Open Access Article
Open Access ArticleSynthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate
Hui Zhanga,
Xiaoyan Wanga,
Na Lia,
Jiaohui Xiaa,
Qingmei Menga,
Jincheng Dingb and
Jie Lu *a
*a
aDepartment of Resources and Environmental Engineering, Shandong University of Technology, 12 Zhangzhou Road, Zibo 255049, China. E-mail: lujiesdut@163.com; Fax: +86-533-2781664; Tel: +86-533-2783397
bCollege of Chemical Engineering, Shandong University of Technology, Zibo 255049, China
First published on 5th October 2018
Abstract
In this study, graphene oxide (GO), titanium dioxide (TiO2) and TiO2/GO nanocomposites were synthesized as the catalysts for photoreduction of endocrine disrupting heavy metal ions in reverse osmosis concentrates (ROC). The morphology, structure and chemical composition of these catalysts were characterized by scanning electron microscopy, transmission electron microscopy, powder X-ray diffraction, Brunauer–Emmett–Teller analysis, Barrett–Joyner–Halenda, Fourier transform infrared spectroscopy and Raman spectroscopy. The photocatalytic experiments showed that TiO2/GO nanocomposites exhibit a higher photoreduction performance than pure TiO2 and GO. Under the optimal conditions, the removal rates of Cd2+ and Pb2+ can reach 66.32 and 88.96%, respectively, confirming the effectiveness of photoreduction to reduce the endocrine disrupting heavy metal ions in ROC resulted from the combined adsorption–reduction with TiO2/GO nanocomposites.
1 Introduction
Reverse osmosis (RO) membrane technology is used widely in the treatment of wastewater and potable water, from which the reverse osmosis concentrate (ROC) is produced.1 However, the treatment of ROC is still a challenge.2 The ROC contains high salinity and elevated levels of ions and organics. The organics including endocrine disrupting chemicals (EDCs), emerging contaminants, persistent organic pollutants (PPCPs), pharmaceuticals, etc., can cause significant damages to the environment. Untreated or improperly managed ROC can generate severe ecotoxicological risks and result in adverse environmental effects.3,4In particular, EDCs consist of a large group of emerging contaminants and are widely distributed in various surface and ground waters, which can disrupt the hormonal system and generate a negative health effect on reproductive, neurological and immune systems.5,6 Generally, EDCs can be classified into five categories: pesticides (atrazine, lindane et al.), persistent organic pollutants (such as DDT and its metabolites), industrial compounds (PCBs, PBBs et al.), chemical substances (phthalates et al.) and heavy metals.7,8 Furthermore, the endocrine systems of organisms and human as well as the reproductive system could be affected by heavy metals. Additionally, cadmium (Cd) as the most toxic heavy metal was found in the ROC. Cd-based compounds are harmful and they can become concentrated in the ecosystem. Similarly, lead (Pb) causes neurological disorders and kidney damage, and can influence the intellectual development of humans.9 Heavy metals would get converted into different forms like Cd2+ and Pb2+ which are highly toxic in comparison to the metal atoms. Even if the concentration of heavy metal ions is not beyond the permissible limit, they could still cause environmental problems.10 Therefore, great attention has been paid to the removal of Cd2+ and Pb2+ from wastewater around the world in recent years. A variety of methods have been used to remove the heavy metal ions such as ion exchange, absorption, membrane filtration, precipitation, photocatalytic reduction, the electrochemical process, etc.11 Among those processes, photocatalytic reduction has been viewed as a safe, simple, efficient, nontoxic and economical method to reduce heavy metal ions.12 In particular, photocatalytic reduction exhibits unique advantages in treatment of low-metal ions-concentration wastewater.
It is well known that TiO2 is a strong and common photocatalyst due to its high photocatalytic efficiency, chemical stability and antibacterial property.13,14 It can absorb light energy to move the electrons up to higher energy levels, generating electron–hole pairs. TiO2 photocatalyst was irradiated by UV light to generate electron–holes which have a high redox ability to degrade pollutants. However, in the practical application, the photocatalytic efficiency of bare TiO2 is low due to a large band gap, the rapid recombination of photogenerated electron–hole pairs, low affinity and poor selection for contaminants.15,16
In order to enhance the photocatalytic ability of TiO2, a variety of approaches was applied to develop TiO2-based composites by designing and modifying TiO2, such as doping with metal or nonmetallic elements, coupling with the semiconductor or incorporating nanostructured carbon materials and so on.14,17,18 In particular, graphene oxide (GO) has drawn much attention as a high potential and efficient material to improve the photocatalytic efficiency of TiO2, since its large specific surface area can facilitate the distribution of TiO2.19,20 In addition, GO can serve as an electronic transfer medium, thus reducing the rate of recombination of electron–hole pairs. The charge transfer rate of electrons will be enhanced due to narrow band gap.21 What's more, the organic pollutants such as EDCs could be absorbed through π–π interactions on the surface of GO.22 Moreover, GO shows a high UV light transparency benefitted from its thin-layered structure which permits UV-light absorption by TiO2.23 Therefore, GO would improve the photocatalytic activity of TiO2 greatly. Similar to GO, rGO showed advantageous enhancement of photocatalytic activity in a number of studies because it can promote charge separation and electron transfer. Due to its outstanding properties, rGO has been investigated as an efficient carbon-based hybrid nanocomposite to improve electronic or photocatalytic performance of materials.24–26 However, the key point is the oxygenous functional groups on the GO surface, such as carboxylic (–COOH), hydroxyl (–OH) and carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, which played an important role in the absorption. Due to the decrease of oxygenous functional groups, rGO showed a poor adsorption capacity than GO.27,28 Therefore, GO is an ideal substrate to adsorb heavy metal ions because its strong absorption affinity. In general, various studies have already been reported for adsorption of heavy metal ions by GO and GO-based composites. Cui et al. reported the EDTA functionalized magnetic GO for removal of Pb(II), Hg(II) and Cu(II) from wastewater.29 Fang et al. demonstrated GO–NH2 as an adsorbent for the removal of Co(II) ions.30 In our study, TiO2/GO nanocomposites were used to adsorb and reduce heavy metal ions.
