Open Access Article
Open Access ArticleFirst in situ vesicular self-assembly of ‘binols’ generated by a two-component aerobic oxidation reaction†‡
Braja G. Bag *,
Subrata Ghorai,
Saikat K. Panja,
Shaishab K. Dinda and
Koushik Paul
*,
Subrata Ghorai,
Saikat K. Panja,
Shaishab K. Dinda and
Koushik Paul
Department of Chemistry and Chemical Technology, Vidyasagar University, Midnapore 721102, WB, India. E-mail: brajagb@gmail.com
First published on 16th August 2018
Abstract
Generation of vesicular self-assemblies from natural and synthetic components has been in the frontiers of research in recent years for an improved understanding of the self-assembly process and also because of its prospective and realized applications in the areas of advanced materials, biotechnology and medicine. In the present work, we report the first example of the in situ generation of vesicular self-assemblies during an aerobic coupling reaction. The two precursor 2-naphthol units, having hydrogen bond donor–acceptor groups with appended alkyl chains, yielded binol (2,2′-dihydroxy-1,1′-binaphthyl) derivatives by aerobic coupling that spontaneously self-assembled in situ, yielding vesicular self-assemblies and gels. The morphology of the self-assemblies has been characterized by various optical, electron and atomic force microscopic techniques. The vesicular self-assemblies obtained in the liquids were capable of entrapping fluorophores such as rhodamine-B and carboxy fluorescein including the anticancer drug doxorubicin. The entrapped fluorophores could also be released by sonication or by rupture of vesicles. The supramolecular gels obtained in binary solvent mixtures showed improved gelation abilities with increase in the alkyl chain lengths as reflected by their minimum gelator concentration (mgcs) values, gel to sol transition temperatures (Tgel) and rheology properties. The results described here are also the first demonstration of gelation during an aerobic coupling reaction.
1 Introduction
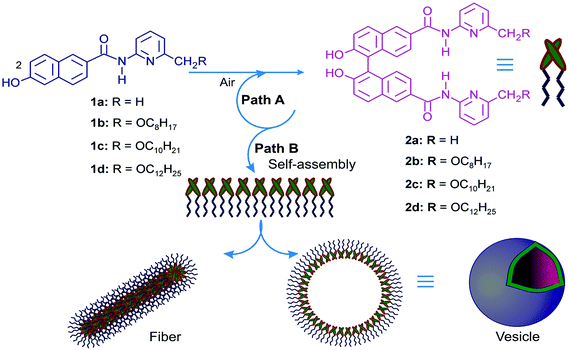
Self-assembly of natural and synthetic molecules yielding supramolecular structures of varied dimensions in the nano to micrometer range has been an area of increasing significance in recent years. These studies have helped scientists to improve the understanding of the structure property relationships that resulted in such supramolecular structures in liquid medium.1–10 Spontaneous self-assembly of molecules, yielding vesicles, is of special significance due to its tremendous application in the areas of targeted drug delivery, medical implants, nanoscale reactors, tissue engineering, gene therapy, theranostics, enzyme entrapment, magnetic resonance imaging, etc.11–18 Literature studies reveal that vesicular self-assemblies have been reported from a wide range of amphiphilic compounds.19–29 Though, the formation of nano-sized spherical assemblies and vesicles in water from long alkyl-chained compounds having different head groups are known, according to our knowledge, aerobic coupling of two components leading to the formation of vesicular self-assemblies and supramolecular nano-architectures in organic media is unprecedented.1–15Binols, obtained by coupling reaction of two β-naphthol precursors, have found application in the design of asymmetric catalysts for a wide range of chemical transformations.30–36 The demonstration of self-replication on non-enzymatic model systems in its minimal form require: (1) suitable complementary recognition sites in the template molecule to complex the precursor molecules by non-covalent interactions and (2) a suitable chemical reaction for the formation of a chemical bond.37–41 Computer modeling studies using PCWin® software indicated that the binol derivatives of the type 2 will be able to form a termolecular complex with two precursor 2-naphthol derivatives via complementary H-bond donor–acceptor sites thereby facilitating the autocatalytic template directed synthesis (Fig. 1 and Scheme S1‡). While carrying out aerobic binol coupling reactions with the 2-naphthol derivatives having H-bond donor–acceptor sites and appended alkyl chains (1b–d), we serendipitously discovered that the binol derivatives (2b–d) spontaneously self-assembled yielding supramolecular gels in the reaction medium. Herein we report the spontaneous formation of vesicular self-assemblies based on three binol derivatives having H-bond donor–acceptor groups with appended alkyl chains in binary solvent mixtures. The self-assemblies have been characterized by atomic force, electron and optical microscopic techniques, dynamic light scattering (DLS) and X-ray diffraction studies. Entrapment of fluorophores, including drugs and their subsequent release, has also been demonstrated. Moreover, gel-to-sol transition temperatures, various thermodynamic parameters and the rheological properties were investigated for the supramolecular gels obtained in the binary solvent mixtures.
