturation. Some possible mechanisms underlying the trend of reactivity are illustrated in Scheme 1. The desorption process was the reverse reaction of adsorption, in which H2S was released from adsorbent at a high temperature during regeneration.
Conclusions
Amine-modified MCM-41 adsorbents (APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41) were successfully prepared. XRD demonstrated that all amine-modified adsorbents retained mesoporous silica of MCM-41. The BET specific surface areas of the modified adsorbents decreased, and surface areas and pore size also decreased owing to the loading of APTMS, PEI and AAPTS in the pore canals of MCM-41. The fixed-bed adsorption desulfurization experiments showed that H2S removal improved significantly after modification. The desulfurization performances decreased in the order APTMS/MCM-41 > AAPTS/MCM-41 > PEI/MCM-41 > MCM-41. The saturated adsorption time was 186 min with a total gas volume of 3720 mL, and the saturated H2S capacity and H2S removal rate maximized to 134.4 mg g−1 and 54.2%, respectively. Regeneration of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 was achieved by maintaining at 423, 523 and 373 K in nitrogen for 3 h, respectively. The recovered saturated H2S capacities of APTMS/MCM-41, PEI/MCM-41 and AAPTS/MCM-41 after regeneration time of 3 h were still much higher than that of MCM-41, indicating high regenerability of amine-modified MCM-41 adsorbents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Mentoring Project of China Petroleum, the Chemical Industry Federation (2016-09-27), and the Natural Science Foundation of Heilongjiang Province (QC2017005).References
- Y. Sun, X. W. Liu, W. Su, Y. Zhou and L. Zhou, Appl. Surf. Sci., 2007, 253, 5650–5655 CrossRef CAS.
- S. Moioli, A. Giuffrida, M. C. Romano, L. A. Pellegrini and G. Lozza, Appl. Energy, 2016, 183, 1452–1470 CrossRef CAS.
- S. J. Majeed, J. Nat. Gas Sci. Eng., 2016, 36, 175–183 CrossRef.
- M. Hamdine, M. C. Heuzey and A. Bégin, Int. J. Biol. Macromol., 2005, 37, 134–142 CrossRef CAS PubMed.
- M. Behl, J. Yeom, Q. Lineberry, P. K. Jain and M. A. Shannon, Nat. Nanotechnol., 2012, 7, 810–815 CrossRef CAS PubMed.
- M. M. Wu, L. Shi, W. Su, T. T. Lim, A. Vedsha, F. Yu, H. L. Fan and J. Mi, Chem. Eng. J., 2018, 353, 273–287 CrossRef CAS.
- M. M. Wu, T. Li, H. Yu. Li, H. L. Fan and J. Mi, Energy Fuels, 2017, 31, 13921–13932 CrossRef CAS.
- M. M. Wu, B. W. Chang, T. T. Lim, W. D. Oh, J. X. Lei and J. Mi, J. Hazard. Mater., 2018, 360, 391–401 CrossRef CAS PubMed.
- Z. B. Huang, B. S. Liu, F. Wang and R. Amin, Appl. Surf. Sci., 2015, 353, 1–10 CrossRef CAS.
- Q. Chen, F. Fan, D. Long, X. Liu, X. Liang and W. Qiao, et al., Ind. Eng. Chem. Res., 2010, 49, 11408–11414 CrossRef CAS.
- B. Yoosuk, T. Wongsanga and P. Prasassarakich, Fuel, 2016, 168, 47–53 CrossRef CAS.
- A. S. Khder, H. Hma and M. S. Elshall, Appl. Catal., A, 2012, 411, 77–86 CrossRef.
- A. Marcilla, A. Gómez-Siurana, D. Berenguer, I. Martínez-Castellanos and M. I. Beltrán, Toxicol. Rep., 2015, 2, 152–164 CrossRef CAS PubMed.
- F. Farjadian, P. Ahmadpour, S. M. Samani and M. Hosseini, Microporous Mesoporous Mater., 2015, 213, 30–39 CrossRef CAS.
- M. Nikoorazm, A. Ghorbani-Choghamarani, H. Mahdavi and S. M. Esmaeili, Microporous Mesoporous Mater., 2015, 211, 174–181 CrossRef CAS.
- T. Yasmin and K. Müller, Microporous Mesoporous Mater., 2015, 208, 83–92 CrossRef CAS.
- T. Intana, K. Föttinger, G. Rupprechter and P. Kongkachuichay, Colloids Surf., A, 2015, 467, 157–165 CrossRef CAS.
- P. Benito, M. Gregori, S. Andreoli, G. Fornasari, F. Ospitali and S. Millefanti, et al., Catal. Today, 2015, 246, 108–115 CrossRef CAS.
- D. Marino, N. G. Gallegos, J. F. Bengoa, A. M. Alvarez, M. V. Cagnoli, S. G. Casuscelli, E. R. Herrero and S. G. Marchetti, Catal. Today, 2008, 133–135, 632–638 CrossRef CAS.
- M. F. Mota, A. C. L. PatrãCio, M. M. D. Silva and M. G. Freire Rodrigues, Mater. Sci. Forum, 2014, 805, 667–671 Search PubMed.
- X. Xu, C. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Energy Fuels, 2015, 16, 1463–1469 CrossRef.
- S. Huh, J. W. Wiench, J. C. Yoo, M. Pruski and V. S. Y. Lin, Chem. Mater., 2003, 15, 4247–4256 CrossRef CAS.
- M. M. da Silva, A. C. L. Patrício, A. K. F. de Sousa, M. G. Freire Rodrigues and M. L. P. da Silva, Mater. Sci. Forum, 2015, 805, 657–661 Search PubMed.
- C. García-Sancho, R. Moreno-Tost, J. Mérida-Robles, J. Santamaría-González, A. Jiménez-López and P. Maireles-Torres, Appl. Catal., A, 2012, 433, 179–187 CrossRef.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- K. Parida, K. G. Mishra and S. K. Dash, J. Hazard. Mater., 2012, 241, 395–403 CrossRef PubMed.
- X. Chen, X. L. Wang, G. Ran, Z. Cui, S. H. Chen and K. Xie, Fine Chemicals, 2016, 33(2), 188–194 CrossRef CAS PubMed.
- H. Zhao, J. Hu, J. Wang, L. Zhou and H. Liu, Acta Phys.-Chim. Sin., 2007, 23, 801–806 CrossRef CAS.
- J. Cheriaa, M. Khaireddine, M. Rouabhia and A. Bakhrouf, Sci. World J., 2012, 2012, 512454 Search PubMed.
- K. S. N. Kamarudin and N. Alias, Fuel Process. Technol., 2013, 106, 332–337 CrossRef CAS.
- A. S. Khder, H. Hma and M. S. Elshall, Appl. Catal., A, 2012, 411, 77–86 CrossRef.
- A. Endo, M. Yamada, S. Kataoka, T. Sano, Y. Inagi and A. Miyaki, Colloids Surf., A, 2010, 357, 11–16 CrossRef CAS.
- J. J. Zhang, L. Wang, H. Song and H. L. Song, Res. Chem. Intermed., 2015, 41, 6087–6104 CrossRef CAS.
- J. J. Zhang, G. J. Wang, W. Y. Wang, H. Song and F. S. Li, Res. Chem. Intermed., 2016, 42, 6003–6012 CrossRef CAS.
- D. T. Dixon, D. H. Herzog, D. S. Twinning, S. J. J. Titinchi, M. Piet, H. S. Abbo, O. Bolland and W. Schwieger, Energy Procedia, 2014, 63, 8153–8160 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |