Open Access Article
Open Access ArticleMulticomponent synthesis of pyrano[2,3-c]coumarins†
Wan-Chi Hsieh,
Chi-Hui Lin,
Yu-Ju Yang and
Ding-Yah Yang *
*
Department of Chemistry, Tunghai University, No. 1727, Sec. 4, Taiwan Boulevard, Xitun District, Taichung 40704, Taiwan. E-mail: yang@thu.edu.tw; Fax: 886-4-2359-0426; Tel: +886-4-2359-7613
First published on 22nd November 2018
Abstract
A base-catalyzed, pseudo-four-component reaction of 4-hydroxycoumarin, two molecules of acetone, and amine towards the synthesis of pyrano[2,3-c]coumarins is reported. The mechanism of this multicomponent reaction is proposed. The reaction is further extended to the preparation of coumarin-substituted pyrano[2,3-c]coumarins by a base-catalyzed, pseudo four-component reaction of two molecules of 4-hydroxycoumarin and two molecules of acetone.
Introduction
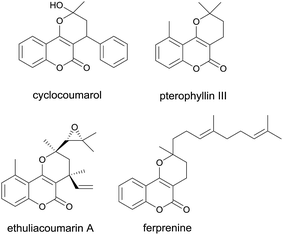
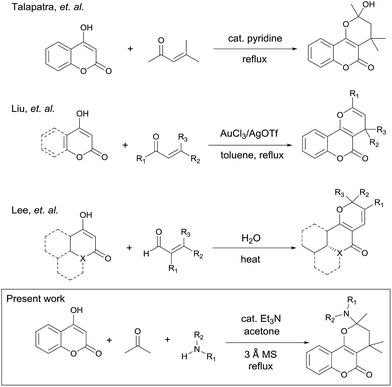
Coumarins and pyrans are privileged chemical entities in most biologically important heterocycles.1 When coumarins and pyrans are fused together in a molecule, the resulting pyranocoumarins typically exhibit more effective properties than when they are alone.2 In particular, the angularly fused pyrano[3,2-c]coumarins represent an indispensable and integral part of a variety of biologically active compounds. They have been shown to possess a wide range of biological activities including antibacterial,3a antifungal,3b anticancer,3c anti-inflammatory, and antioxidants activities.3d For instance, cyclocoumarol4 is a semiacetalic form of warfarin was recently found to inhibit the cyclooxygenase-2 (Fig. 1).4a Pterophyllin III (ref. 5a and b) possesses inhibitory activity against the proliferation of human lymphocytes.5a Ethuliacoumarin A is one of the important examples of pyranocoumarins which shows the anticoagulant effects and interferes with the life cycle of parasitic trematodes.6 Ferprenine7 is an effective inhibitor of vitamin K epoxide reductase complex subunit 1 that helps in clotting. In light of their associated broad ranges of pharmacological activities, the synthesis of pyrano[3,2-c]coumarins has attracted considerable attention of organic and medicinal chemists. One of the earlier preparations of pyrano[3,2-c]coumarins was reported by Talapatra8 in 1984. It involved the condensation of 4-hydroxycoumarin (1a) with mesityl oxide to afford the hemiketal 2a, as shown in Scheme 1. Subsequently, Liu9 has shown that pyrano[3,2-c]coumarins can be prepared by gold(III)-catalyzed tandem conjugate addition of 4-hydroxycoumarins with α,β-unsaturated ketones. Recently, Lee10 has demonstrated that substituted pyranocoumarins can be effectively prepared by coupling of 4-hydroxycoumarins with α,β-unsaturated aldehydes in water medium.Among various synthetic approaches to these compounds, few utilized multicomponent reactions (MCRs) of readily accessible starting materials. Further, most preparations of pyrano[3,2-c]coumarins employed reagents or catalysts that are either expensive or less environmentally benign.11 Thus, the search for a cost-effective and simple protocol for the synthesis of pyrano[3,2-c]coumarins remains desirable. In our continuing effort to develop new MCRs for the preparation of the coumarin-based heterocycles12 to investigate their potential biological or functional properties, here we report the efficient synthesis of pyrano[3,2-c]coumarins via a base-catalyzed, pseudo four-component reaction of 4-hydroxycoumarin, acetone, and amine, as well as a base-catalyzed, pseudo four-component reaction of 4-hydroxycoumarin and acetone. A plausible mechanism is also proposed on the basis of the experimental results.
Results and discussion
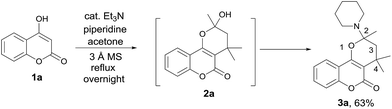
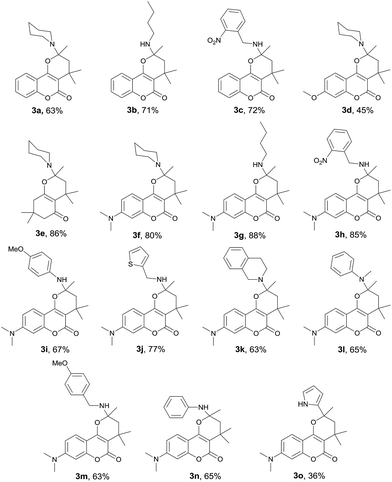
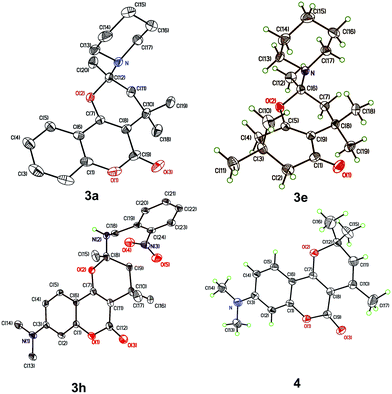
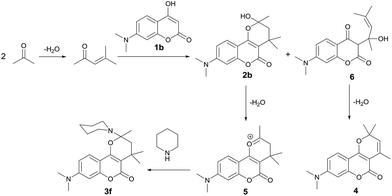
To realize the preparation of pyrano[2,3-c]coumarins via a MCR manner, we speculated that the common substrate mesityl oxide (4-methylpent-3-en-2-one) employed by Talapatra and Lee can be generated in situ by a base-catalyzed aldol condensation of two molecules of acetone. Thus, by heating of 4-hydroxycoumarin (1a) in acetone in the presence of trimethylamine as a base, we expected that hemiketal (2a) may be obtained in a pseudo-three-component reaction. The resulting hemiketal (2a) can presumably undergo dehydration to generate the oxonium ion which may be trapped by an external nucleophile such as an amine to afford a nitrogen-containing heterocycle. To test the aforementioned hypothesis, we initiated our studies by refluxing of 4-hydroxycoumarin (1a) and piperidine in the presence of a catalytic amount of trimethylamine and some 3 Å molecular sieves in acetone overnight, as shown in Scheme 2. To our delight, a pseudo-four-component product, that is, pyrano[2,3-c]coumarin 3a was obtained in 63% yield. Further studies indicate that compound 3 can be readily accessed by refluxing 4-hydroxycoumarin, a primary or secondary amine, and a catalytic amount of trimethylamine in acetone overnight.Fig. 2 lists the structures and yields of the synthesized pyranocoumarins 3a–o. The molecular structures of 3a–o were elucidated by 1H and 13C NMR spectroscopy. In proton NMR spectra, a characteristic AB quartet absorption peaks appeared at a chemical shift of 2.39–2.03 and 2.12–1.55 ppm were assigned to the two methylene hydrogens on C-3 position (see compound 3a in Scheme 2 for atom-numbering). The molecular structures of 3a, 3e, and 3h were further verified by X-ray crystallography13 as depicted in Fig. 3. Our studies indicate that 4-hydroxycoumarin substrate with an electron-donating substituent generally gave a better yield than that of an electron-withdrawing one. As for amine substrates, the primary amine normally gave higher yields than the secondary amines and anilines. When the amine was replaced with pyrrole, the corresponding pyranocoumarin 3o was obtained, although with a lower yield. No expected product was detected when the amine was replaced with either furan, thiophene or phenol. For most of the reactions, the formation of a trace by-product was constantly observed. This by-product was isolated and subsequently identified to be 8-(dimethylamino)-2,2,4-tri methyl-2H,5H-pyrano[3,2-c]chromen-5-one (4) whose X-ray crystal structure is also shown in Fig. 3.13 Isolation of the compound 4 provides crucial insights into the mechanism of this multicomponent reaction.
Scheme 3 depicts a plausible mechanism of this pseudo-four-component synthesis of the pyrano[2,3-c]coumarin 3f. It starts with the base-catalyzed aldol condensation of two molecules of acetone to yield the 4-methylpent-3-en-2-one. The subsequent conjugate addition of 7-N,N-dimethylamino-4-hydroxycoumarin (1b) to 4-methylpent-3-en-2-one yields the major hemiketal 2b. Dehydration of 2b gives the reactive oxonium ion 5 which is then trapped by piperidine to furnish the final product 3f. Alternatively, the simple addition of 4-hydroxycoumarin to 4-methylpent-3-en-2-one yields the minor tertiary alcohol 6, which further undergoes dehydration and follows by electrocyclic reaction to give the by-product 4. Since the ketone substrate of this MCR is limited to acetone only, few positions on pyran moiety of the pyrano[2,3-c]coumarins are available for variation. Nevertheless, the bond formation during the construction of the pyranocoumarin scaffold in this pseudo-four-component reaction is highly atom-economical, generating two C–C bonds, one C–O bond, and one C–N bond in the final product.
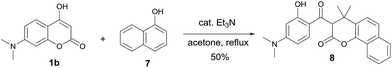
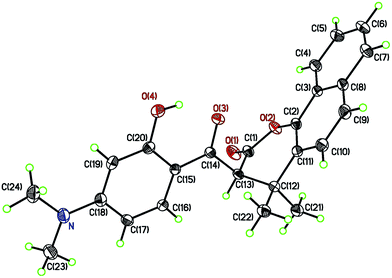
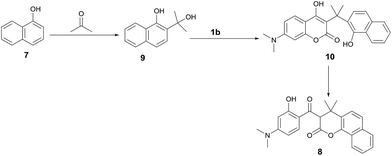
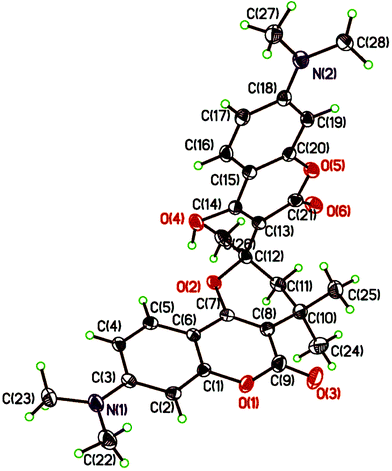
In an effort to further explore the scope of this pseudo-four-component reaction, the amine was replaced with 1-naphthalenol (7) in expectation to obtain a different product. Under these reaction conditions, the isolated compound was found to be the β-keto ester 8 (Scheme 4). The molecular structure of 8 was confirmed by the X-ray crystal analysis as shown in Fig. 4.13 The crystal structure and 1H NMR spectrum of β-keto ester 8 indicates that it exists exclusively in the keto form in both solid state and solution, and the two keto groups are almost orthogonal to each other. Scheme 5 depicts the proposed mechanism for the formation of 8. It presumably involves the first coupling of 1-naphthalenol (7) with acetone to give the alcohol 9. The subsequent condensation of 9 with 7-N,N-dimethylamino-4-hydroxycoumarin (1b) affords the intermediate 10. Final intramolecular ring-opening of the coumarin lactone ring of 10 by the nearby 1-naphthalenol hydroxyl group furnishes the final product β-keto ester 8.
