Open Access Article
Open Access ArticleSynthesis, structure, and luminescence characteristics of far-red emitting Mn4+-activated LaScO3 perovskite phosphors for plant growth
Liangling Sun,
Balaji Devakumar,
Heng Guo ,
Jia Liang,
Bin Li,
Shaoying Wang,
Qi Sun and
Xiaoyong Huang
,
Jia Liang,
Bin Li,
Shaoying Wang,
Qi Sun and
Xiaoyong Huang *
*
College of Physics and Optoelectronics, Taiyuan University of Technology, Taiyuan 030024, PR China. E-mail: huangxy04@126.com
First published on 25th September 2018
Abstract
Far-red emitting phosphors LaScO3:Mn4+ were successfully synthesized via a high-temperature solid-state reaction method. The X-ray powder diffraction confirmed that the pure-phase LaScO3:Mn4+ phosphors had formed. Under 398 nm excitation, the LaScO3:Mn4+ phosphors emitted far red light within the range of 650–800 nm peaking at 703 nm (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 cm−1) due to the 2Eg → 4A2g transition, which was close to the spectral absorption center of phytochrome PFR located at around 730 nm. The optimal doping concentration and luminescence concentration quenching mechanism of LaScO3:Mn4+ phosphors was found to be 0.001 and electric dipole–dipole interaction, respectively. And the CIE chromaticity coordinates of the LaScO3:0.001Mn4+ phosphor were (0.7324, 0.2676). The decay lifetimes of the LaScO3:Mn4+ phosphors gradually decreased from 0.149 to 0.126 ms when the Mn4+ doping concentration increased from 0.05 to 0.9 mol%. Crystal field analysis showed that the Mn4+ ions experienced a strong crystal field in the LaScO3 host. The research conducted on the LaScO3:Mn4+ phosphors illustrated their potential application in plant lighting to control or regulate plant growth.
225 cm−1) due to the 2Eg → 4A2g transition, which was close to the spectral absorption center of phytochrome PFR located at around 730 nm. The optimal doping concentration and luminescence concentration quenching mechanism of LaScO3:Mn4+ phosphors was found to be 0.001 and electric dipole–dipole interaction, respectively. And the CIE chromaticity coordinates of the LaScO3:0.001Mn4+ phosphor were (0.7324, 0.2676). The decay lifetimes of the LaScO3:Mn4+ phosphors gradually decreased from 0.149 to 0.126 ms when the Mn4+ doping concentration increased from 0.05 to 0.9 mol%. Crystal field analysis showed that the Mn4+ ions experienced a strong crystal field in the LaScO3 host. The research conducted on the LaScO3:Mn4+ phosphors illustrated their potential application in plant lighting to control or regulate plant growth.
Introduction
Nowadays, to meet the continuous increasing needs of people, the greenhouse industry has been developed. In the agriculture field, when it comes to conditions for plant growth, moderate sunlight, air, and moderate moisture are usually mentioned as the basic conditions.1–4 The blue (≈400–500 nm), red (≈600–690 nm) and far red (≈700–740 nm) light in sunlight can affect the growth process of natural plants, such as phototropic processes and photomorphogenesis.5–7 There exist two kinds of phytochrome, PR and PFR, and PR is the biologically inactive state, whereas PFR is the biologically active state.4,8 These two phytochromes can be converted into each other by absorbing different wavelengths of light, in which the PR was sensitive to red light and can turn into PFR by absorbing light with wavelength peaking at around 660 nm, whereas PFR should absorb far red light peaking at around 730 nm to switch to PR.9,10 It is known that in order to blossom, short-day plants need to stay in the dark for a longer time than long-day plants.11–13 So plant growth progress can be controlled by changing the spectral composition in artificial light.The proportion of red light in the natural sunlight is higher than far red light.9 Meanwhile, with the development of science and technology in the world, the far red light become even less due to the night lighting, which means the far-red light is insufficient for plant cultivation and even influence the entire life style of the plant.4 Thus, finding artificial light to meet the requirement of plant growth is urgent, especially in the greenhouse industry. In the past, the light emitted by traditional gas-discharge lamps cannot match well with the absorption spectrum of phytochrome, especially the PFR. But the solid state light-emitting diodes (LEDs) device with long lifetime and low power consumption,14–21 which can exhibit various colors by coating different phosphors onto the blue/near-ultraviolet LED chip,22–30 can make up for this drawback. That means the light from the specific LEDs can match well with the absorption spectrum of phytochrome. Many research efforts have already been conducted to develop the red phosphors, such as K2NaAlF6:Mn4+ (630 nm),31 Na3MgZr(PO4)3:Eu3+ (611 nm),32 and Sr2MgAl22O36:Mn4+ (658 nm),33 which emit red light in the range of 610–660 nm. In contrast, relatively less attention has been paid to the far-red phosphors with emission wavelength within 660–730 nm, which can be used for the plant growth. So it is important to find novel phosphors that can emit far-red light to meet the requirement of far-red light.