O) groups, which played an important role in the absorption. Due to the decrease of oxygenous functional groups, rGO showed a poor adsorption capacity than GO.27,28 Therefore, GO is an ideal substrate to adsorb heavy metal ions because its strong absorption affinity. In general, various studies have already been reported for adsorption of heavy metal ions by GO and GO-based composites. Cui et al. reported the EDTA functionalized magnetic GO for removal of Pb(II), Hg(II) and Cu(II) from wastewater.29 Fang et al. demonstrated GO–NH2 as an adsorbent for the removal of Co(II) ions.30 In our study, TiO2/GO nanocomposites were used to adsorb and reduce heavy metal ions.
TiO2/GO nanocomposites have attracted much attention for degrading pollutants recently. R. Raliya and co-workers reported that TiO2/GO suspension applied for adsorptive and photocatalytic removal of methyl orange.31 H. Yadav et al. prepared TiO2/GO photocatalysts for the degradation of benzene gas, showing a significant degradation compared to pure TiO2 nanoparticles.32 V. Bhatia and co-workers used TiO2/graphene oxide composites as a catalyst for the degradation of atenolol.33
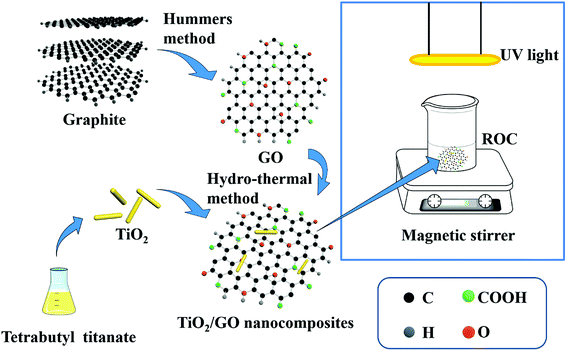
In the present work, GO and TiO2 were synthesized by the modified Hummers method and hydro-thermal method, respectively. Then, TiO2/GO nanocomposites photocatalyst was prepared by hydro-thermal method. Meanwhile, the morphology and structure of GO, TiO2 and TiO2/GO nanocomposites were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), powder X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), Barrett–Joyner–Halenda (BJH), Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy. After that, the photocatalytic effects of synthesized TiO2/GO nanocomposites on heavy metal ions reduction were evaluated in batch modes under different conditions. Besides, an optimal experiment condition was obtained to maximize the reduction efficiency of heavy metal ions by TiO2/GO nanocomposites in the photocatalytic reduction reaction. The synthesis route of TiO2/GO nanocomposites and photocatalytic experiments were illustrated in Fig. 1.
2 Materials and methods
2.1 Synthesis of photocatalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–1
5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). After the ultrasonic treatment, the mixture was dispersed in DI water completely. The supernatant of GO aqueous colloidal suspension was decanted and the centrifuged impurities were discarded. Then, the obtained GO aqueous colloidal suspension was added drop wise into liquid nitrogen and placed in a freeze drier for about 20 h to remove water. The final product is GO. Besides, the concentration of GO aqueous colloidal suspension was about 5 mg mL−1. For comparison, the reduced graphene oxide (rGO) was prepared by thermal treatment at 400 °C in nitrogen atmosphere according to the method described in the ref. 35.
10). After the ultrasonic treatment, the mixture was dispersed in DI water completely. The supernatant of GO aqueous colloidal suspension was decanted and the centrifuged impurities were discarded. Then, the obtained GO aqueous colloidal suspension was added drop wise into liquid nitrogen and placed in a freeze drier for about 20 h to remove water. The final product is GO. Besides, the concentration of GO aqueous colloidal suspension was about 5 mg mL−1. For comparison, the reduced graphene oxide (rGO) was prepared by thermal treatment at 400 °C in nitrogen atmosphere according to the method described in the ref. 35.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 15
6, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 15
8, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 15
10, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 15
12, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, and the corresponding products were denoted as TiO2/GO-6, TiO2/GO-8, TiO2/GO-10, TiO2/GO-12, TiO2/GO-14, respectively. Moreover, titanium dioxide/reduced graphene oxide (TiO2/rGO) nanocomposites were prepared by thermal treatment at 400 °C in nitrogen atmosphere.