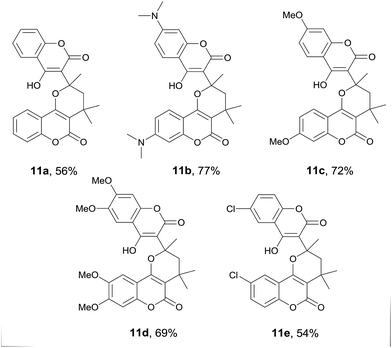
Interestingly, when 4-hydroxycoumarin (1a) was refluxed in acetone for 24 hours in the absence of any external nucleophile such as a primary amine, the major product obtained was found to be the pyrano[2,3-c]coumarin 11a. Fig. 5 lists the structures and yields of the prepared pyranocoumarins 11a–e. Similar to pyranocoumarins 3, the 4-hydroxycoumarin substrate with an electron-donating substituent generally gave a higher yield than that of an electron-withdrawing one. A representative X-ray crystal structure of 11b is shown in Fig. 6.13 Apparently, the proposed intermediate 5 (Scheme 3) generated in situ in the reaction was trapped by a second molecule of 4-hydroxycoumarin to give the observed product. This pseudo-four-component reaction of two molecules of 4-hydroxycoumarin and two molecules of acetone provides quick access to coumarin-substituted pyrancoumarins with extreme simplicity and high atom economy, forming three C–C bonds and one C–O bond in the final product.
Conclusions
In summary, we have demonstrated that pyrano[2,3-c]coumarins 3a–o can be efficiently synthesized via pseudo-four-component reaction of 4-hydroxycoumarin, two molecules of acetone, and a primary or secondary amine in the presence of a catalytic amount of trimethylamine as a base in acetone under refluxed conditions. Moreover, the coumarin-substituted pyrano[2,3-c]coumarins 11a–e can be readily constructed in good to excellent yields via pseudo-four-component reaction of two molecules of 4-hydroxycoumarin and two molecules of acetone in acetone under refluxed conditions.Experimental
4.1. General
Melting points were determined on a Mel-Temp melting point apparatus in open capillaries and are uncorrected. Infrared (IR) spectra were recorded using 1725XFT-IR spectrophotometer. High resolution mass spectra (HRMS) were obtained on a Thermo Fisher Scientific Finnigan MAT95XL spectrometer using magnetic sector analyzer 1H NMR (300 or 400 MHz) and 13C NMR (75 or 100 MHz) spectra were recorded on a Varian Unity 300 or Bruker 400 spectrometer. Chemical shifts were reported in parts per million on the δ scale relative to an internal standard (tetramethylsilane, or appropriate solvent peaks) with coupling constants given in hertz. 1H NMR multiplicity data are denoted by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel 60G-254 plates (25 mm) and developed with the solvents mentioned. Visualization was accomplished by using portable UV light, ninhydrin spray, or iodine chamber. Flash chromatography was performed in columns of various diameters with Merck silica gel (230–400 mesh ASTM 9385 kieselgel 60H) by elution with the solvent systems. Solvents, unless otherwise specified, were reagent grade and distilled once prior to use. All new compounds exhibited satisfactory spectroscopic and analytical data.4.2. General procedure for the synthesis of 3
To a mixture of 4-hydroxycoumarin (1.00 mmol, 1 equiv.) in acetone (10 mL) was added amine (2 equiv.), Et3N (0.5 equiv.), and 3 Å molecular sieves (3.0 g) at room temperature. The resulting mixture was then refluxed overnight. The progress of the reaction was monitored by TLC. After cooled down to room temperature, the mixture was filtered, and the filtrate was concentrated in vacuo. The residue was re-dissolved in water (30 mL) and the product was extracted by DCM (15 mL × 3). The combined organic layer was then dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography to give the desired compound 3.4.3. 8-(Dimethylamino)-2,2,4-trimethylpyrano[3,2-c]chromen-5(2H)-one (4)