Mn4+ ions in particular host materials with octahedral structures can emit red light and even deep red light due to the 2Eg → 4A2g transition,34–37 such as Sr4Al14O25:Mn4+ (652 nm),38 NaMgGdTeO6:Mn4+ (697 nm),39 Ca3La2W2O12:Mn4+ (711 nm),40 La2MTiO6:Mn4+ (M = Mg and Zn; 710 nm),41 and Ba2GeO4:Mn4+ (667 nm).42 Considering that the structure of the LaScO3 compound with [ScO6] octahedral structure is similar to LaAlO3 and Mn4+-doped LaAlO3 phosphors can emit far-red emission, so the LaScO3 compound has been chosen as the host for Mn4+ doping.43,44 Thus, in this work, LaScO3 and Mn4+ ions had been chosen for the host material and activator, respectively, and a series of LaScO3:xMn4+ (x = 0.0005–0.0090) phosphors with different Mn4+ doping concentration had been synthesized. These prepared phosphors can be well excited by 398 nm and emit far-red light peaking at 703 nm with CIE chromaticity coordinates of (0.7324, 0.2676). The crystal structure of the LaScO3, decay lifetimes and luminescence properties had also been investigated in detail, and the results indicated the LaScO3:Mn4+ phosphors could be serve as the far-red emitting phosphor in plant growth LEDs.
Experimental section
LaScO3:xMn4+ (x = 0.0005–0.009) phosphors were prepared by a facile solid-state reaction method using Sc2O3 (analytical reagent, AR), MnCO3 (AR), and La2O3 (99.99%) as the starting materials. The raw materials were weighed according to the stoichiometric ratio. The mixed starting materials was ground in an agate mortar and transferred to Al2O3 crucible to sinter at 1500 °C for 10 h in air. And then, the products were cooled down to room temperature and reground into powders to get the target phosphors LaScO3:xMn4+.The X-ray powder diffraction (XRD) results of LaScO3:xMn4+ phosphors were measured on an X-ray diffractometer (Bruker D8 Advance) with Cu-Kα radiation. The room-temperature photoluminescence (PL)/PL excitation (PLE) spectra and luminescence decay lifetimes of LaScO3:xMn4+ phosphors were recorded by using the same Edinburgh FS5 spectrofluorometer, equipping with a 150 W continued-wavelength Xenon lamp and a pulsed Xenon lamp, respectively. The internal quantum efficiency (IQE) of the LaScO3:0.001Mn4+ phosphors were measured by the Edinburgh FS5 spectrofluorometer with a integrating sphere.
Results and discussion
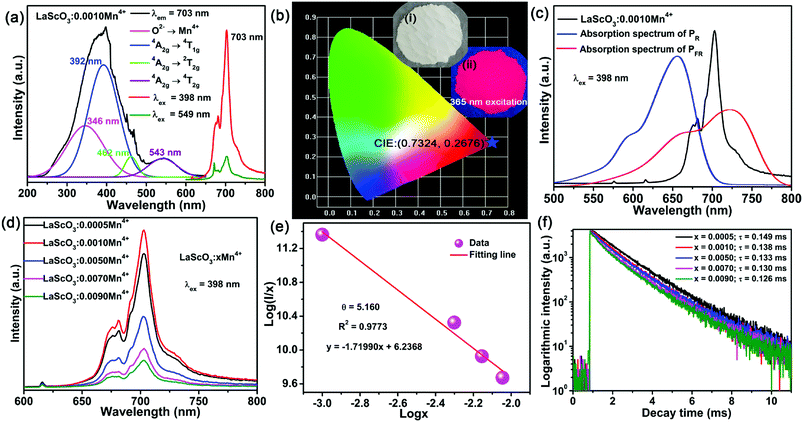
Fig. 1 showed the XRD patterns of the LaScO3:xMn4+ (x = 0, 0.0005, 0.001, and 0.005) phosphors and the standard data of LaScO3 (JCPDS: 26-1148). It could be seen that all the diffraction peaks of the samples matched well with the standard data of LaScO3. There was no excess crystal phase formation, which illustrated the Mn4+ ions could be successfully doped into the LaScO3 host. Mn4+ ions can replace specific cationic sites of the specific host, where cationic sites have a coordination number (CN) of six, such as Nb5+, Zr4+, Ti4+, Te6+, and W6+ ions.39,40,45–48 Considering the cationic radius and the coordination environment of Mn4+ (r = 0.535 Å, CN = 6) and Sc3+ (r = 0.745 Å, CN = 6),43,49,50 the Mn4+ ions could replace the Sc3+ sites in the LaScO3 host.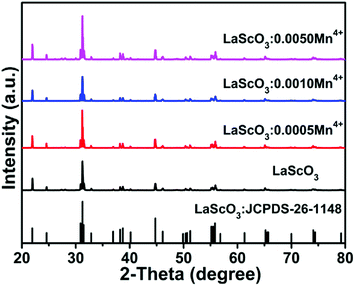 | ||
| Fig. 1 XRD patterns of LaScO3:xMn4+ (x = 0, 0.0005, 0.001, and 0.005) phosphors and the stand pattern of LaScO3 (JCPDS: 26–1148). | ||
In order to identify the structure of the as-prepared sample, the Rietveld refinement for the LaScO3 host and LaScO3:0.001Mn4+ phosphors were conducted. Fig. 2(a) and (b) depict the Rietveld refinement results of LaScO3 host and LaScO3:0.001Mn4+, respectively. From the values of Rp and Rwp (Rp = 8.13% and Rwp = 7.55% for LaScO3 host; Rp = 4.86% and Rwp = 3.50% for LaScO3:0.001Mn4+ phosphors), we could know that the obtained results were reliable. The refined crystallographic parameters of the LaScO3 host and LaScO3:0.001Mn4+ phosphors were listed in Table 1 and Table 2, respectively. It could be seen that the parameters of LaScO3 host and LaScO3:0.001Mn4+ phosphors changed slightly due to the difference in ions radii between Sc3+ and Mn4+ ions. The crystal systems of the LaScO3 host and LaScO3:0.001Mn4+ phosphors were orthorhombic with a space group of Pbnm. Fig. 2(c) and (d) show the crystal structure of the LaScO3 host and the [ScO6] octahedron, respectively. In the unit cell, one Sc3+ ion was surrounded by six O2− ions to form [ScO6] octahedral units and the Mn4+ ions could substitute for Sc3+ ions to form the LaScO3:Mn4+ compound. All the results illustrated the formation of LaScO3:Mn4+ phosphors.
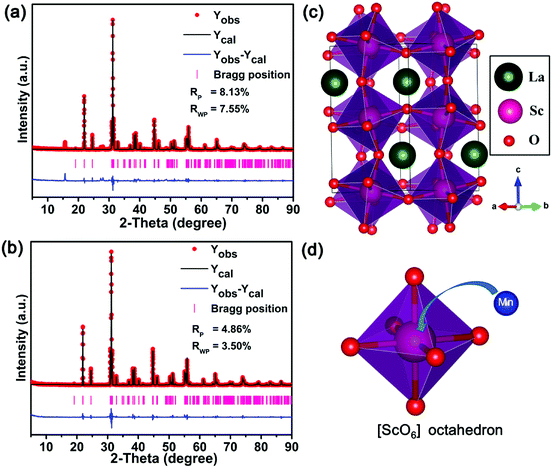 | ||
| Fig. 2 Rietveld refinement of LaScO3 host (a) and LaScO3:0.001Mn4+ (b) using the Fullprof suit software. The structure of (c) LaScO3 host and (d) [ScO6] octahedron. | ||
| Formula | LaScO3 |
| Crystal system | Orthorhombic |
| Space group | pbnm |
| Lattice parameters | a = 5.68010(17) Å, b = 5.79022(16) Å, c = 8.0952(2) Å, V = 266.244(13) Å3 |
| Atom | x | y | z | Occ. | Uaniso (Å2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| U11 | U22 | U33 | U12 | U13 | U23 | |||||
| La1 | 0.01060 | 0.95680 | 0.25 | 1 | 0.01218 | 0.00649 | −0.00259 | −0.00656 | 0.00000 | 0.00000 |
| Sc1 | 0.00000 | 0.5 | 0.00000 | 1 | 0.00448 | −0.00362 | 0.00289 | −0.00681 | −0.00587 | −0.00140 |
| O1 | 0.71276 | 0.28618 | 0.01023 | 1 | −0.05698 | 0.04639 | −0.04425 | −0.01988 | 0.00000 | 0.00000 |
| O2 | 0.90808 | 0.52065 | −0.01828 | 1 | 0.04604 | −0.02305 | 0.00770 | 0.02371 | 0.02416 | 0.03004 |
| Formula | LaScO3:0.001Mn4+ |
| Crystal system | Orthorhombic |
| Space group | pbnm |
| Lattice parameters | a = 5.67879(18) Å, b = 5.79140(16) Å, c = 8.0956(3), V = 266.251(14) Å3 |
| Atom | x | y | z | Occ. | Uaniso (Å2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| U11 | U22 | U33 | U12 | U13 | U23 | |||||
| La1 | 0.00909 | 0.95617 | 0.25 | 1 | 0.00781 | −0.00411 | 0.00299 | −0.00040 | 0.00000 | 0.00000 |
| Sc1 | 0.00000 | 0.5 | 0.00000 | 0.999 | 0.01333 | −0.00843 | −0.00312 | 0.00057 | 0.00554 | −0.00207 |
| Mn1 | 0.00000 | 0.5 | 0.00000 | 0.001 | 0.01333 | −0.00843 | −0.00312 | 0.00057 | 0.00554 | −0.00207 |
| O1 | 0.91109 | 0.52509 | 0.25000 | 1 | −0.00405 | −0.02970 | −0.00531 | −0.00072 | 0.00000 | 0.00000 |
| O2 | 0.70010 | 0.30343 | 0.01124 | 1 | 0.02081 | 0.03995 | −0.02703 | −0.02709 | −0.00394 | 0.00625 |
The PLE and PL spectra of the LaScO3:0.001Mn4+ phosphors were shown in Fig. 3(a). When monitored at 703 nm, the obtained excitation spectrum of the Mn4+ ions in LaScO3 was an asymmetric absorption band, which could be fitted to four peaks though Gaussian fitting, namely, peaks at 346 nm (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 cm−1; Mn4+–O2− transition), 392 nm (25
902 cm−1; Mn4+–O2− transition), 392 nm (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 cm−1; 4A2g → 4T1g dominant transition), 462 nm (21
510 cm−1; 4A2g → 4T1g dominant transition), 462 nm (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 cm−1; 4A2g → 2T2g transition), and 543 nm (18
645 cm−1; 4A2g → 2T2g transition), and 543 nm (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cm−1; 4A2g → 4T2g transition).48,51 Under the excitation of 398 nm or 549 nm, the LaScO3:0.001Mn4+ phosphors emitted deep red light peaking at 703 nm (2Eg → 4A2g transition; see Fig. 3(a)) with the CIE chromaticity coordinates of (0.7324, 0.2676) (see Fig. 3(b)). Fig. 3(c) shows the absorption spectra of PR and PFR as well as the emission spectrum of the LaScO3:0.001Mn4+ phosphor. Obviously, the PL spectrum of the LaScO3:0.001Mn4+ phosphor was located within the absorption spectrum of the PFR. Importantly, the dominant emission peak of LaScO3:0.001Mn4+ phosphor at 703 nm was close to the peak wavelength (around 730 nm) of the absorption spectrum of PFR. So it was meaningful to apply LaScO3:Mn4+ phosphors to plant growth LEDs.