14, and the corresponding products were denoted as TiO2/GO-6, TiO2/GO-8, TiO2/GO-10, TiO2/GO-12, TiO2/GO-14, respectively. Moreover, titanium dioxide/reduced graphene oxide (TiO2/rGO) nanocomposites were prepared by thermal treatment at 400 °C in nitrogen atmosphere.2.2 Characterization
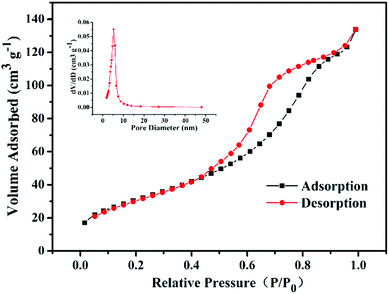
The morphology and structure of GO and TiO2/GO nanocomposites was characterized by SEM (Sirion 200FEI, Netherlands) and TEM (Tecnai F20, 200 kV). The crystalline pattern of samples was investigated by XRD (Cu Kα radiation, Panalytical X PertPro). The BET surface area was determined by N2 adsorption/desorption on an ASAP2020 surface area analyzer using the adsorption date branch in the relative P/P0 pressure range going from 0.0 to 1.0. The pore size distribution of TiO2/GO nanocomposites was obtained by BJH model. In addition, the FTIR (spectral resolution of 0.09 cm−1) spectroscopy was used to determine the functional groups of GO, TiO2/GO and TiO2/rGO nanocomposites from 7800 to 40 cm−1 on a Nicolet 5700 spectrometer (Thermo, Waltham, MA, USA). Raman spectra were obtained using a Horiba Jobin Yvon LABRAM HR Evolution at 785 nm.2.3 Photocatalytic experiments
ROC was obtained from a petrochemical wastewater treatment plant located in Shandong, China. All the photocatalytic experiments were performed at room temperature in a 500 mL Pyrex breaker. In addition, a high-pressure 500 W Hg lamp was used as the UV light source (maximum energy at 365 nm). First of all, a predetermined amount of photocatalyst was added to wastewater (250 mL). Besides, H2SO4 and NaOH were used to adjust the pH value of wastewater. Before UV irradiation, the wastewater was stirred in the dark for 0.5 h to reach adsorption/desorption equilibrium. Subsequently, irradiation started and samples were collected at a specified interval. Besides, the absorbance of Cd2+ and Pb2+ was determined by a flame atomic absorption spectrophotometer of A3 series with a Beijing Purkinje General instrument (China). And the standard curve method was adopted to determine the heavy metal ions concentration of untreated and treated samples.3 Results and discussion
3.1 Characterization of photocatalyst
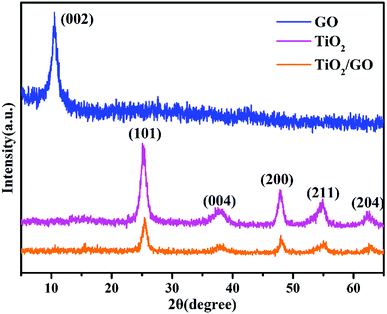
From the XRD pattern of TiO2, the characteristic peaks at 2θ values of 25.28°, 37.77°, 47.99°, 54.98° and 62.63° represent the crystal planes of (101), (004), (200), (211) and (204) of anatase TiO2, respectively,37 which are in good agreement with the standard JCPDS Card no. 21-1272. Obviously, peaks of TiO2/GO-8 nanocomposites are similar to TiO2. However, the diffraction peak of GO at 2θ = 10.54° disappeared because the regular stack of GO sheets had been destroyed due to the intercalation of TiO2 nanoparticles.38
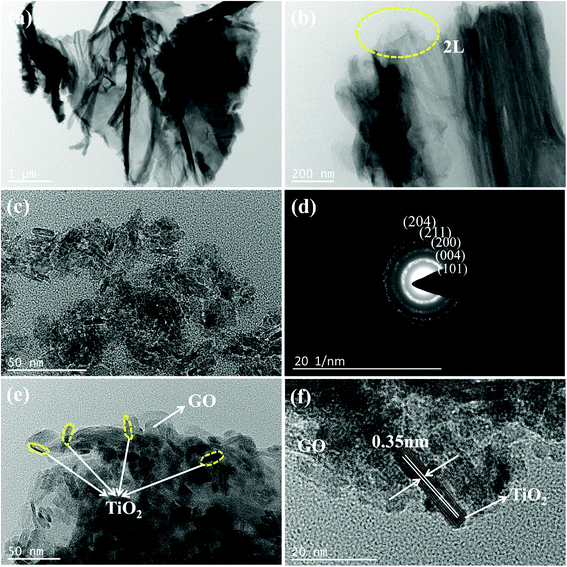 | ||
| Fig. 4 TEM images of GO (a and b), TiO2 with the SAED pattern (c and d), and TiO2/GO-8 nanocomposites (e and f). | ||
As shown in Fig. 4c, the morphology and size of the TiO2 nanoparticles are similar. The TiO2 is a rod-like nanoparticle with the average length of about 10 nm and width of about 4 nm. The selected area electron diffraction (SAED) pattern of TiO2 (Fig. 4d) revealed that TiO2 has a polycrystalline structure according to a series of concentric rings with different radii. It has been calculated that the distances between the various diffraction rings and diffraction center are 0.352, 0.237, 0.190, 0.166 and 0.148 nm, respectively, corresponding to the lattice spacings of (101), (004), (200), (211) and (204) planes of anatase TiO2 (JCPDS Card no. 21-1272). Besides, it is consistent with the results of XRD analysis.