2 Results and discussion
2.1 Syntheses
Syntheses of compounds 1a–1d were carried out from 6-hydroxy-2-naphthoic acid 4 and 2-amino-6-picoline 6a following a procedure optimized in our laboratory.42 The intermediate acid chloride 5 was synthesized in excellent yields from 4 in four steps and then reacted with 2-amino-6-picoline or with its derivatives (6a–6d) in the presence of triethyl amine as a base to produce 7a–7d in 80–90% yields. On reductive debenzylation of 7b–7d with H2/Pd(C) the precursors 1b–1d were obtained in 70–80% yields. The binol derivatives 2a–2d were synthesized by aerial coupling of 1a–1d in chloroform at room temperature using CuCl(OH)·TMEDA as a catalyst in 80–87% isolated yields. The intermediate acid chloride 5 on reaction with n-octanol in the presence of triethyl amine as a base produced 8 in 88% yield. Reductive debenzylation using H2/Pd(C) produced 9 in 80% yield. Aerial coupling of 9 at room temperature in the presence of CuCl(OH)·TMEDA as a catalyst produced 3 in 90% isolated yield.2.2 Self-assembly studies
Compounds of the type 2a, having H-bonding recognition sites, were chosen as templates for autocatalysis via Path A during aerobic coupling of 1a in the presence of CuCl(OH)·TMEDA (Fig. 1).43–45 But, compound 1a was soluble only in polar solvents such as aliphatic alcohols, DMSO, DMF and remained insoluble in chloroform or dichloromethane. We anticipated that compound 1b having the attached octyloxy chain will have better solubility in aprotic nonpolar solvents. Indeed the compound 1b was soluble in aprotic solvents like chloroform, dichloromethane, and mixtures of chloroform–hexanes, etc. While carrying out aerobic oxidative coupling of 1b in hexane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
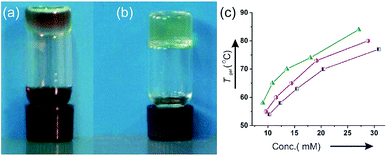
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of CuCl(OH)·TMEDA, formation of a heterogeneous soft solid-like material was observed during the progress of the reaction. Monitoring the solution kinetics of the reaction by HPLC (Fig. 2a and S7‡) was disrupted as well. To explain this observation, we isolated the product 2b in pure form and dissolved the compound (10 mM) contained in a vial in the same solvent hexane–chloroform (6
1) in the presence of CuCl(OH)·TMEDA, formation of a heterogeneous soft solid-like material was observed during the progress of the reaction. Monitoring the solution kinetics of the reaction by HPLC (Fig. 2a and S7‡) was disrupted as well. To explain this observation, we isolated the product 2b in pure form and dissolved the compound (10 mM) contained in a vial in the same solvent hexane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under hot condition and allowed it to cool at room temperature. On turning the vial upside down after 2 h, no flow of the material was observed and it formed a transparent supramolecular gel (Fig. 2b).
1) under hot condition and allowed it to cool at room temperature. On turning the vial upside down after 2 h, no flow of the material was observed and it formed a transparent supramolecular gel (Fig. 2b).
To find out the effect of the longer alkyl chains in the self-assembly process, the decyloxy derivative 2c and dodecyloxy derivative 2d were also synthesized35 and the self-assembly properties of all the three derivatives 2b–2d were studied in different liquids. All the three alkyl chained derivatives were highly soluble in polar organic liquids such as DMF and DMSO. Colloidal suspensions were obtained in DMSO–water and DMF–water binary solvent mixtures. Interestingly, all the derivatives 2b–2d self-assembled in all the seven alkane–chloroform binary solvent mixtures studied forming transparent gels (Table 1). For 2b, the minimum gelator concentrations (MGCs) were in the range of 3.5–4.9 mM in the alkane–chloroform binary solvent mixtures studied. With increasing alkyl chain length from 2b to 2d, a gradual decrease of MGCs was observed (Table 1) indicating better gelation abilities of the longer alkyl chained derivatives. Repeated heating and cooling experiments carried out with all the gels indicated that all the gels were thermo-reversible in nature. The gel to sol transition temperatures (Tgel) increased with increasing concentration of the gelators. A positive free energy change calculated from the plot of Tgel vs. concentration indicated stability of the gels (Fig. S1‡).
| Entry | Solventa | 2bb | 2cb | 2db |
|---|---|---|---|---|
| a Hexane to dodecane mentioned in entries 1–5 are all normal alkanes. Entry 6: C6H12 = cyclohexane, entry 7: C7H14 = methyl cyclohexane.b MGC/Tgel: The minimum gelator concentration (MGC), defined as the minimum concentration required for the formation of a gel, is given in millimolar (mM) unit for each gelator in the binary solvent mixture and its gel to sol transition temperature Tgel in °C at MGC. In entry 8 and 9 colloidal suspensions were obtained at 5% w/v concentration. | ||||
| 1 | Hexane–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.7/50 | 4.0/51 | 3.7/53 |
| 2 | Heptane–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.5/53 | 2.9/55 | 2.2/58 |
| 3 | Octane–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.9/54 | 4.0/56 | 3.6/60 |
| 4 | Decane–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.7/59 | 3.2/61 | 2.7/64 |
| 5 | Dodecane–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3.7/63 | 2.9/65 | 2.7/68 |
| 6 | C6H12–CHCl3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.5/48 | 4.1/50 | 3.9/52 |
| 7 | C7H14–CHCl3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.7/50 | 4.0/53 | 3.7/55 |
| 8 | DMSO–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.0/— | 5.0/— | 5.0/— |
| 9 | DMF–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.0/— | 5.0/— | 5.0/— |
The gel to sol transition temperatures (Tgel) increased with increasing concentrations of the gelators in different neat liquids and also in binary liquid mixtures. These data were utilized to calculate the related thermodynamic parameters such as enthalpy, entropy and Gibbs free energy change (ΔH°, ΔS° and ΔG°). The positive free energy changes during gel to sol transition in organic binary liquid mixture cyclohexane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were nearly identical though their enthalpy and entropy changes were slightly different (Table 2). The free energy changes (ΔG°) calculated during gel to sol transformations were positive in all the cases which is indicative of the stability of the gel phase.
1) were nearly identical though their enthalpy and entropy changes were slightly different (Table 2). The free energy changes (ΔG°) calculated during gel to sol transformations were positive in all the cases which is indicative of the stability of the gel phase.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 298 K
1) at 298 K
| Entry | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | ΔG° (kJ mol−1) |
|---|---|---|---|
| 2b | 44.528 | 97.92 | 15.3478 |
| 2c | 41.321 | 87.00 | 15.398 |
| 2d | 43.139 | 90.50 | 16.150 |
2.3 Morphology of the self-assemblies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DMF–water (5
1), DMF–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), cyclohexane–chloroform (6
1), cyclohexane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), n-octane–chloroform (6
1), n-octane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
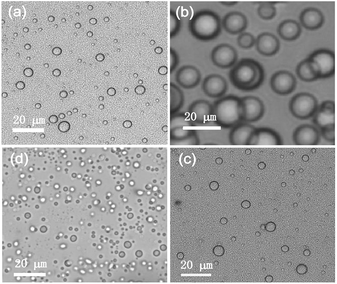
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) etc. below their MGCs were investigated. Spherical self-assemblies of average diameter of 3.6 μm were observed in the binary solvent mixtures (Fig. 4 and S3‡). No fibrillar network was observed in the native state. Thus the observation of small percentage of fibrillar network in the dried self-assemblies by AFM (discussed earlier) might be due to a change in morphology during solvent evaporation. Nanosized objects were not observed due to the size limitations using visible light. This limitation could be overcome by electron microscopy of the dried samples of 2b–2d as discussed below.
1) etc. below their MGCs were investigated. Spherical self-assemblies of average diameter of 3.6 μm were observed in the binary solvent mixtures (Fig. 4 and S3‡). No fibrillar network was observed in the native state. Thus the observation of small percentage of fibrillar network in the dried self-assemblies by AFM (discussed earlier) might be due to a change in morphology during solvent evaporation. Nanosized objects were not observed due to the size limitations using visible light. This limitation could be overcome by electron microscopy of the dried samples of 2b–2d as discussed below.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.11% w/v), n-octane–chloroform (6
1 v/v, 0.11% w/v), n-octane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
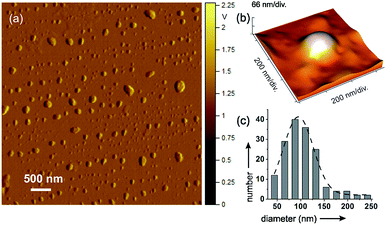
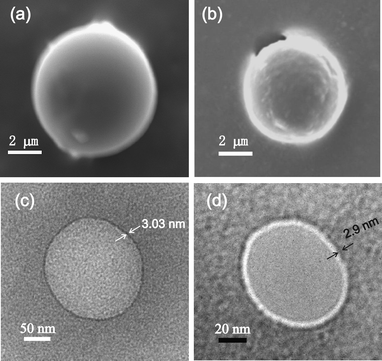
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.11% w/v) were studied by scanning electron microscopy (SEM). SEM studies of the dried samples in different organic binary liquid mixtures revealed nano-sized vesicular self-assemblies along with microsized vesicular self-assemblies (Fig. 5a and b).