416 cm−1; 4A2g → 4T2g transition).48,51 Under the excitation of 398 nm or 549 nm, the LaScO3:0.001Mn4+ phosphors emitted deep red light peaking at 703 nm (2Eg → 4A2g transition; see Fig. 3(a)) with the CIE chromaticity coordinates of (0.7324, 0.2676) (see Fig. 3(b)). Fig. 3(c) shows the absorption spectra of PR and PFR as well as the emission spectrum of the LaScO3:0.001Mn4+ phosphor. Obviously, the PL spectrum of the LaScO3:0.001Mn4+ phosphor was located within the absorption spectrum of the PFR. Importantly, the dominant emission peak of LaScO3:0.001Mn4+ phosphor at 703 nm was close to the peak wavelength (around 730 nm) of the absorption spectrum of PFR. So it was meaningful to apply LaScO3:Mn4+ phosphors to plant growth LEDs.
To find the optimal doping concentration of Mn4+ ions in LaScO3:Mn4+ phosphors, a series of LaScO3:xMn4+ (x = 0.0005–0.009) phosphors were synthesized and the corresponding PL spectra were shown in Fig. 3(d). It could be found that the PL intensity of the LaScO3:xMn4+ phosphors firstly increased as Mn4+ concentration increased and then decreased, and the optimal-doping concentration reached when x = 0.001, which was attributed to the concentration quenching in the LaScO3:xMn4+ phosphors. The IQE of the LaScO3:0.001Mn4+ phosphor was 15%. The optical doping concentration of Mn4+ is much lower than rare-earth doped phosphors, because the d-electron wave functions of transition metals (e.g., Mn4+ ions) extend more widely than 4f electrons of rare earths (e.g., Eu3+ and Tb3+ ions).52,53 Due to the energy transfer between the nearest Mn4+ and ends with energy transfer to traps or killing sites, concentration quenching occurs in LaScO3:xMn4+ phosphors.47,54 Considering the concentration quenching occurred in LaScO3:xMn4+ phosphors, the critical distance (Rc) of Mn4+ ions in the LaScO3:xMn4+ phosphors was calculated using the following equation:55,56
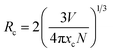 | (1) |
To determine whether the electric multipole interaction was electric dipole–dipole (d–d), or dipole–quadrupole (d–q), or quadrupole–quadrupole (q–q) interactions, the following equation could be used to get the exact form:58,59
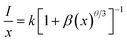 | (2) |
Fig. 3(f) exhibits the luminescence decay curves of Mn4+ 703 nm emissions in LaScO3:xMn4+ (x = 0.0005–0.009) phosphors. The decay lifetimes of the LaScO3:xMn4+ samples were obtained by the following double-exponential model:60
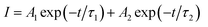 | (3) |
To explore the influence of the octahedral coordination environment of LaScO3:xMn4+ phosphors on the 3d3 energy level of Mn4+ ions, Fig. 4(b) shows the Tanabe–Sugano energy-level diagram of Mn4+ ions in the octahedral site of LaScO3 host together with the simple energy level diagram of Mn4+ ions. Based on the 4A2g → 4T2g (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cm−1) transition energy gap, the crystal-field strength (Dq) of the LaScO3:xMn4+ phosphors can be roughly estimated by the following equation:61
416 cm−1) transition energy gap, the crystal-field strength (Dq) of the LaScO3:xMn4+ phosphors can be roughly estimated by the following equation:61
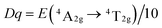 | (4) |
Based on the PLE spectrum of the LaScO3:0.001Mn4+ phosphors, the energy difference between the 4A2g → 4T1g (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 cm−1) and 4A2g → 4T2g (18
510 cm−1) and 4A2g → 4T2g (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cm−1) was about 7094 cm−1. Therefore, the Racah parameter B can be obtained by the following expression:62
416 cm−1) was about 7094 cm−1. Therefore, the Racah parameter B can be obtained by the following expression:62
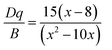 | (5) |
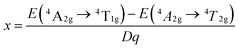 | (6) |
From the PL spectrum of the LaScO3:0.001Mn4+ phosphors, the energy of the 2Eg → 4A2g (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 cm−1) was acquired and the Racah parameter B was evaluated by the following expression:47,63