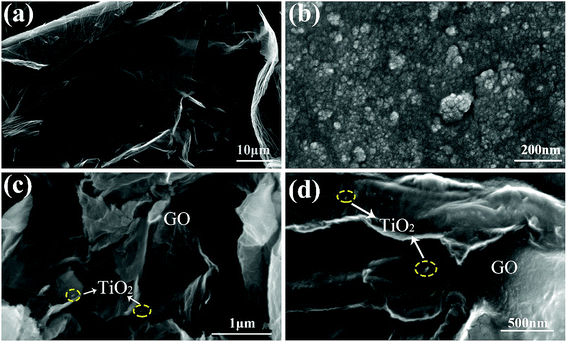
Fig. 3c and d shows that the layers of GO curled and the well dispersed TiO2 anchored on the GO planes. This result proved that the TiO2 was successfully loaded onto the GO planes. Most of TiO2 nanoparticles are situated on the edge of GO sheets as shown in Fig. 4e. Fig. 4f shows the lattice fringe is 0.35 nm, which corresponds to the lattice spacing of the (101) plane of anatase TiO2. It is obvious that GO barely affects the morphology and crystalline pattern of TiO2.
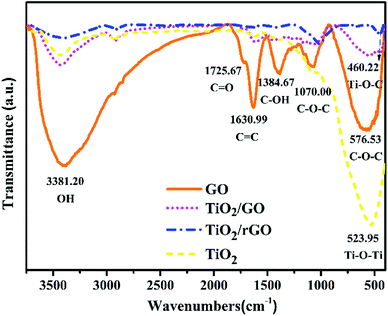
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. Other bands at 1725 and 3381 cm−1 correspond to stretching vibration of oxygen containing functional groups of carboxyl (C
C stretching vibration. Other bands at 1725 and 3381 cm−1 correspond to stretching vibration of oxygen containing functional groups of carboxyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and hydroxyl (OH), respectively.39 The co-existence of these two stretching vibrations shows the carboxyl (COOH) group on GO. As a result, those polar functional groups can provide anchoring sites for the adsorption of TiO2 on the GO.
O) and hydroxyl (OH), respectively.39 The co-existence of these two stretching vibrations shows the carboxyl (COOH) group on GO. As a result, those polar functional groups can provide anchoring sites for the adsorption of TiO2 on the GO.
By comparison with the GO spectrum, a small amount of oxygen-containing functional groups still exist on the surface of TiO2/GO and TiO2/rGO nanocomposites. For the FTIR spectra of TiO2/GO-8 and TiO2/rGO nanocomposites, the broad band at 460.22 cm−1 was originated from the stretching Ti–O–C.40 Besides, the absorption band near 523 cm−1 corresponded to the Ti–O vibration.41 Furthermore, peak strength of TiO2/rGO nanocomposites is lower than TiO2/GO nanocomposites and some of the peaks even disappear. From the FTIR spectrum of TiO2/rGO nanocomposites, few peaks indicating the oxygen-containing functional groups can be seen after reduction. These oxygen-containing functional groups demonstrate the successful preparation of GO and provide anchoring sites for the adsorption of heavy metal ions on the TiO2/GO-8 nanocomposites. It had been confirmed that the oxygen-containing functional groups played an important role in the adsorption of heavy metal ions.42
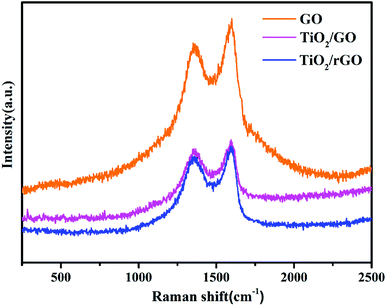
| Samples | WD (cm−1) | WG (cm−1) | ID | IG | ID/IG |
|---|---|---|---|---|---|
| GO | 1363 | 1600 | 925 | 1032 | 0.896 |
| TiO2/GO | 1358 | 1595 | 481 | 519 | 0.927 |
| TiO2/rGO | 1344 | 1593 | 446 | 491 | 0.908 |
3.2 Photocatalytic performance of TiO2/GO nanocomposites
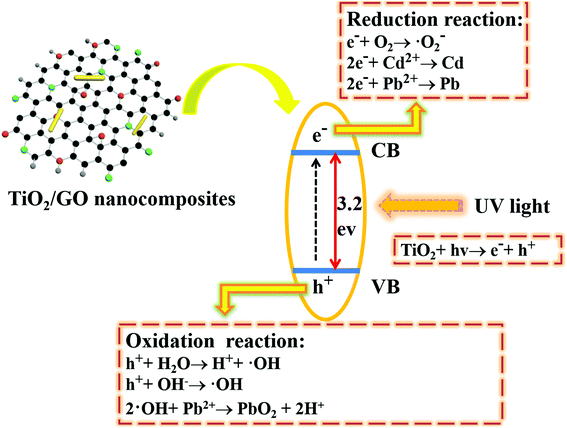
| TiO2/GO + hv → e− + h+ | (1) |
| e− + O2 → ·O2− | (2) |
| 2e− + Cd2+ → Cd | (3) |
| 2e− + Pb2+ → Pb | (4) |
| h+ + H2O → H+ + ·OH | (5) |
| h+ + OH− → ·OH | (6) |
| 2·OH + Pb2+ → PbO2 + 2H+ | (7) |