Rf = 0.8 (30% EtOAc/hexanes); yellow solid; 1H NMR (CDCl3, 300 MHz) δ 7.61 (d, J = 9.0 Hz, 1H), 6.59 (dd, J = 9.0, 2.4 Hz, 1H), 6.46 (d, J = 2.4 Hz, 1H), 5.13 (q, J = 1.5 Hz, 1H), 3.05 (s, 6H), 2.20 (d, J = 1.5 Hz, 3H), 1.46 (s, 6H); HRMS (EI) m/z calcd for C17H19NO3 [M+] 285.1365 found 285.1367.4.4. 3-(4-(Dimethylamino)-2-hydroxybenzoyl)-4,4-dimethyl-3,4-dihydro-2H-benzo[h]chromen-2-one (8)
Rf = 0.5 (30% EtOAc/hexanes); light pink solid; 193 mg; yield 50%; mp 103–104 °C; 1H NMR (CDCl3, 400 MHz) δ 12.50 (s, 1H), 8.31 (dd, J = 8.8, 1.2 Hz, 1H), 7.84 (dd, J = 8.8, 1.2 Hz, 1H), 7.69 (d, J = 8.8 Hz, 1H), 7.66 (d, J = 8.8 Hz, 1H), 7.58–7.50 (m, 2H), 7.42 (d, J = 8.8 Hz, 1H), 6.25 (dd, J = 9.2, 2.4 Hz, 1H), 6.06 (d, J = 2.4 Hz, 1H), 4.62 (s, 1H), 3.06 (s, 6H), 1.54 (s, 3H), 1.49 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 194.1, 165.8, 165.6, 156.3, 145.2, 133.4, 132.4, 127.5, 126.7, 126.5, 125.3, 124.6, 123.4, 121.4, 121.2, 110.5, 104.5, 97.9, 56.1, 40.0, 37.8, 29.5, 24.2; IR ν (KBr) 2925, 1749, 1627, 1529, 1364, 1268, 1218, 1148, 812 cm−1; HRMS (EI) m/z calcd for C24H23NO4 [M+] 389.1627 found 389.1633.4.5. General procedure for the synthesis of 11
To a mixture of 4-hydroxycoumarin (2.00 mmol, 1 equiv.) in acetone (20 mL) was add Et3N (0.25 equiv.) and 3 Å molecular sieves (3.0 g) at room temperature. The resulting mixture was refluxed in acetone for 24 h. After cooled down to room temperature, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was re-dissolved in water (30 mL) and the product was extracted by DCM (15 mL × 3). The combined organic layer was then dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography to give the desired compound 11.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under Grant No. MOST 107-2113-M-029-002 and MOST 106-2632-M-029-001.Notes and references
- G. Zhang, Y. Zhang, J. Yan, R. Chen, S. Wang, Y. Ma and R. Wang, J. Org. Chem., 2012, 77, 878–888 CrossRef CAS PubMed.
- (a) D. P. Chakraborty, A. Das Gupta and P. K. Bose, Ann. Biochem. Exp. Med., 1957, 17, 59–62 CAS; (b) D. E. Galinis, R. W. Fuller, T. C. McKee, J. H. Cardellina, R. J. Gulakowski, J. B. McMahon and M. R. Boyd, J. Med. Chem., 1996, 39, 4507–4510 CrossRef CAS PubMed; (c) L. Xie, Y. Takeuchi, L. M. Cosentino, A. T. McPhail and K. H. Lee, J. Med. Chem., 2001, 44, 664–671 CrossRef CAS PubMed; (d) R. S. Mali, P. P. Joshi, P. K. Sandhu and A. Manekar-Tilve, J. Chem. Soc., Perkin Trans. 1, 2002, 1, 371–376 RSC; (e) D. N. Nicolaides, D. R. Gautam, K. E. Litinas, D. J. Hadjipavlou-Litina and K. C. Flyakakidou, Eur. J. Med. Chem., 2004, 39, 323–332 CrossRef CAS PubMed.
- (a) M. A. Musa, J. S. Cooperwood and M. O. F. Khan, Curr. Med. Chem., 2008, 15, 2664–2679 CrossRef CAS PubMed; (b) P. C. B. Page, L. F. Appleby, D. Day, Y. Chan, B. R. Buckley, S. M. Allin and M. J. McKenzie, Org. Lett., 2009, 11, 1991–1993 CrossRef PubMed; (c) A. Lacy and R. O'Kennedy, Curr. Pharm. Des., 2004, 10, 3797–3811 CrossRef CAS PubMed; (d) E. Melliou, P. Magiatis, S. Mitaku, A.-L. Skaltsounis, E. Chinou and I. Chinou, J. Nat. Prod., 2005, 68, 78–82 CrossRef CAS PubMed.