225 cm−1) was acquired and the Racah parameter B was evaluated by the following expression:47,63
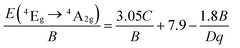 | (7) |
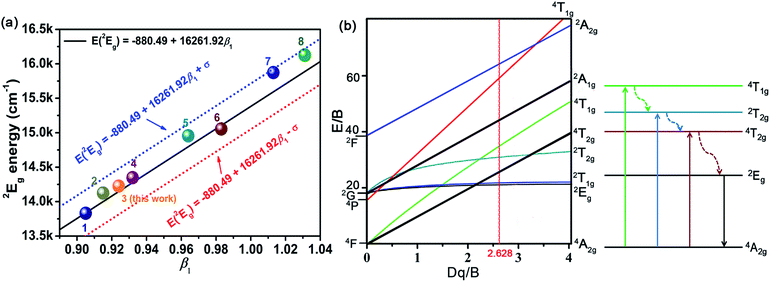
According to the eqn (4)–(7), the crystal field parameters Dq, B, and C were 1842, 701, and 3006 cm−1, respectively. Thus the value of the Dq/B, which represented the intensity of the crystal field, was about 2.628 (>2.2), indicating that the Mn4+ ions experienced strong crystal strength in LaScO3 host than those in Y3Al5O12 (Dq/B = 1.98),64 Ba2YSbO6 (Dq/B = 1.88),65 and Ca2LaNbO6 (Dq/B = 2.31).66
The emission energy of the 2Eg → 4A2g transition of Mn4+ ions in specific host could be affected by the nephelauxetic effect.67 The nephelauxetic effect can be described as the parameter β1, which was established by Brik et al.63 And the value of β1 can be calculated by the following expression:68
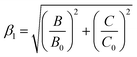 | (8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261.92β1 ± σ; σ = 332 cm−1)] between the 2Eg energy level of Mn4+ ions in different hosts.63 Fig. 4(a) depicts the relationship between the 2Eg energy level of Mn4+ ions and β1 in different hosts. All the points were located around the line E(2Eg) = −880.49 + 16
261.92β1 ± σ; σ = 332 cm−1)] between the 2Eg energy level of Mn4+ ions in different hosts.63 Fig. 4(a) depicts the relationship between the 2Eg energy level of Mn4+ ions and β1 in different hosts. All the points were located around the line E(2Eg) = −880.49 + 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261.92β1, indicating the data were acceptable. The detailed data of these phosphors, which used in Fig. 4(a), were listed in Table 3. It can be seen clearly from Table 3 and Fig. 4(a) that with the increasing of β1, the value of the energy level of 2Eg increased. And the value of the nephelauxetic effect in LaScO3:xMn4+ phosphors was close to the La(MgTi)1/2O3:Mn4+, NaMgGdTeO6:Mn4+, and SiTiO3:Mn4+, indicating the similar coordination environments of Mn4+ ions in these phosphors. Based on the Tanabe–Sugano energy-level diagram in the left diagram of Fig. 4(b), the simple schematic diagram of the Mn4+ energy level transition in the LaScO3 host was illustrated in the right diagram of Fig. 4(b). The electrons at the ground state 4A2g (from the 4F term) absorbed the energy of the excited light (398 nm or 549 nm) and then were pumped to the excited level 4T1g (from the 4F term), 2T2g (from the 2G term), and 4T2g (from the 4F term), after which the electrons could relax to the lowest excited state level 2Eg (from the 2G term) through non-radiative transitions process 4T1g → 2T2g → 4T2g → 2Eg, and finally released the energy by radiation transitions process with a red light emission at 703 nm.61
261.92β1, indicating the data were acceptable. The detailed data of these phosphors, which used in Fig. 4(a), were listed in Table 3. It can be seen clearly from Table 3 and Fig. 4(a) that with the increasing of β1, the value of the energy level of 2Eg increased. And the value of the nephelauxetic effect in LaScO3:xMn4+ phosphors was close to the La(MgTi)1/2O3:Mn4+, NaMgGdTeO6:Mn4+, and SiTiO3:Mn4+, indicating the similar coordination environments of Mn4+ ions in these phosphors. Based on the Tanabe–Sugano energy-level diagram in the left diagram of Fig. 4(b), the simple schematic diagram of the Mn4+ energy level transition in the LaScO3 host was illustrated in the right diagram of Fig. 4(b). The electrons at the ground state 4A2g (from the 4F term) absorbed the energy of the excited light (398 nm or 549 nm) and then were pumped to the excited level 4T1g (from the 4F term), 2T2g (from the 2G term), and 4T2g (from the 4F term), after which the electrons could relax to the lowest excited state level 2Eg (from the 2G term) through non-radiative transitions process 4T1g → 2T2g → 4T2g → 2Eg, and finally released the energy by radiation transitions process with a red light emission at 703 nm.61