1 v/v, 0.11% w/v) were studied by scanning electron microscopy (SEM). SEM studies of the dried samples in different organic binary liquid mixtures revealed nano-sized vesicular self-assemblies along with microsized vesicular self-assemblies (Fig. 5a and b).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.11% w/v), n-octane–chloroform (6
1 v/v, 0.11% w/v), n-octane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 0.11% w/v), we performed high resolution transmission electron microscopy (HRTEM). Nano-sized vesicular self-assemblies of 2b–2d were observed by HRTEM in the dried samples prepared from the dilute colloid of 2b–2d in binary organic solvent mixtures (Fig. 5c and d). A distinct periphery in sharp contrast to the inner core confirmed the vesicular nature of the spherical self-assemblies. The membrane thickness of the vesicular self-assemblies was 3.03 nm for 2d. With a molecular length of 3.03 nm, a monolayer vesicular membrane is supported. All these observations support the results obtained by scanning electron microscopy, atomic force microscopy and optical microscopy studies (discussed earlier).
1 v/v, 0.11% w/v), we performed high resolution transmission electron microscopy (HRTEM). Nano-sized vesicular self-assemblies of 2b–2d were observed by HRTEM in the dried samples prepared from the dilute colloid of 2b–2d in binary organic solvent mixtures (Fig. 5c and d). A distinct periphery in sharp contrast to the inner core confirmed the vesicular nature of the spherical self-assemblies. The membrane thickness of the vesicular self-assemblies was 3.03 nm for 2d. With a molecular length of 3.03 nm, a monolayer vesicular membrane is supported. All these observations support the results obtained by scanning electron microscopy, atomic force microscopy and optical microscopy studies (discussed earlier).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) revealed peaks at 2θ = 3.05° and 2.9° corresponding to d values of 2.9 nm and 3.03 nm respectively that also supported a monolayer vesicular self-assembly (Fig. S4‡) as depicted in Fig. 1 (Path B).
1) revealed peaks at 2θ = 3.05° and 2.9° corresponding to d values of 2.9 nm and 3.03 nm respectively that also supported a monolayer vesicular self-assembly (Fig. S4‡) as depicted in Fig. 1 (Path B).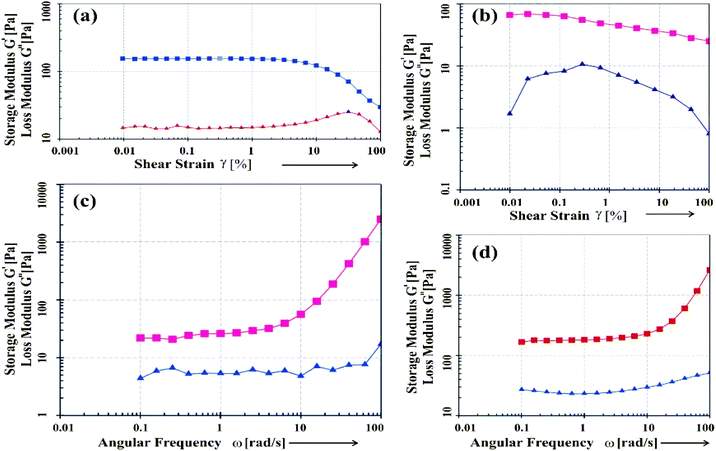 | ||
Fig. 6 Rheological plots of the gels of 2c, 2d in octane–chloroform (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): (a and b) G′, G′′ vs. shear strain (%), and (c and d) G′, G′′ vs. angular frequency. 1): (a and b) G′, G′′ vs. shear strain (%), and (c and d) G′, G′′ vs. angular frequency. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 6). In amplitude sweep experiment applying the shear strain ramp (0.01–100%), it was observed that with increasing strain, G′ started to decrease in a critical strain after passing the linear region. For both the samples, no crossover point (G′ = G′′) was observed indicating that both the gels were very strong. The longer linear region observed for the gel from 2d (Fig. 6a) compared to that from 2c (Fig. 6b) indicated that 2d with a longer alkyl chain was a better gelator compared to 2c. This result is also supported by the gel to sol transition temperatures (Tgel) (as discussed previously in Section 2).