The dark absorption and photoreduction activity of Cd2+ and Pb2+ using TiO2, GO and TiO2/GO nanocomposites was studied. Fig. 10 and 11 illustrate the concentration of Cd2+ and Pb2+ under various operating conditions, respectively. The initial concentrations of Cd2+ and Pb2+ in the ROC were 0.155 and 0.334 mg L−1, respectively. Fig. 10a and 11a shows that both pure GO and TiO2 could reduce Cd2+ and Pb2+ to some extent. Moreover, compared with pure GO and TiO2, the photoreduction capability was increased significantly with the catalysis of TiO2/GO nanocomposites. There was a large specific surface area on TiO2/GO nanocomposites and the combination of GO with TiO2 led to a stronger redox ability. Besides, the functional groups of TiO2/GO nanocomposites outnumbered the unmodified TiO2, which helps the TiO2/GO nanocomposites contact fully with the heavy metal ions. Furthermore, GO had a stronger adsorption of Pb2+ than Cd2+, resulting in the higher photoreduction of Pb2+ than Cd2+. This is probably attributed to the selective adsorption of the specified heavy metal ion by GO.47
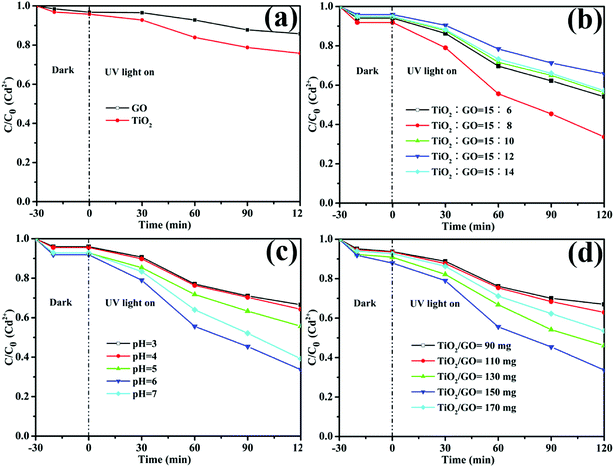 | ||
| Fig. 10 The relative concentration of Cd2+ under different conditions. (a) Pure TiO2 and GO, (b) the mass ration of TiO2 to GO in TiO2/GO nanocomposites, (c) pH value and (d) the amount of TiO2/GO. | ||
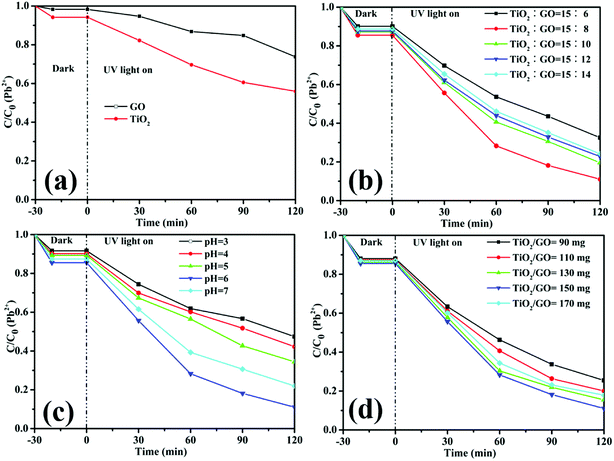 | ||
| Fig. 11 The relative concentration of Pb2+ under different conditions. (a) Pure TiO2 and GO, (b) the mass ration of TiO2 to GO in TiO2/GO nanocomposites, (c) pH value and (d) the amount of TiO2/GO. | ||
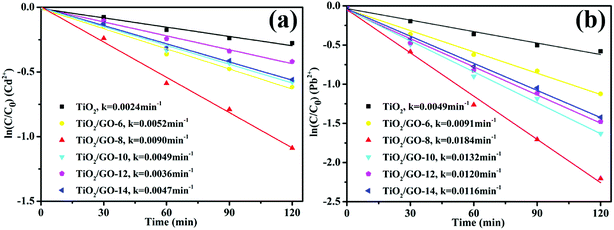
Fig. 10b and 11b illustrate that the photoreduction activities of Cd2+ and Pb2+ obtained at the mass ratio of TiO2 to GO of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 are higher than those obtained at other mass ratios of TiO2 to GO. With the amount of GO increasing, the reaction rates and photoreduction activities of TiO2/GO nanocomposites increase firstly and then decrease. The TiO2/GO-8 nanocomposites displayed the highest photoreduction capability with maximal apparent rate constant k values of 0.0090 (Cd2+) and 0.0184 (Pb2+) min−1. GO created a large surface area to improve the photocatalytic activity of TiO2. When GO was introduced into TiO2, the absorbance edge of TiO2 shifted to the higher wavelength region due to the formation of Ti–O–C bond in TiO2/GO nanocomposites, which helps to enhance the absorption ability of the photocatalysts.21 This indicates that the introduction of appropriate amount of GO has a positive influence on improving photoreduction performance of nanocomposites. However, further increasing the amount of GO lead to a significant decrease of photoreduction activity, probably owning to the reduced catalytic active sites and increased charge carriers recombination rate caused by excessive GO loading. The higher GO content introduced into TiO2 could increase the probability of collision between photogenerated electron–hole pairs, which would increase the charge carrier recombination rate increased from the conduction band to valence band and ultimately lower the photocatalytic performance of the catalyst.32 Besides, in the TiO2/GO-10, TiO2/GO-12 and TiO2/GO-14 nanocomposites, excess black GO would absorb a lot of light, which inhibited the efficient absorption of UV irradiation light by TiO2.48 Therefore, the optimal dosage of GO is 80 mg in the hydro-thermal reaction to prepare TiO2/GO nanocomposites, and the corresponding product (TiO2/GO-8 nanocomposites) presented the best photoreduction activity. It showed a significant synergetic effect of GO with TiO2 in the photoreduction process.