| No. | Hosts | Dq/cm−1 | B cm−1 | C /cm−1 | Dq/B | β1 | E(2Eg)/cm−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | SiTiO3 | 1818 | 719 | 2839 | 2.529 | 0.905 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 (723 nm) 831 (723 nm) |
69 |
| 2 | La(MgTi)1/2O3 | 2053 | 700 | 2959 | 2.933 | 0.915 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 (708 nm) 124 (708 nm) |
9 |
| 3 | LaScO3 | 1842 | 701 | 3006 | 2.628 | 0.924 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 225 (703 nm) 225 (703 nm) |
This work |
| 4 | NaMgGdTeO6 | 2083 | 727 | 2971 | 2.865 | 0.932 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 (697 nm) 347 (697 nm) |
39 |
| 5 | Y2Ti2O7 | 2000 | 600 | 3500 | 3.333 | 0.964 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 956 (669 nm) 956 (669 nm) |
63 and 70 |
| 6 | CaZrO3 | 1850 | 754 | 3173 | 2.454 | 0.983 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 054 (664 nm) 054 (664 nm) |
67 |
| 7 | K2SiF6 | 2197 | 599 | 3750 | 3.668 | 1.013 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 (630 nm) 873 (630 nm) |
63 |
| 8 | KTeF5 | 2267 | 567 | 3904 | 3.998 | 1.031 | 16 129 (620 nm) | 71 |
Conclusions
In conclusion, LaScO3:xMn4+ (x = 0.0005–0.009) far-red emitting phosphors have been successfully synthesized via high-temperature solid-state reaction process. The crystal structure of the host and LaScO3:0.001Mn4+ phosphors had been discussed and the XRD patterns of the LaScO3:xMn4+ proved that they all were pure phase. Monitored at 703 nm, the obtained PLE spectrum of the LaScO3:0.001Mn4+ phosphors exhibited four Gaussian fitting peaks, centering at 346 nm (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 cm−1; Mn4+–O2− transition), 392 nm (25
902 cm−1; Mn4+–O2− transition), 392 nm (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 cm−1; 4A2g → 4T1g dominant transition), 462 nm (21
510 cm−1; 4A2g → 4T1g dominant transition), 462 nm (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 cm−1; 4A2g → 2T2g transition), and 543 nm (18
645 cm−1; 4A2g → 2T2g transition), and 543 nm (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cm−1; 4A2g → 4T2g transition). Upon 398 nm excitation, the PL spectrum of LaScO3:0.001Mn4+ phosphors showed a far-red emission peaking at 703 nm within the 650–800 nm range, which was very close to the central absorption wavelength of the PFR at around 730 nm. The optimal doping concentration of Mn4+ ion was 0.001 in LaScO3 host and the electric d–d interaction contributed to the concentration quenching mechanism. All the results indicated that the LaScO3:Mn4+ phosphors are promising far-red emitting materials for plant growth LEDs application.
416 cm−1; 4A2g → 4T2g transition). Upon 398 nm excitation, the PL spectrum of LaScO3:0.001Mn4+ phosphors showed a far-red emission peaking at 703 nm within the 650–800 nm range, which was very close to the central absorption wavelength of the PFR at around 730 nm. The optimal doping concentration of Mn4+ ion was 0.001 in LaScO3 host and the electric d–d interaction contributed to the concentration quenching mechanism. All the results indicated that the LaScO3:Mn4+ phosphors are promising far-red emitting materials for plant growth LEDs application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51502190), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, No. 2017-skllmd-01).Notes and references
- R. J. Bula, R. C. Morrow, T. W. Tibbitts and D. J. Barta, HortScience, 1991, 26, 203–205 Search PubMed.
- D. E. Macey and J. T. Arnott, Can. J. For. Res., 1986, 16, 949–954 CrossRef.
- C. A. Jaleel, P. Manivannan, B. Sankar, A. Kishorekumar, R. Gopi, R. Somasundaram and R. Panneerselvam, Colloids Surf., B, 2007, 60, 7–11 CrossRef PubMed.
- C. Kami, S. Lorrain, P. Hornitschek and C. Fankhauser, Curr. Top. Dev. Biol., 2010, 91, 29–66 Search PubMed.
- L. Ma, D.-j. Wang, Z.-y. Mao, Q.-f. Lu and Z.-h. Yuan, Appl. Phys. Lett., 2008, 93, 144101 CrossRef.
- J. Chen, N. Zhang, C. Guo, F. Pan, X. Zhou, H. Suo, X. Zhao and E. M. Goldys, ACS Appl. Mater. Interfaces, 2016, 8, 20856–20864 CrossRef PubMed.