- (a) G. Cravotto, G. M. Nano, S. Tagliapietra, G. Palmisano and T. Pilati, J. Heterocycl. Chem., 2001, 38, 965–971 CrossRef CAS; (b) A. M. Rayar, N. Lagarde, F. Martin, F. Blanchard, B. Liagre, C. Ferroud, J.-F. Zagury, M. Montes and M. S.-I. Veitía, Eur. J. Med. Chem., 2018, 146, 577–587 CrossRef CAS PubMed.
- (a) M. Ikawa, M. A. Stahmann and K. P. Link, J. Am. Chem. Soc., 1944, 66, 902–906 CrossRef CAS; (b) G. Appendino, G. Cravotto, G. B. Giovenzana and G. Palmisano, J. Nat. Prod., 1999, 62, 1627–1631 CrossRef CAS; (c) H. A. Oketch-Rabah, E. Lemmich, S. F. Dossaji, T. G. Theander, C. E. Olsen, C. Cornett, A. Kharazmi and S. B. Christensen, J. Nat. Prod., 1997, 60, 458–461 CrossRef CAS PubMed.
- M. M. Kady, L. Brimer, P. Furu, E. Lemmich, H. M. Nielsen, S. T. Thiilborg, O. Thastrup and S. B. Christensen, Planta Med., 1992, 58, 334–337 CrossRef CAS PubMed.
- (a) M. Aragno, S. Tagliapietra, G. M. Nano and G. Ugazio, Res. Commun. Chem. Pathol. Pharmacol., 1988, 59, 399–402 CAS; (b) M.-S. Louvet, G. Gault, S. Lefebvre, F. Popowycz, M. Boulven, S. Besse, E. Benoit, V. Lattard and D. Grancher, Phytochemistry, 2015, 118, 124–130 CrossRef CAS PubMed.
- (a) B. Talapatra, S. K. Mandal, K. Biswas, R. Chakrabarti and S. K. Talapatra, J. Indian Chem. Soc., 2001, 78, 765–771 CAS; (b) S. K. Talapatra, R. Chakrabarti, P. K. Mukhopadhyay, P. K. Das and B. Talapatra, Heterocycles, 1984, 22, 519–526 CrossRef CAS.
- Y. Liu, J. Zhu, J. Qian, B. Jiang and Z. Xu, J. Org. Chem., 2011, 76, 9096–9101 CrossRef CAS PubMed.
- E. J. Jung, B. H. Park and Y. R. Lee, Green Chem., 2010, 12, 2003–2011 RSC.
- (a) R. Sarma, M. M. Sarmah, K. C. Lekhok and D. Prajapati, Synlett, 2010, 19, 2847–2852 Search PubMed; (b) S. Ahadi, M. Zolghadr, H. R. Khavasi and A. Bazgir, Org. Biomol. Chem., 2013, 11, 279–286 RSC.
- (a) J.-J. Chen, K.-T. Li and D.-Y. Yang, Org. Lett., 2011, 13, 1658–1661 CrossRef CAS PubMed; (b) K.-T. Li, Y.-B. Lin and D.-Y. Yang, Org. Lett., 2012, 14, 1190–1193 CrossRef CAS PubMed; (c) C.-H. Lin and D.-Y. Yang, Org. Lett., 2013, 15, 2802–2805 CrossRef CAS PubMed; (d) W.-C. Lin and D.-Y. Yang, J. Org. Chem., 2013, 78, 11798–11806 CrossRef CAS PubMed.
- Crystallographic data (excluding structure factors) for 3a, 3e, 3h, 4, 8, and 11b have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC-1853612, -1846223, -1846224, -1846226, -1853613, and -1846225, respectively.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1853612, 1853613 and 1846223–1846226. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra06666c |
| This journal is © The Royal Society of Chemistry 2018 |