8 are higher than those obtained at other mass ratios of TiO2 to GO. With the amount of GO increasing, the reaction rates and photoreduction activities of TiO2/GO nanocomposites increase firstly and then decrease. The TiO2/GO-8 nanocomposites displayed the highest photoreduction capability with maximal apparent rate constant k values of 0.0090 (Cd2+) and 0.0184 (Pb2+) min−1. GO created a large surface area to improve the photocatalytic activity of TiO2. When GO was introduced into TiO2, the absorbance edge of TiO2 shifted to the higher wavelength region due to the formation of Ti–O–C bond in TiO2/GO nanocomposites, which helps to enhance the absorption ability of the photocatalysts.21 This indicates that the introduction of appropriate amount of GO has a positive influence on improving photoreduction performance of nanocomposites. However, further increasing the amount of GO lead to a significant decrease of photoreduction activity, probably owning to the reduced catalytic active sites and increased charge carriers recombination rate caused by excessive GO loading. The higher GO content introduced into TiO2 could increase the probability of collision between photogenerated electron–hole pairs, which would increase the charge carrier recombination rate increased from the conduction band to valence band and ultimately lower the photocatalytic performance of the catalyst.32 Besides, in the TiO2/GO-10, TiO2/GO-12 and TiO2/GO-14 nanocomposites, excess black GO would absorb a lot of light, which inhibited the efficient absorption of UV irradiation light by TiO2.48 Therefore, the optimal dosage of GO is 80 mg in the hydro-thermal reaction to prepare TiO2/GO nanocomposites, and the corresponding product (TiO2/GO-8 nanocomposites) presented the best photoreduction activity. It showed a significant synergetic effect of GO with TiO2 in the photoreduction process.
Besides, the effect of pH on the reduction of Cd2+ and Pb2+ was shown in Fig. 10c and 11c, respectively. It is obvious that the pH value had a great influence on the removal rate of heavy metal ions. One reason is that the acidity can influence the surface active sites of TiO2/GO nanocomposites. Another reason is that the pH value was related to the state of heavy metal ions in the solution. Clearly, the photoreduction activity of Cd2+ and Pb2+ were both improved by increasing the pH value to 6. However, further increasing the value of pH will bring about a decrease of photoreduction activity, probably owning to that both ions would be hydrolyzed to produce hydroxide precipitation.49 Therefore, the optimum pH is 6 for photoreduce Cd2+ and Pb2+.
As shown in Fig. 10d and 11d, the photoreduction activity of Pb2+ and Cd2+ obtained at the TiO2/GO nanocomposites dosage of 150 mg are higher than those obtained at other photocatalyst dosages. The results may be explained by the surface active sites of TiO2/GO nanocomposites and the phenomenon of light scattering. Higher dosage might cause the aggregation of the photocatalyst, which could reduce the surface active sites of the TiO2/GO nanocomposites. Additionally, the increase of the photocatalyst dosage resulted in the light scattering phenomenon, which could reduce the efficiency of light radiation. Therefore, 150 mg of TiO2/GO nanocomposites exhibited the highest activity in photocatalytic reduction.