- M. J. Kasperbauer, Plant Physiol., 1987, 85, 350–354 CrossRef PubMed.
- P. F. Devlin, J. M. Christie and M. J. Terry, J. Exp. Bot., 2007, 58, 3071–3077 CrossRef PubMed.
- Z. Zhou, J. Zheng, R. Shi, N. Zhang, J. Chen, R. Zhang, H. Suo, E. M. Goldys and C. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 6177–6185 CrossRef PubMed.
- A. M. Srivastava, M. G. Brik, H. A. Comanzo, W. W. Beers, W. E. Cohen and T. Pocock, ECS J. Solid State Sci. Technol., 2017, 7, R3158–R3162 CrossRef.
- T. Nakajima and T. Tsuchiya, ACS Appl. Mater. Interfaces, 2015, 7, 21398–21407 CrossRef PubMed.
- G. D. Massa, HortScience, 2008, 43, 1951–1956 Search PubMed.
- H. Smith, Nature, 2000, 407, 585–591 CrossRef PubMed.
- X. Huang, Nat. Photonics, 2014, 8, 748–749 CrossRef.
- C. Yu, Z. Yang, J. Qiu, Z. Song and Z. Dacheng, J. Am. Ceram. Soc., 2018, 101, 612–623 CrossRef.
- X. Wu, Y. Jiao, O. Hai, Q. Ren, F. Lin and H. Li, J. Alloys Compd., 2018, 730, 521–527 CrossRef.
- J. Han, L. Li, M. Peng, B. Huang, F. Pan, F. Kang, L. Li, J. Wang and B. Lei, Chem. Mater., 2017, 29, 8412–8424 CrossRef.
- D. Wen, H. Kato, M. Kobayashi, S. Yamamoto, M. Mitsuishi and M. Kakihana, J. Mater. Chem. C, 2017, 5, 4578–4583 RSC.
- P. Du, L. Luo, X. Huang and J. S. Yu, J. Colloid Interface Sci., 2018, 514, 172–181 CrossRef PubMed.
- P. Du, X. Huang and J. S. Yu, Inorg. Chem. Front., 2017, 4, 1987–1995 RSC.
- X. Huang, J. Alloys Compd., 2017, 690, 356–359 CrossRef.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef.
- X. Huang and H. Guo, Dyes Pigm., 2018, 154, 82–86 CrossRef.
- S. G. Prasanna Kumar, R. Hari Krishna, N. Kottam, P. Krishna Murthy, C. Manjunatha, R. Preetham, C. Shivakumara and T. Thomas, Dyes Pigm., 2018, 150, 306–314 CrossRef.
- J. Zhong, S. Zhou, D. Chen, J. Li, Y. Zhu, X. Li, L. Chen and Z. Ji, Dalton Trans., 2018, 47, 8248 RSC.
- X. Huang, H. Guo and B. Li, J. Alloys Compd., 2017, 720, 29–38 CrossRef.
- X. Huang, B. Li, H. Guo and D. Chen, Dyes Pigm., 2017, 143, 86–94 CrossRef.
- H. Guo, B. Devakumar, B. Li and X. Huang, Dyes Pigm., 2018, 151, 81–88 CrossRef.
- X. Huang, S. Wang, B. Li, Q. Sun and H. Guo, Opt. Lett., 2018, 43, 1307–1310 CrossRef PubMed.
- L. Y. Wang, E. H. Song, T. T. Deng, Y. Y. Zhou, Z. F. Liao, W. R. Zhao, B. Zhou and Q. Y. Zhang, Dalton Trans., 2017, 46, 9925–9933 RSC.
- G. Zhu, Z. Li, C. Wang, F. Zhou, Y. Shi, Y. Wen and S. Xin, J. Mater. Sci.: Mater. Electron., 2017, 29, 2216–2221 CrossRef.
- R. Cao, M. Peng, E. Song and J. Qiu, ECS J. Solid State Sci. Technol., 2012, 1, R123–R126 CrossRef.
- Q. Sun, S. Wang, B. Li, H. Guo and X. Huang, J. Lumin., 2018, 203, 371–375 CrossRef.
- J. Liang, P. Du, H. Guo, L. Sun, B. Li and X. Huang, Dyes Pigm., 2018, 157, 40–46 CrossRef.
- K. Sankarasubramanian, B. Devakumar, G. Annadurai, L. Sun, Y.-J. Zeng and X. Huang, RSC Adv., 2018, 8, 30223–30229 RSC.
- X. Huang, J. Liang, B. Li, L. Sun and J. Lin, Opt. Lett., 2018, 43, 3305–3308 CrossRef PubMed.
- M. Peng, X. Yin, P. A. Tanner, M. G. Brik and P. Li, Chem. Mater., 2015, 27, 2938–2945 CrossRef.
- K. Li, H. Lian and R. V. Deun, J. Lumin., 2018, 198, 155–162 CrossRef.
- X. Huang and H. Guo, Dyes Pigm., 2018, 152, 36–42 CrossRef.
- Y. Takeda, H. Kato, M. Kobayashi, H. Kobayashi and M. Kakihana, Chem. Lett., 2015, 44, 1541–1543 CrossRef.