1) (Fig. 6). In amplitude sweep experiment applying the shear strain ramp (0.01–100%), it was observed that with increasing strain, G′ started to decrease in a critical strain after passing the linear region. For both the samples, no crossover point (G′ = G′′) was observed indicating that both the gels were very strong. The longer linear region observed for the gel from 2d (Fig. 6a) compared to that from 2c (Fig. 6b) indicated that 2d with a longer alkyl chain was a better gelator compared to 2c. This result is also supported by the gel to sol transition temperatures (Tgel) (as discussed previously in Section 2).The frequency sweep experiments (Fig. 6c and d), were carried out by applying angular frequency = 0.01–100 rad s−1, at a constant temperature (25 °C). An increase in G′ and G′′ were observed with increase in angular frequency. This can be explained by the fact that, with an increase in oscillatory frequency, the gel network vibrate rapidly and were unable to rearrange themselves in the imposed motion. As a result, it showed solid like behavior at higher frequency compared to lower frequency. Thus to breakdown the gel network, greater shear strain is required at higher frequency than that of lower frequency.47,48 Thus, both in frequency sweep as well as amplitude sweep experiments, an increase in G′ and G′′ were observed with increase in frequency and % shear strain respectively. These results indicated the gel behavior and elastic nature of the organogels in the binary liquid mixtures studied.
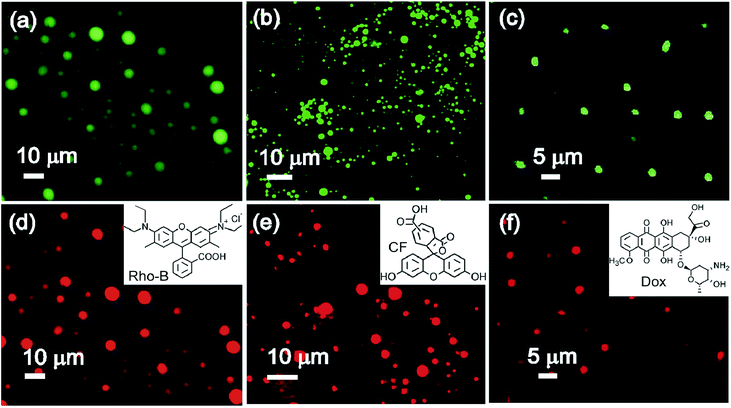
2.3.9.1 Entrapment of fluorophores. Initial entrapment studies of binol derived vesicular self-assemblies of 2c and 2d were carried out with a cationic fluorophore rhodamine B (Rho-B) hydrochloride and an anionic fluorophore 5,6-carboxy-fluorescein (CF). Bright green and red fluorescence observed from the vesicular self-assemblies under fluorescence light indicated that both the fluorophores Rho-B and CF were entrapped inside the vesicular self-assemblies of 2c and 2d (Fig. 8a–e). Such observation have been made by us previously with the vesicular self-assemblies of naturally occurring diterpenic and hydroxy-triterpenic acids.49–51 These observations inspired us to examine the entrapment of doxorubicin (Dox), a well-known anticancer drug having fluorescence properties. A solution of 2c (47 mM) and Dox (0.47 mM) in DMSO–water (5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
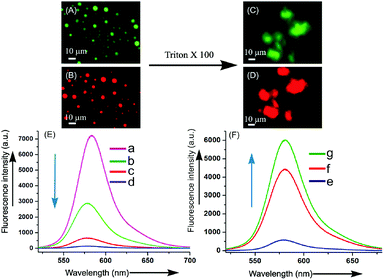
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at 45 °C was cooled at room temperature and the resulting colloidal suspension was examined after 4 h. Bright fluorescence from the core of the vesicular self-assemblies of 2c under epifluorescence microscope confirmed the entrapment of Dox inside the vesicles (Fig. 8c and f). Quenching of fluorescence at 560 nm during entrapment of Rho-B inside the vesicles compared to free Rho-B (λex = 520 nm) was also observed (Fig. 9A, B and E) confirming the entrapment of Rho-B inside the vesicles. Successful entrapment of guest molecules including drugs inside the vesicular self-assemblies of binol derivatives having an average diameter <10 μm and their release studies open up its use in cell signaling,52 clinical diagnosis53 including targeted delivery through blood capillaries.54–57
1 v/v) at 45 °C was cooled at room temperature and the resulting colloidal suspension was examined after 4 h. Bright fluorescence from the core of the vesicular self-assemblies of 2c under epifluorescence microscope confirmed the entrapment of Dox inside the vesicles (Fig. 8c and f). Quenching of fluorescence at 560 nm during entrapment of Rho-B inside the vesicles compared to free Rho-B (λex = 520 nm) was also observed (Fig. 9A, B and E) confirming the entrapment of Rho-B inside the vesicles. Successful entrapment of guest molecules including drugs inside the vesicular self-assemblies of binol derivatives having an average diameter <10 μm and their release studies open up its use in cell signaling,52 clinical diagnosis53 including targeted delivery through blood capillaries.54–57
2.3.9.2 Release of entrapped guest molecules. To examine the release of the entrapped guest molecules from the vesicular self-assemblies of binol derivatives, sonication of the Rho-B entrapped vesicular self-assemblies of 2d was carried out in an ultrasonicator bath. Gradual increase in fluorescence emission intensity at 560 nm (λex = 520 nm) indicted the release of entrapped Rho-B from the vesicular self-assemblies (Fig. S6‡). For chemical rupture of the Rho-B entrapped vesicular self-assemblies, the Rho-B (0.41 mM) entrapped vesicular self-assemblies of 2d (41 mM) were treated with a small amount of triton X-100 (0.41 mM), a well-known vesicle rupturing agent. Disappearance of the bright fluorescent spherical images within 2–3 hours with concomitant appearance of an intense background under epifluorescence microscope indicated the lysis of the vesicles (Fig. 9C, D and F) with concomitant release of the fluorophore Rho-B.55
3 Conclusions
In conclusion, spontaneous formation of vesicular self-assemblies during an aerobic coupling reaction of two monomeric components has been demonstrated. According to our knowledge, this is the first report of the formation of vesicular self-assemblies and gels from compounds obtained by in situ coupling of two components. Detailed characterization of the vesicular self-assemblies has been carried out by atomic force, optical, electron microscopic techniques and X-ray diffraction studies. Entrapment of cationic fluorophore rhodamine B, anionic fluorophore carboxyfluorescein and a commonly used anticancer drug doxorubicin in the self-assemblies has been demonstrated. The entrapped fluorophores could be released by sonication and by rupture of vesicles. Gel to sol transition temperatures data for the gels enabled us to calculate the ΔG, ΔH and ΔS values during gel to sol transitions. Improved gelation abilities of the gelators with an increase in the alkyl chain lengths were also observed, as reflected by their minimum gelator concentration, Tgel values and rheology properties. Dynamic rheology experiments carried out with the gels showed no cross over points between the storage (G′) and the loss (G′′) moduli indicating that the supramolecular gels obtained in the binary solvent mixtures were very strong in nature.4 Experimental section
4.1 General information
For general information regarding conditions for NMR, optical microscopy, fluorescence microscopy, SEM, HRTEM, AFM, DLS, XRD and rheological studies, please see ESI.‡4.2 Synthesis
General procedure for the syntheses of binol derivatives 2a–2d and the model compound 3 by taking the synthesis of 2d from 1d as a representative example:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as a reaction medium in the presence of CuCl(OH)·TMEDA (0.006 g, 0.027 mmol) was 0.056 g (80%) as a white solid. Mp > 360 °C, FTIR (neat, cm−1) νmax: 3363, 2964, 1672, 1611, 1533. 1H NMR (400 MHz, DMSO-d6): δ = 8.524 (s, 2H), 8.042 (d, J = 8.2 Hz, 2H), 7.92 (d, J = 8.8 Hz, 2H), 7.72–7.53 (m, 4H), 7.38 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 7.4 Hz, 2H), 4.43 (broad s, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.44, 157.1, 155.47, 151.98, 138.90, 136.25, 131.03, 129.57, 128.23, 127.47, 125.01, 124.68, 119.78, 119.45, 115.60, 111.98, 24.04 ppm. HRMS (ESI): m/z calcd for C34H26N4O4Na [M + Na+] 554.5947; found 554.5949.
1 v/v) as a reaction medium in the presence of CuCl(OH)·TMEDA (0.006 g, 0.027 mmol) was 0.056 g (80%) as a white solid. Mp > 360 °C, FTIR (neat, cm−1) νmax: 3363, 2964, 1672, 1611, 1533. 1H NMR (400 MHz, DMSO-d6): δ = 8.524 (s, 2H), 8.042 (d, J = 8.2 Hz, 2H), 7.92 (d, J = 8.8 Hz, 2H), 7.72–7.53 (m, 4H), 7.38 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 7.4 Hz, 2H), 4.43 (broad s, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ = 166.44, 157.1, 155.47, 151.98, 138.90, 136.25, 131.03, 129.57, 128.23, 127.47, 125.01, 124.68, 119.78, 119.45, 115.60, 111.98, 24.04 ppm. HRMS (ESI): m/z calcd for C34H26N4O4Na [M + Na+] 554.5947; found 554.5949.Conflicts of interest
“There are no conflicts to declare”.Acknowledgements
DST Fast-Track (SR/FTP/CS-42/2001), SERB (EMR/2016/001123), Indo-Srilanka project (DST/INT/SL/P25/2016), UGC-SAP and DST-FIST, Vidyasagar University are gratefully acknowledged for providing financial support and infrastructural facilities. S. K. D., K. P., S. G. thank CSIR and S. G., S. K. P. thank UGC-BSR for providing research fellowships.References
- S. Bhattacharya and S. K. Samanta, Chem. Rev., 2016, 116, 11967–12028 CrossRef PubMed.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef PubMed.
- A. Sorrenti, O. Illa and R. M. Ortuno, Chem. Soc. Rev., 2013, 42, 8200–8219 RSC.
- M. B. Avinash and T. Govindaraju, Acc. Chem. Res., 2018, 51, 414–426 CrossRef PubMed.
- L. Wang and Q. Li, Chem. Soc. Rev., 2018, 47, 1044–1097 RSC.
- C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef PubMed.
- E. Carretti, M. Bonini, L. Dei, B. H. Berrie, L. V. Angelova, P. Baglioni and R. G. Weiss, Acc. Chem. Res., 2010, 43, 751–760 CrossRef PubMed.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1444 CrossRef PubMed.
- A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644–656 CrossRef PubMed.
- B. G. Bag and R. Majumdar, Chem. Rec., 2017, 17, 841–873 CrossRef PubMed.
- P. Moitra, K. Kumar, P. Kondaiah and S. Bhattacharya, Angew. Chem., Int. Ed., 2014, 53, 1113–1117 CrossRef PubMed.
- S. K. Misra, P. Moitra, B. S. Chhikara, P. Kondaiah and S. Bhattacharya, J. Mater. Chem., 2012, 22, 7985–7998 RSC.
- D. M. Vriezema, P. M. L. Garcia, N. S. Oltra, N. S. Hatzakis, S. M. Kuiper, R. J. M. Nolte, A. E. Rowan and J. C. M. V. Hest, Angew. Chem., Int. Ed., 2007, 46, 7378–7382 CrossRef PubMed.
- S. K. Misra, P. Kondaiah, S. Bhattacharya and C. N. R. Rao, Small, 2012, 8, 131–143 CrossRef PubMed.
- J. Mayr, C. Saldias and D. D. Diaz, Chem. Soc. Rev., 2018, 47, 1484–1515 RSC.
- Z. Yang, G. Liang, L. Wang and B. Xu, J. Am. Chem. Soc., 2006, 128, 3038–3043 CrossRef PubMed.
- P. Broz, S. Driamov, J. Ziegler, N. Ben-Haim, S. Marsch, W. Meier and P. Hunziker, Nano Lett., 2006, 6, 2349–2353 CrossRef PubMed.
- F. J. Ostos, J. A. Lebrjn, M. L. Moy, M. L. Ljpez, A. Sanchez, A. Clavero, C. B. G. Calderjn, I. V. Rosado and P. L. Cornejo, Chem.–Asian J., 2017, 12, 679–689 CrossRef PubMed.
- Y. Jang and J. A. Champion, Acc. Chem. Res., 2016, 49, 2188–2198 CrossRef PubMed.
- D. Astruc, E. Boisselier and C. Ornelas, Chem. Rev., 2010, 110, 1857–1959 CrossRef PubMed.
- R. Dong, Y. Zhou and X. Zhu, Acc. Chem. Res., 2014, 47, 2006–2016 CrossRef PubMed.
- D. S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef PubMed.
- M. Suzuki and K. Hanabusa, Chem. Soc. Rev., 2009, 38, 967–975 RSC.
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef PubMed.
- T. S. Ingole, S. S. Kale, S. S. Babu and G. J. Sanjayan, Chem. Commun., 2016, 52, 10771–10774 RSC.
- W. Cai, G. T. Wang, Y. X. Xu, X. K. Jiang and Z. T. Li, J. Am. Chem. Soc., 2008, 130, 6936–6937 CrossRef PubMed.
- J. Zhou, G. Yu and F. Huang, Chem. Soc. Rev., 2017, 46, 7021–7053 RSC.
- Z. A. Ahmady and K. Kostarelos, Chem. Rev., 2016, 116, 3883–3918 CrossRef PubMed.
- X. Chi, H. Zhang, G. I. V. Zúñiga, G. M. Peters and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 5829–5832 CrossRef PubMed.
- B. H. Lipshutz, B. James, S. Vance and I. Carrico, Tetrahedron Lett., 1997, 38, 753–756 CrossRef.
- D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef PubMed.
- M. M. Pereira, J. F. Calvete, R. M. B. Carrilho and A. R. Abreu, Chem. Soc. Rev., 2013, 42, 6990–7027 RSC.
- S. Schenker, A. Zamfir, M. Freund and S. B. Tsogoeva, Eur. J. Org. Chem., 2011, 12, 2209–2222 CrossRef.
- Y. Chen, S. Yekta and A. K. Yudin, Chem. Rev., 2003, 103, 3155–3212 CrossRef PubMed.
- M. Shibasaki and S. Matsunaga, Chem. Soc. Rev., 2006, 35, 269–279 RSC.
- J. M. Brunel, Chem. Rev., 2007, 107, PR1–PR45 CrossRef.
- G. Von Kiedrowski, Bioorg. Chem. Front., 1993, 3, 113–146 Search PubMed.
- L. E. Orgel, Acc. Chem. Res., 1995, 28, 109–118 CrossRef PubMed.
- D. N. Reinhoudt, D. M. Rudkevich and F. de Jong, J. Am. Chem. Soc., 1996, 118, 6880–6889 CrossRef.
- J. S. Nowick, Q. Feng, T. Tjivikua, P. Ballester and J. Rebek Jr, J. Am. Chem. Soc., 1991, 113, 8831–8839 CrossRef.
- B. G. Bag and G. von Kiedrowski, Pure Appl. Chem., 1996, 68, 2145–2152 Search PubMed.
- B. G. Bag, S. Ghorai, S. K. Panja, S. K. Dinda and K. Paul, Prayogik Rasayan, 2017, 1, 74–79 Search PubMed.
- D. Sievers and G. von Kiedrowski, Nature, 1994, 389, 221–224 CrossRef PubMed.
- N. Giuseppone, Acc. Chem. Res., 2012, 45, 2178–2188 CrossRef PubMed.
- M. Noji, M. Nakajima and K. Koga, Tetrahedron Lett., 1994, 35, 7983–7984 CrossRef.
- M. Laupheimer, K. Jovic, F. E. Antunes, M. G. M. Miguel and C. Stubenrauch, Soft Matter, 2013, 9, 3661–3670 RSC.
- P. Patra, A. P. Rameshbabu, D. Das, S. Dhara, A. B. Panda and S. Pal, Polym. Chem., 2016, 7, 5426–5435 RSC.
- X. Ran, Y. Li, Q. Gao, W. Qiu and L. Guo, Asian J. Org. Chem., 2017, 6, 95–101 CrossRef.
- B. G. Bag, A. C. Barai, K. Wijesekera and P. Kittakoop, ChemistrySelect, 2017, 2, 4969–4973 CrossRef.
- B. G. Bag, S. Das, S. N. Hasan and A. C. Barai, RSC Adv., 2017, 7, 18136–18143 RSC.
- B. G. Bag and R. Majumdar, RSC Adv., 2014, 4, 53327–53334 RSC.
- B. Sun and D. T. Chiu, Langmuir, 2004, 20, 4614–4620 CrossRef PubMed.
- R. Xu, N. Takahashi and R. J. Simpson, J. Clin. Invest., 2016, 4, 1152–1162 CrossRef PubMed.
- M. Antonietti and S. Foerster, Adv. Mater., 2003, 15, 1323–1333 CrossRef.
- S. Dinda, M. Ghosh and P. K. Das, Langmuir, 2016, 32, 6701–6712 CrossRef PubMed.
- J. Naskar and A. Banerjee, Chem.–Asian J., 2009, 4, 1817–1823 CrossRef PubMed.
- J. Naskar, S. Roy, A. Joardar, S. Das and A. Banerjee, Org. Biomol. Chem., 2011, 9, 6610–6615 RSC.
Footnotes |
| † This paper is dedicated to Professor Guenter von Kiedrowski on his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06488a |
| This journal is © The Royal Society of Chemistry 2018 |

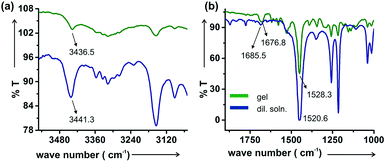
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency is at lower wave number (1676.8 cm−1) in the gel state than that in the diluted state (1685.5 cm−1). In contrast the N–H bending is at higher wavenumber (1528.3 cm−1) in the gel state than that after dilution (1520.6 cm−1).
O stretching frequency is at lower wave number (1676.8 cm−1) in the gel state than that in the diluted state (1685.5 cm−1). In contrast the N–H bending is at higher wavenumber (1528.3 cm−1) in the gel state than that after dilution (1520.6 cm−1).