4 Conclusion
In this study, GO, TiO2 and TiO2/GO nanocomposites photocatalysts were synthesized by means of Hummers, hydro-thermal and freeze-drying method, respectively. Moreover, the characterization showed that the TiO2/GO nanocomposites had stable structures and good photocatalytic activity. Especially, GO could limit the recombination of photo-generated electron–hole pairs effectively and enhance the photocatalytic activity of TiO2 remarkably. In the photocatalytic reduction experiments, the TiO2/GO nanocomposites showed a higher photocatalytic activity than pure GO and TiO2. TiO2/GO-8 exhibit the highest reaction rate and more than 66.32% Cd2+ and 88.96% Pb2+ were reduced using a 0.6 g L−1 concentration of TiO2/GO nanocomposites at the pH 6 after 120 min irradiation. The combination of GO and TiO2 promoted the reduction of heavy metal ions. In a word, TiO2/GO nanocomposites were a favorable photocatalyst for the reduction of heavy metal ions to reduce their toxicity in ROC. This study provides a feasible method to solve the problem that low concentration heavy metal ions are difficult to reduce in sewage, thereby benefitting the human health, social development, and the reuse of wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province, China (Grant No. ZR2015EL044, ZR2013BL010) and SDUT & Zibo City Integration Development Project (Grant No. 2016ZBXC116).References
- X. Wei, P. Gu and G. Zhang, Desalination, 2014, 352, 18–26 CrossRef CAS.
- J. Lu, L. Fan and F. A. Roddick, Chemosphere, 2013, 93, 683–688 CrossRef CAS PubMed.
- G. Naidu, S. Jeong, Y. Choi and S. Vigneswaran, J. Membr. Sci., 2016, 524, 565–575 CrossRef.
- H. Luo, H. Li, Y. Lu, G. Liu and R. Zhang, Desalination, 2017, 408, 52–59 CrossRef CAS.
- J. O. Tijani, O. O. Fatoba, O. O. Babajide and L. F. Petrik, Environ. Chem. Lett., 2016, 14, 27–49 CrossRef CAS.
- C. Jung, A. Son, N. Her, K. D. Zoh, J. Cho and Y. Yoon, J. Ind. Eng. Chem., 2015, 27, 1–11 CrossRef CAS.
- M. Gmurek, M. Olak-Kucharczyk and S. Ledakowicz, Chem. Eng. J., 2017, 310, 437–456 CrossRef CAS.
- M. Llorca, M. Badia-Fabregat, S. Rodríguez-Mozaz, G. Caminal, T. Vicent and D. Barceló, Chemosphere, 2017, 184, 1054–1070 CrossRef CAS PubMed.
- M. A. P. Cechinel, S. M. A. G. U. D. A. Souza and A. U. D. Souza, J. Cleaner Prod., 2014, 65, 342–349 CrossRef CAS.
- C. F. Carolin, P. S. Kumar, A. Saravanan, G. J. Joshiba and M. Naushad, J. Environ. Chem. Eng., 2017, 5, 2782–2799 CrossRef CAS.
- C. Xiong, W. Wang, F. Tan, F. Luo, J. Chen and X. Qiao, J. Hazard. Mater., 2015, 299, 664–674 CrossRef CAS PubMed.
- R. Daghrir, P. Drogui and D. Robert, Ind. Eng. Chem. Res., 2013, 52, 3581–3599 CrossRef CAS.
- W. K. Jo, S. Kumar, M. A. Isaacs, A. F. Lee and S. Karthikeyan, Appl. Catal., B, 2016, 201, 159–168 CrossRef.
- G. Peng, A. Li, D. D. Sun and W. J. Ng, J. Hazard. Mater., 2014, 279, 96–104 CrossRef PubMed.
- C. Lai, M. M. Wang, G. M. Zeng, Y. G. Liu, D. L. Huang, C. Zhang, R. Z. Wang, P. Xu, M. Cheng, C. Huang, H. P. Wu and L. Qin, Appl. Surf. Sci., 2016, 390, 368–376 CrossRef CAS.
- L. Lin, H. Wang, W. Jiang, A. R. Mkaouar and P. Xu, J. Hazard. Mater., 2017, 333, 162–168 CrossRef CAS PubMed.
- Z. Zhao, J. Sun, S. Xing, D. Liu, G. Zhang, L. Bai and B. Jiang, J. Alloys Compd., 2016, 679, 88–93 CrossRef CAS.
- M. C. Rosu, M. Coros, F. Pogacean, L. Magerusan, C. Socaci, A. Turza and S. Pruneanu, Solid State Sci., 2017, 70, 13–20 CrossRef CAS.
- Q. Deng, C. Chen, Q. Lei, Ji. Liang, T. Zhang and J. Jiang, RSC Adv., 2018, 8, 23382–23389 RSC.
- J. Wang, R. Liu and X. Yin, J. Chem. Eng., 2018, 63, 409–416 CrossRef CAS.