- R. Cao, W. Luo, Q. Xiong, S. Jiang, Z. Luo and J. Fu, Chem. Lett., 2015, 44, 1422–1424 CrossRef.
- D. Lybye, P. Finn Willy and M. Mogens, Solid State Ionics, 2000, 128, 91–103 CrossRef.
- J. Du, O. Q. De Clercq, K. Korthout and D. Poelman, Materials, 2017, 10, 1422 CrossRef PubMed.
- C. Wu, J. Li, H. Xu, J. Wu, J. Zhang, Z. Ci, L. Feng, C. Cao, Z. Zhang and Y. Wang, J. Alloys Compd., 2015, 646, 734–740 CrossRef.
- M. H. Du, J. Mater. Chem. C, 2014, 2, 2475–2481 RSC.
- L. Qin, S. Bi, P. Cai, C. Chen, J. Wang, S. I. Kim, Y. Huang and H. J. Seo, J. Alloys Compd., 2018, 755, 61–66 CrossRef.
- R. Cao, Z. Shi, G. Quan, T. Chen, S. Guo, Z. Hu and P. Liu, J. Lumin., 2017, 188, 577–581 CrossRef.
- R. Cao, Y. Ye, Q. Peng, G. Zheng, H. Ao, J. Fu, Y. Guo and B. Guo, Dyes Pigm., 2017, 146, 14–19 CrossRef.
- B. Wang, H. Lin, F. Huang, J. Xu, H. Chen, Z. Lin and Y. Wang, Chem. Mater., 2016, 28, 3515–3524 CrossRef.
- B. Wang, H. Lin, J. Xu, H. Chen and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 22905–22913 CrossRef PubMed.
- U. B. Humayoun, S. N. Tiruneh and D.-H. Yoon, Dyes Pigm., 2018, 152, 127–130 CrossRef.
- M. Inokuti and F. Hirayama, J. Chem. Phys., 1965, 43, 1978–1989 CrossRef.
- S. Zhang, Y. Hu, H. Duan, Y. Fu and M. He, J. Alloys Compd., 2017, 693, 315–325 CrossRef.
- B. Li, X. Huang, H. Guo and Y. Zeng, Dyes Pigm., 2018, 150, 67–72 CrossRef.
- X. Huang, B. Li and H. Guo, J. Alloys Compd., 2017, 695, 2773–2780 CrossRef.
- K. Li, H. Lian and R. Van Deun, Dalton Trans., 2018, 47, 2501–2505 RSC.
- C. Wang, Y. Jin, Y. Lv, G. Ju, L. Chen, Z. Li and Y. Hu, J. Lumin., 2017, 192, 337–342 CrossRef.
- S. K. Hussain, T. T. H. Giang and J. S. Yu, J. Alloys Compd., 2018, 739, 218–226 CrossRef.
- S. Liang, M. Shang, H. Lian, K. Li, Y. Zhang and J. Lin, J. Mater. Chem. C, 2016, 4, 6409–6416 RSC.
- X. Zhang, J. Nie, S. Liu, Y. Li and J. Qiu, J. Am. Ceram. Soc., 2017, 1–9 Search PubMed.
- C. Yang, Z. Zhang, G. Hu, R. Cao, X. Liang and W. Xiang, J. Alloys Compd., 2017, 694, 1201–1208 CrossRef.
- M. G. Brik, S. J. Camardello and A. M. Srivastava, ECS J. Solid State Sci. Technol., 2014, 4, R39–R43 CrossRef.
- L. Zhou, C. Shen, L. Shen, S. Liu, J. Liu, L. Ding, J. Du, W. Xiang and X. Liang, J. Alloys Compd., 2018, 769, 686–693 CrossRef.
- J. Zhong, D. Chen, S. Yuan, M. Liu, Y. Yuan, Y. Zhu, X. Li and Z. Ji, Inorg. Chem., 2018, 57, 8978–8987 CrossRef PubMed.
- Z. Lu, H. Wang, D. Yu, T. Huang, L. Wen, M. Huang, L. Zhou and Q. Wang, Opt. Laser Technol., 2018, 108, 116–123 CrossRef.
- M. G. Brik and A. M. Srivastava, ECS J. Solid State Sci. Technol., 2013, 2, R148–R152 CrossRef.
- M. G. Brik, S. J. Camardello, A. M. Srivastava, N. M. Avram and A. Suchocki, ECS J. Solid State Sci. Technol., 2015, 5, R3067–R3077 CrossRef.
- Z. Bryknar, V. Trepakov, Z. Potůček and L. Jastrabík, J. Lumin., 2000, 87–89, 605–607 CrossRef.
- M. G. Brik, A. M. Srivastava and N. M. Avram, Opt. Mater., 2011, 33, 1671–1676 CrossRef.
- T. T. Deng, E. H. Song, J. Su, Y. Y. Zhou, L. Y. Wang, S. Ye and Q. Y. Zhang, J. Mater. Chem. C, 2018, 6, 4418–4426 RSC.
| This journal is © The Royal Society of Chemistry 2018 |