- R. Atchudan, T. Edison, S. Perumal, D. Karthikeyan and Y. Lee, J. Photochem. Photobiol., A, 2017, 333, 92–104 CrossRef CAS.
- J. S. Lee, K. H. You and C. B. Park, Adv. Mater., 2012, 24, 1084–1088 CrossRef CAS PubMed.
- Y. Sang, Z. Zhao, J. Tian, P. Hao, H. Jiang, H. Liu and J. P. Claverie, Small, 2014, 10, 3775–3782 CrossRef CAS PubMed.
- L. Fan, X. Li, X. Song, N. Hu, D. Xiong, A. Koo and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 2637–2648 CrossRef CAS PubMed.
- X. Song, X. Li, Z. Bai, B. Yan, D. Xiong, L. Lin, H. Zhao, D. Li and Y. Shao, Carbon, 2018, 133, 14–22 CrossRef CAS.
- D. Xiong, X. Li, Z. Bai and S. Lu, Small, 2018, 14, 1703419 CrossRef PubMed.
- W. Peng, H. Li, Y. Liu and S. Song, J. Mol. Liq., 2017, 230, 496–504 CrossRef CAS.
- Z. Li, F. Chen, L. Yuan, Y. Liu, Y. Zhao, Z. Chai and W. Shi, Chem. Eng. J., 2012, 210, 539–546 CrossRef CAS.
- L. Cui, Y. Wang, L. Gao, L. Hu, L. Yan, Q. Wei and B. Du, Chem. Eng. J., 2015, 281, 1–10 CrossRef CAS.
- F. Fang, L. Kong, J. Huang, S. Wu, K. Zhang, X. Wang, B. Sun, Z. Jin, J. Wang, X. Huang and J. Liu, J. Hazard. Mater., 2014, 270, 1–10 CrossRef CAS PubMed.
- R. Raliya, C. Avery, S. Chakrabarti and P. Biswas, Appl. Nanosci., 2017, 7, 253–259 CrossRef CAS.
- H. Yadav and J. Kim, J. Alloys Compd., 2016, 688, 123–129 CrossRef CAS.
- V. Bhatia, G. Malekshoar, A. Dhir and A. Ray, J. Photochem. Photobiol., A, 2017, 332, 182–187 CrossRef CAS.
- M. Nawaz, W. Miran, J. Jang and D. S. Lee, Appl. Catal., B, 2017, 203, 85–95 CrossRef CAS.
- Z. Zhang, J. Zhao, J. Zhou, Y. Zhao, X. Tang and S. Zhuo, Energy Storage Mater., 2017, 8, 35–41 CrossRef.
- W. Liu, J. Cai, Z. Ding and Z. Li, Appl. Catal., B, 2015, 174–175, 421–426 CrossRef CAS.
- J. Ge, Y. Ping, G. Liu, G. Qiao, E. J. Kim and M. Wang, Mater. Lett., 2016, 181, 216–219 CrossRef CAS.
- P. Gao, Z. Liu, M. Tai, D. D. Sun and W. Ng, Appl. Catal., B, 2013, 138, 17–25 CrossRef.
- B. Qiu, Y. Zhou, Y. Ma, X. Yang, W. Sheng, M. Xing and J. Zhang, Sci. Rep., 2015, 5, 8591–8596 CrossRef CAS PubMed.
- J. Hu, H. Li, Q. Wu, Y. Zhao and Q. Jiao, Chem. Eng. J., 2015, 263, 144–150 CrossRef CAS.
- J. Fu, G. Kyzas, Z. Cai, E. Deliyanni, W. Liu and D. Zhao, Chem. Eng. J., 2018, 335, 290–300 CrossRef CAS.
- W. Tang, G. Zeng, J. Gong, J. Liang, P. Xu, C. Zhang and B. Huang, Sci. Total Environ., 2014, 468–469, 1014–1027 CrossRef CAS PubMed.
- R. Atchudan and A. Pandurangan, Microporous Mesoporous Mater., 2013, 167, 162–175 CrossRef CAS.
- W. Yang, Y. Li and Y. Lee, Appl. Surf. Sci., 2016, 380, 249–256 CrossRef CAS.
- Y. Yang, L. Luo, M. Xiao, H. Li, X. Pan and J. Fang, Mater. Sci. Semicond. Process., 2015, 40, 183–193 CrossRef CAS.
- J. Henych, V. Štengl, A. Mattsson, J. Tolasz and L. Österlund, J. Hazard. Mater., 2018, 359, 482–490 CrossRef CAS PubMed.
- W. Peng, H. Li, Y. Liu and S. Song, J. Mol. Liq., 2017, 230, 496–504 CrossRef CAS.
- Y. Zhu, Y. Wang, W. Yao, R. Zong and Y. Zhu, RSC Adv., 2015, 5, 29201–29208 RSC.
- J. Zhao, Y. Niu, R. Ben, H. Chen, S. Zhang, J. Jin and Y. Zhang, Chem. Eng. J., 2018, 347, 574–584 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |