Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced photocatalytic performance of WON@porous TiO2 nanofibers towards sunlight-assisted degradation of organic contaminants†
Yahia H. Ahmad a,
Assem T. Mohameda,
Mostafa H. Sliemb,
Aboubakr M. Abdullahb and
Siham Y. Al-Qaradawi
a,
Assem T. Mohameda,
Mostafa H. Sliemb,
Aboubakr M. Abdullahb and
Siham Y. Al-Qaradawi *a
*a
aDepartment of Chemistry and Earth Sciences, College of Arts and Sciences, Qatar University, Doha 2713, Qatar. E-mail: siham@qu.edu.qa
bCenter for Advanced Materials, Qatar University, Doha 2713, Qatar
First published on 21st September 2018
Abstract
In the last few decades, TiO2 has been widely used in different types of photocatalytic applications. However, the relatively large optical band gap (∼3.2 eV), low charge carrier mobility and consequently its low quantum efficiency limit its photocatalytic activity. Herein, we construct a novel nanostructured heterojunction of WON/TiO2 nanofibers (NFs) by integration of TiO2 nanofibers synthesized by electrospinning of a polymer solution containing a titanium(IV) butoxide precursor with WON nanoparticles fabricated via annealing of a WO3 precursor in dry ammonia at 700 °C. The synthesized photocatalysts were characterized using different spectroscopic techniques. Their photocatalytic performance towards the degradation of methyl orange, methylene blue, and phenol as model contaminants was investigated and the charge transfer process was elucidated and compared to that of a TiO2/WO3 heterojunction.
Introduction
Environmental pollution caused by rapid worldwide industrial growth is considered as one of the most critical environmental issues especially those accompanied with bioaccumulation and toxicity. The lack of effective treatment at the contamination sources causes the release of hazardous materials into the water effluents. Some of these water contaminants such as heavy metal ions and organic dyes are extremely toxic and non-biodegradable. Different structures of organic dyes can exist in water which makes their removal a complicated issue. These dyes can undergo hydrolysis, oxidation or other chemical reaction which lead to formation of toxic by-products that severely affect human and aquatic life. Total removal of these dyes from water is a challenging issue due to their high solubility and high persistency. In this regard, different procedures have been devoted to decontaminate water from these hazardous materials. Among them, elector-coagulation, reverse osmosis, electro-oxidation, flocculation, adsorption and chemical oxidation. However, the aforementioned techniques have drawbacks of high cost in addition to the formation of deleterious by-products.Recently, degradation of organic pollutants assisted only by solar energy as a clean renewable source has received considerable interest. In this respect, different types of photocatalysts have been devoted for this aim including plasmonic metals-based materials,1–4 metal–organic frameworks (MOFs),5–10 organic polymers based compounds,11,12 graphene and carbon nitride-based materials,13–15 metal complexes,16,17 hybrid compounds, and composites.18–20 Among them, metal oxides such as TiO2, WO3, CeO2, Cu2O and ZnO have been extensively used as photocatalysts for such application type.21 Owing to its chemical stability, rational resistance to photo-corrosion, high abundance, non-toxicity, and low cost, TiO2 is considered as one of the most widely used semiconductors in photocatalysis. In addition, TiO2 and TiO2 based catalysts can create hydroxyl radicals (·OH) that can efficiently degrade many pollutants. Several factors can influence the photocatalytic activity of TiO2, among them, the crystalline phase, morphology, surface area, exposed crystal facets, defects density, type of defects, and the degree of crystallinity.22–24 Nevertheless, the wide optical band gap (∼3.2 eV) which limits its absorption to UV region (∼4% sunlight), low electron mobility, short hole diffusion length, relatively fast recombination rate of photo-generated electron–hole pairs and consequently the low quantum efficiency lessen its photocatalytic performance.25,26 Different strategies have been devoted to decrease the band gap of TiO2 and enhance its photocatalytic performance, for instance, doping with metal27–29 and nonmetal,30–32 surface sensitization, coupling with other semiconductors, creation of lattice defects and fabrication of different morphologies, such as nanospheres,33 nanowires,34 nanofibers,35 nanotubes,36,37 nanorods,38 nanobelts,39 mesoporous TiO2,40 and 2D TiO2.41 Coupling of TiO2 with a semiconductor with more negative conduction band can greatly enhance photoactivity by improving the electron–hole separation, increasing the life time of charge carriers by decreasing the rate of charge recombination, and consequently facilitating the interfacial charge transfer between the semiconductor and the adsorbed molecules. Many semiconductors have been coupled with TiO2 to enhance its photocatalytic performance, among them, oxides such as ZnO,42 GeO2,43 Fe2O3,44 sulfides such as FeS2,45 MoS2,46 Cu2ZnSnS4,47 and wide variety of other semiconductors as BiVO4,48 C3N4,49 and CdSe.50 WO3 is a semiconductor with moderate band gap (∼2.8 eV), it is also chemically stable in acidic and nearly acidic media. It exhibits high rate of recombination of photo-generated charge carriers, the factor which greatly limits its performance in photocatalytic applications.51,52 Doping of WO3 with metal or non-metal can modify the band gap energy and hence widen its restricted absorption range from UV to visible region and enhance its photocatalytic activity as compared to WO3.53 Substitution of O with the less electronegative N to form oxynitride, WOXNY shifts the valence band to more negative potential due to contribution of N 2p in the valence band and consequently decreases the optical band gap.54 Different synthesis procedures have been devoted for the synthesis of tungsten oxynitride (WOXNY) such as DC magnetron sputtering,55 metal–organic chemical vapor deposition (MOCVD)56 and the more common method, annealing under ammonia atmosphere.57,58 The performance of WOXNY in energy conversion and storage has been reported, however its application in photocatalysis was not reported before. Triggered by this, herein we reported facile synthesis of a novel TiO2/WON heterojunction by hybridization of nanoporous TiO2 nanofibers synthesized from electrospinning of polyvinylpyrrolidone solution of titanium(IV) butoxide with tungsten oxynitride (WOXNY) synthesized by high temperature ammonolysis of WO3 precursor. The synthesized photocatalyst was characterized using different techniques UV-vis, SEM-EDX, TEM, BET, XRD, XPS, and Raman spectroscopy. Moreover, its photocatalytic activity for degradation of methyl orange, methylene blue, and phenol as model contaminants was also examined. Finally, a mechanism that accounts for the charge transfer and photocatalytic activity of WON/TiO2 was proposed compared to that of WO3/TiO2 heterojunction.
Experimental
Materials synthesis
Materials characterization
The morphology and composition of the as-synthesized materials was examined via field emission scanning electron microscope (FESEM, Philips XL-30, FEI Co., USA) equipped with an energy dispersive X-ray spectrometer (EDX). X-ray diffraction pattern, XRD was recorded using X'Pert-Pro MPD diffractometer (PANalytical Co., Netherlands) via using of Cu Kα X-ray source (λ = 1.54059 Å) as the X-ray source at the 2θ range 10–80. Raman spectra were investigated using a DXR 2 Raman Microscope (Thermo Fisher Scientific, USA) using a 780 nm laser source for excitation. The chemical composition and the valence states of elements were examined via XPS spectrophotometer Kratos Axis Ultra XPS equipped with a monochromatic Al Kα radiation source (1486.6 eV) under UHV environment (ca. 5 × 10−9 torr). All binding energies were calibrated to the C 1s peak at 284.8 eV. The BET surface area was obtained using N2 adsorption isotherms and samples were degassed for 24 h at 100 °C under vacuum before carrying out the measurements.Electrochemical impedance measurements
All electrochemical measurements were performed at room temperature into. A Pt wire, saturated calomel electrode (SCE) and the photocatalyst deposited on FTO glass were used as auxiliary, reference, and working electrodes, respectively. Electrochemical impedance spectroscopy (EIS) measurements were performed conventional three electrode cell using a Gamry electrochemical analyser (reference 3000, Gamry Co., USA) using AC voltage pulse with 5 mV amplitude in the frequency domain from 0.01 Hz to 100 kHz. Electrochemical impedance spectroscopy (EIS) measurements were carried out in each electrolyte solution at frequencies ranging from 0.1 Hz to 100 kHz with an AC voltage pulse of 10 mV amplitude.Photocatalytic activity measurements
Photocatalytic performance of different used photocatalysts was examined by degradation of methyl orange, MO and methylene blue, MB. A 100 W Xe arc lamp (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013, Abet Technologies Inc., Milford, USA) was used as the light source. The light intensity was 100 mW cm−2. 50 mg of the photocatalyst was dispersed in an aqueous solution of MO (100 mL, 10 mg L−1), MB (100 mL, 10 mg L−1), and phenol (100 mL, 40 mg L−1) Prior to the light irradiation, the mixtures were magnetically stirred for 30 min in dark in order to reach the adsorption–desorption equilibrium between the photocatalyst and synthetic dye. At different time intervals, aliquots (4 mL) of the test solution were sampled and centrifuged to remove the photocatalyst, then concentration was monitored by measuring the absorbance at λmax 463 nm, 664 nm, and 270 nm for MO, MB, and phenol, respectively via UV-vis spectrophotometer (Agilent 8453, China).
013, Abet Technologies Inc., Milford, USA) was used as the light source. The light intensity was 100 mW cm−2. 50 mg of the photocatalyst was dispersed in an aqueous solution of MO (100 mL, 10 mg L−1), MB (100 mL, 10 mg L−1), and phenol (100 mL, 40 mg L−1) Prior to the light irradiation, the mixtures were magnetically stirred for 30 min in dark in order to reach the adsorption–desorption equilibrium between the photocatalyst and synthetic dye. At different time intervals, aliquots (4 mL) of the test solution were sampled and centrifuged to remove the photocatalyst, then concentration was monitored by measuring the absorbance at λmax 463 nm, 664 nm, and 270 nm for MO, MB, and phenol, respectively via UV-vis spectrophotometer (Agilent 8453, China).
Results and discussion
Fig. 1a FE-SEM image of the electrospun TiO2 NFs after annealing in air at 450 °C for 2 h. TiO2 NFs show high aspect ratio with uniform smooth surfaces with few beads. The length of fibers ranges from few micrometers to tens of micrometers, whereas their diameters show wide distribution ranging from 40 to 150 nm.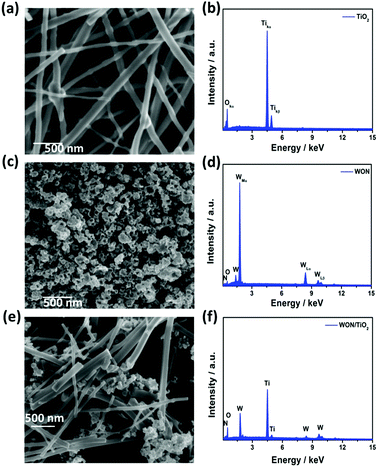 | ||
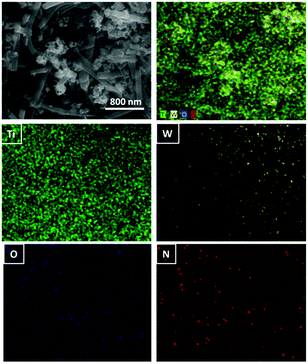
| Fig. 1 SEM Images of (a) TiO2 NFs, (c) WON, and (e) WON/TiO2 and EDX Analysis of (b) TiO2 NFs, (d) WON, and (f) WON/TiO2. | ||
Fig. 1c exhibits the SEM image of WON nanoparticles synthesized by ammonolysis of WO3 nanopowder. The particles exhibit distorted morphology with average particle size less than 100 nm. WON/TiO2 micrograph (Fig. 2e) manifests distribution of WON aggregates among TiO2 nanofibers with significant decrease in the length of TiO2 nanofibers due to ultrasonication effect.
EDX analysis of different materials was conducted to investigate the elemental composition of different photocatalysts. EDX spectrum of TiO2 nanofibers (Fig. 2b) revealed a composition of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O 31.7
O 31.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68.3, and that of WON expressed a composition of (W
68.3, and that of WON expressed a composition of (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 42.8
N 42.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.7
29.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27.5). WON/TiO2 expressed elemental composition of (Ti
27.5). WON/TiO2 expressed elemental composition of (Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 30.8
N 30.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64.3
64.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3) with well distribution of different elements throughout the composite (Fig. 2).
2.3) with well distribution of different elements throughout the composite (Fig. 2).
TEM image of TiO2 (Fig. 3a) indicates that annealed TiO2 showed a wide distribution of diameter ranging from 40 to 170 nm with average diameter of 104 nm (Fig. 3b). HR-TEM image (Fig. 3c) confirms the nanoscale porosity of annealed fibers. TEM image of WON particles showed nanosized particles with average diameter of 37 nm (Fig. 3e) interconnected together in large aggregates (Fig. 3d and f). Lattice resolved HR-TEM image of WON/TiO2 displayed well defined lattice fringes of WON and TiO2 which were assigned to (111) facet of WON with d-spacing of 2.3 Å and (101) of TiO2 with d-spacing of 3.5 Å (Fig. 4).
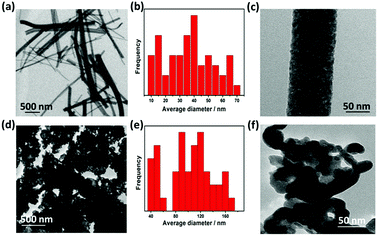 | ||
| Fig. 3 TEM low resolution images (a and d), particle size distribution (b and e), and high resolution images (c and f) of TiO2 NFs and WON, respectively. | ||
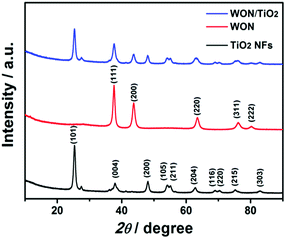
The X-ray diffraction pattern of TiO2 NFs, WON, and WON/TiO2 composite depicts a well crystalline structures (Fig. 5). The diffraction pattern of TiO2 confirms the presence of anatase as a predominant phase (JCPDS no. 00-021-1271) with small diffraction peaks at 27.4° and 36.1° assigned to (110) and (101) planes of rutile phase, respectively. The diffraction pattern of WON matches that of face centered cubic W0.62(N,O) (JCPDS no. 025-1254). The XRD of WON/TiO2 matches the integrated pattern of TiO2 and WON with slight decrease in the crystallite size as revealed from increased values of FWHM of the diffraction peaks.
Raman spectroscopy is hired to investigate the crystallinity and the formation of chemical bonds.59 Fig. S1† presents the Raman spectra of TiO2 NFs, WON, and WON/TiO2 (all details are available in ESI† Section).
The surface composition, chemical bonding and oxidation states of WON/TiO2 heterojunction was investigated compared to TiO2 NFs and WON using XPS. Fig. 6a shows the survey spectrum of WON/TiO2 composite, it demonstrate the existence of Ti and O as main constituents and W and N as minor elements, in addition to presence of surface adsorbed carbon. C 1s peak at 284.6 eV was used for calibration. High resolution spectrum of Ti displayed a well resolved doublet at 458.3 and 464.0 eV corresponding to Ti 2p3/2 and Ti 2p1/2, respectively (Fig. 6b). They are assigned to Ti4+ valence state in TiO2.60 The high resolution XPS spectrum of tungsten W 4f (Fig. 6c) was deconvoluted into four peaks. Two at 37.6 and 35.6, corresponding to 4f5/2 and 4f7/2, respectively and assigned to W6+ oxidation state and the other two peaks at 34.6 and 33.0 eV attributed to lower oxidation state W5+ of oxynitride.58
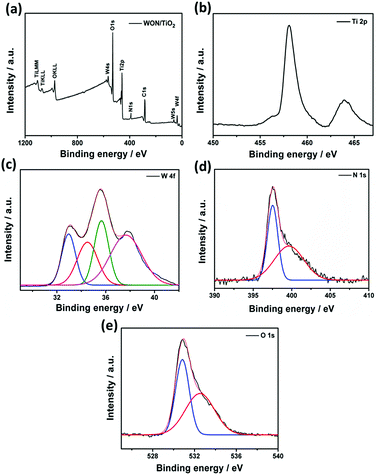 | ||
| Fig. 6 XPS spectrum of (a) WON/TiO2 and high resolution spectra of (b) Ti 2p, (c) W 4f, (d) N 1s, and (e) O 1s. | ||
Two different types of nitrogen can be detected from the XPS of N 1s (Fig. 6d). Intense peak at 397.5 eV corresponding to N bonded to W–O and a smaller peak at 400.0 eV assigned to surface adsorbed nitrogen and/or nitrogen trapped in the surface layers as nitrogen.61 Deconvolution of high resolution XPS spectrum of oxygen reveals two peaks at 530.8 and 532.5 eV corresponding to lattice oxygen O2−, and oxygen bonded to nitrogen (O–N), respectively (Fig. 6e).62,63 The binding energy of Ti 2p was shifted to higher binding energies in the composite WON/TiO2 relative to TiO2 NFs which confirms the interaction between mixed photocatalysts in the composite (Fig. S3†). In addition the binding energies of W 4f7/2 and 4f5/2 were shifted to lower values in WON/TiO2 confirming the interaction between TiO2 and WON (Fig. S4†).64
Fig. 7a represents N2 adsorption/desorption isotherm curves of TiO2 NFs, WON, and WON/TiO2. The calculated specific surface area of WON/TiO2, TiO2 NFs, and WON were 58.22, 52.82, and 8.54 m2 g−1, respectively, whereas the calculated pore size of WON/TiO2 and TiO2 NFs was 6.11 and 8.53 nm, respectively (Fig. 7b). The increased specific surface area of WON/TiO2 can create more active sites which ease the access of reactant molecules and hence enhance the photocatalytic activity.
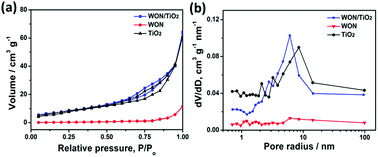 | ||
| Fig. 7 (a) Adsorption/desorption isotherm curves, and (b) BJH pore size curves of TiO2 NFs, WON, and WON/TiO2. | ||
The optical properties of the synthesized photocatalysts were investigated using UV-vis absorption spectroscopy (Fig. 8). TiO2 NFs showed absorption edge at 384 nm whereas WON-5/TiO2 exhibited slight shift in the absorption edge at 398 nm. The absorption spectrum of WON show no absorption edge. It shows light absorption over the whole visible range and this is indicated by its black color. The optical band gap of semiconductors can be calculated using Tauc plots from UV-vis absorption spectrum.
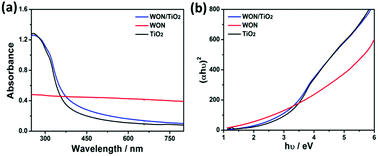 | ||
| Fig. 8 UV-vis absorption spectra (a) of TiO2 NFs, WON, and WON/TiO2 and Tauc plots (b) of TiO2 NFs, WON, and WON/TiO2. | ||
The band gap, Eg can be calculated by:
| αhν = A(hν − Eg)1/2 |
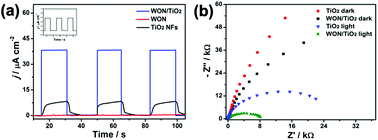
Transient photocurrents vs. time responses of different photoelectrodes in 0.1 M Na2SO4 solution under simulated sunlight illumination are displayed in Fig. 9a. WON/TiO2 photoelectrode expressed better photoswitching with faster response time and enhanced photostability than TiO2 photoelectrode. The coupled photoelectrode also displayed enhanced photocurrent density which is 4.6 and 635 times more greater than TiO2 and WON photoelectrodes, respectively. The extremely low photocurrent density expressed by WON despite its low band gap confirms its poor photo-induced charge separation and consequently faint photocatalytic activity.
Electrochemical impedance spectroscopy, EIS was hired to investigate the charge transfer process at WON/TiO2 and TiO2 photo-electrodes. Fig. 9b displays Nyquist plots of WON/TiO2 and TiO2 in 0.1 M Na2SO4 solution at open circuit potential under dark and simulated solar light illumination. The recorded Nyquist plot of WON/TiO2 exhibited a lower semicircle diameter than TiO2 demonstrating decreased charge transfer resistance and lower conductivity. This proves that the heterojunction facilitates charge separation and transportation relative to pure TiO2.65
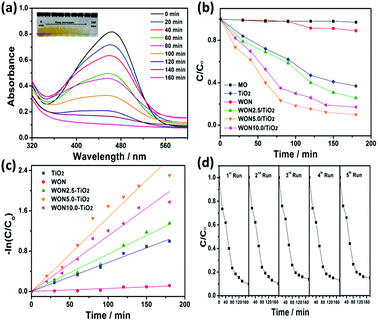
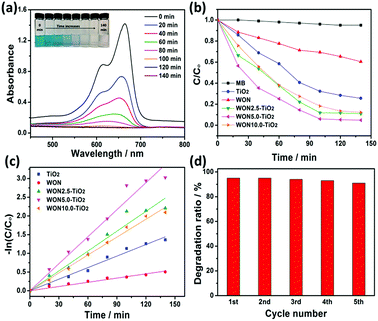
The photocatalytic performance of the synthesized photocatalysts was evaluated by studying degradation of MO and MB as model contaminants. The absorption spectra of MO recorded in the presence of WON-5/TiO2 is given in Fig. 10a. The spectra were recorded from 400–700 nm. From the measured spectra, the maximum absorbance at 464 nm decreases gradually with the irradiation time indicating the degradation of MO.
The degradation efficiency can be evaluated as C/Co, where C and Co are defined as the remnant and initial concentration of the dye, respectively. Fig. 10b represents the photo-degradation rate of MO under simulated sunlight in the absence and presence of TiO2 NFs, WON, and their mixtures in different ratios particularly, 2.5%, 5%, and 10% WON. No detectable photo-degradation was observed for MO over a time interval of 180 min in absence of photocatalyst, which confirms the stability of MO under UV-vis light irradiation and that self-photolysis is negligible during the course of degradation process. Only 10% of MO was degraded in WON after 180 min confirming the weak photocatalytic performance of WON despite its relatively small band gap energy which can be attributed to fast annihilation of the photo-generated hole–electron pairs. Notwithstanding the relatively larger band gap of TiO2 compared to WON, but its photocatalytic activity is higher due to relatively larger life time of charge carriers than WON. Obviously, WON-5/TiO2 exhibited the optimum composition with the best photocatalytic performance among all studied photocatalysts. The degradation ratio of MO over WON-5/TiO2 reached 90% after 180 min under simulated sunlight irradiation, while it needed 180 min for WON-10/TiO2 and WON-2.5/TiO2 to reach about 83% and 74%, respectively. TiO2 and WON expressed degradation rates of 63%, and 11%, respectively after the same time interval (Fig. 10b).
The photodegradation of the studied dyes follows the first order kinetics model: ln(C/Co) = kt where k is the degradation rate constant. The estimated rate constants for the used photocatalysts are 5.92 × 10−4, 5.75 × 10−3, 7.4 × 10−3, 1.47 × 10−2, and 1.1 × 10−2 min−1 for WON, TiO2, WON-2.5/TiO2, WON-5/TiO2, and WON-10/TiO2, respectively (Fig. 10c). The rate constant of WON-5/TiO2 is almost 25 times as greater as WON and 2.5 times that of TiO2. The photo-stability of a photocatalyst can be evaluated by measuring its recycle degradation performance for the contaminant. The first five degradation cycles of WON-5/TiO2 show no significant change in the degradation efficiency of WON-5/TiO2 towards photo-degradation of MO (Fig. 10d).
Typical absorption spectrum of MB in presence of WON-5/TiO2 is given in Fig. 11a. The maximum absorbance at 664 nm decreases gradually with irradiation time indicates the decrease of MB concentration as a result of degradation. The rate of decay of MB over different expressed as C/Co vs. t is given in Fig. 11b. No significant degradation was observed under light irradiation in absence of photocatalyst during the course of experiment which confirms that MB is stable against self-photolysis at the selected range of wavelength. Obviously, WON-5/TiO2 expressed the optimum photocatalytic activity compared to other counterparts. For instance, after 100 min of light irradiation, WON-5/TiO2 manifested highest photocatalytic activity with 94% decay for MB, whereas WON-2.5/TiO2, WON-10/TiO2, TiO2, and WON exhibited 89, 88, 74, and 39% decay at the same time interval.
The plot of ln(C/Co) vs. time for different photocatalysts showed straight lines with a slope equals the rate constant of the photo-degradation reaction, k (Fig. 11c). The values of calculated rate constants for WON, TiO2, WON-2.5/TiO2, WON-5/TiO2, and WON-10/TiO2 are 0.0038, 0.0103, 0.0177, 0.02408, and 0.0159 min−1, respectively. Once more, WON-5/TiO2 show the highest rate constant which is almost 2.5 and 6.5 times as greater as that of TiO2 and WON, respectively.
Durability of the photocatalyst is a key factor that determines the possibility of its commercialization. Repeating of MB degradation experiments for five times showed no considerable decay in the degradation efficiency of WON-5/TiO2 which confirms its stability (Fig. 11d).
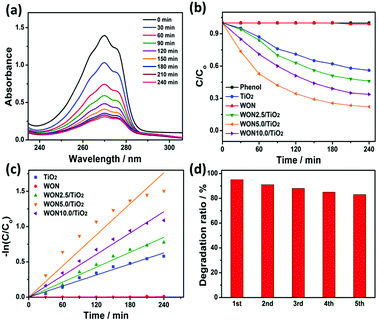
The photocatalytic performance of WON/TiO2 was also examined in the photodegradation of phenol as a model hazardous contaminant. Typical absorption spectra were obtained for WON-5/TiO2 in phenol solution with maximum absorption at 270 nm of decreasing intensity with time (Fig. 12a).
Plotting of C/Co vs. time for different photocatalysts confirms the enhanced photocatalytic activity of WON-5/TiO2 over its counterparts (Fig. 12b). In addition, the estimated rate constant, k for different photocatalysts were 2.46 × 10−5, 2.61 × 10−3, 3.53 × 10−3, 7.35 × 10−3, and 5.05 × 10−3 min−1 for WON, TiO2, WON-2.5/TiO2, WON-5/TiO2, and WON-10/TiO2, respectively (Fig. 12c). Durability tests for WON-5/TiO2 revealed slight decay in its performance over the first 5 cycles probably due to deactivation of photocatalyst active sites by adsorbed degradation products (Fig. 12d).
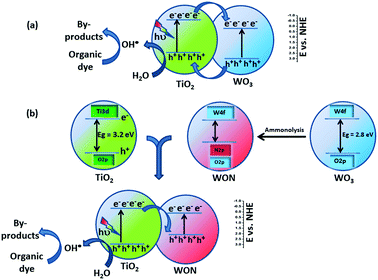
The enhancement in photocatalytic activity of WON/TiO2 can be attributed to the charge transfer taking place at the heterojunction. In case of WO3/TiO2, both the valence band and conduction bands of WO3 exists at more positive potential compared to their counterparts of TiO2. The conduction band, CB of WO3 can act as a sink of electrons and capture the photo-generated CB electrons of TiO2 which lies at a potential of −0.33 eV vs. NHE,64 whereas, the valence band, VB of TiO2 acts as a sink of holes, so holes will transfer from VB of WO3 to the VB of TiO2 that lies at a potential of 2.95 eV vs. NHE and thus enhance electron–hole separation and hence enhance overall photocatalytic activity (Scheme 1a).63,64
After annealing of WO3 in ammonia, nitrogen N3− replace O2− in the lattice of the oxide and hence the VB of WON shifts to more negative potential than that of TiO2 as a result of partial substitution of oxygen with nitrogen accompanied with the contribution of N 2p in the valence band.54,66–68 Hence, the photo-induced holes in the VB of WON cannot transfer to the VB of TiO2 which lies at more negative potential. Interestingly, the enhanced photocatalytic activity of WON/TiO2 heterojunction compared to TiO2 can be ascribed to that the generated charge carriers (electrons) in the CB of TiO2 migrated to the VB WON and participated in the reduction reaction (Scheme 1b). Such mechanism was assumed by many authors to account for photoactivity of different heterojunctions.69,70 The redox potential of (O2/·O2−) is −0.33 eV vs. NHE which is more negative than CB energy of WON, hence the accumulated electrons on the CB of WON cannot reduce O2 to form ·O2−. On the other hand, the redox potential of (H2O/·OH) is 2.4 eV vs. NHE which is less positive than the VB of TiO2 (2.95 V vs. NHE) so ·OH radicals are the expected formed active species.64,71,72
Conclusions
WON/TiO2 nanocomposite was prepared by coupling of electrospun and annealed TiO2 nanofibers with WON synthesized by ammonia annealing of WO3. The nanocomposite displayed lower charge transfer resistance and enhanced photocatalytic activity compared to TiO2 and WON counterparts. WON-5/TiO2 expressed the optimum composition with best performance towards simulated sunlight assisted-photodegradation of MO, MB, and phenol. The photoactivity of WON/TiO2 can be attributed to the enhanced separation of electron–hole pairs, high surface area and porosity, and decreased band gap energy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was made possible by the NPRP Grant No. NPRP 7-485-1-091 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.References
- J. Cai, J. Huang and Y. Lai, J. Mater. Chem. A, 2017, 5, 16412–16421 RSC.
- M. Misra, N. Singh and R. K. Gupta, Catal. Sci. Technol., 2017, 7, 570–580 RSC.
- M. Mittal, A. Gupta and O. P. Pandey, Sol. Energy, 2018, 165, 206–216 CrossRef.
- F. Zheng and Z. Zhu, ACS Appl. Nano Mater., 2018, 1, 1141–1149 CrossRef.
- Q. Li, Z.-L. Fan, D.-X. Xue, Y.-F. Zhang, Z.-H. Zhang, Q. Wang, H.-M. Sun, Z. Gao and J. Bai, J. Mater. Chem. A, 2018, 6, 2148–2156 RSC.
- Q. Li, D.-X. Xue, Y.-F. Zhang, Z.-H. Zhang, Z. Gao and J. Bai, J. Mater. Chem. A, 2017, 5, 14182–14189 RSC.
- M. Liu, X.-F. Yang, H.-B. Zhu, B.-S. Di and Y. Zhao, Dalton Trans., 2018, 47, 5245–5251 RSC.
- N. Zhao, F. Sun, N. Zhang and G. Zhu, Cryst. Growth Des., 2017, 17, 2453–2457 CrossRef.
- X. Liu, J. Luo, Y. Zhu, Y. Yang and S. Yang, J. Alloys Compd., 2015, 648, 986–993 CrossRef.
- X. Liu, W. Gong, J. Luo, C. Zou, Y. Yang and S. Yang, Appl. Surf. Sci., 2016, 362, 517–524 CrossRef.
- N. Xu, R.-L. Wang, D.-P. Li, X. Meng, J.-L. Mu, Z.-Y. Zhou and Z.-M. Su, Dalton Trans., 2018, 47, 4191–4197 RSC.
- S. Zhou, F. Wang, S. Balachandran, G. Li, X. Zhang, R. Wang, P. Liu, Y. Ding, S. Zhang and M. Yang, RSC Adv., 2017, 7, 52375–52381 RSC.
- P. Khare, A. Singh, S. Verma, A. Bhati, A. K. Sonker, K. M. Tripathi and S. K. Sonkar, ACS Sustainable Chem. Eng., 2017, 6, 579–589 CrossRef.
- R. C. Pawar, Y. Pyo, S. H. Ahn and C. S. Lee, Appl. Catal., B, 2015, 176, 654–666 CrossRef.
- A. Anshuman, S. Saremi-Yarahmadi and B. Vaidhyanathan, RSC Adv., 2018, 8, 7709–7715 RSC.
- C.-Y. Liu, L.-Y. Xu, Z.-G. Ren, H.-F. Wang and J.-P. Lang, Cryst. Growth Des., 2017, 17, 4826–4834 CrossRef.
- N. Hussain and V. K. Bhardwaj, Dalton Trans., 2016, 45, 7697–7707 RSC.
- Y. Y. Lee, J. H. Moon, Y. S. Choi, G. O. Park, M. Jin, L. Y. Jin, D. Li, J. Y. Lee, S. U. Son and J. M. Kim, J. Phys. Chem. C, 2017, 121, 5137–5144 CrossRef.
- P. Atkin, T. Daeneke, Y. Wang, B. Carey, K. Berean, R. Clark, J. Ou, A. Trinchi, I. Cole and K. Kalantar-Zadeh, J. Mater. Chem. A, 2016, 4, 13563–13571 RSC.
- C. Liang, C.-G. Niu, X.-J. Wen, S.-F. Yang, M.-C. Shen and G.-M. Zeng, New J. Chem., 2017, 41, 5334–5346 RSC.
- C. Ray and T. Pal, J. Mater. Chem. A, 2017, 5, 9465–9487 RSC.
- M. Dahl, Y. Liu and Y. Yin, Chem. Rev., 2014, 114, 9853–9889 CrossRef PubMed.
- K. Bourikas, C. Kordulis and A. Lycourghiotis, Chem. Rev., 2014, 114, 9754–9823 CrossRef PubMed.
- S. Liu, J. Yu and M. Jaroniec, Chem. Mater., 2011, 23, 4085–4093 CrossRef.
- G. Liu, J. Pan, L. Yin, J. T. Irvine, F. Li, J. Tan, P. Wormald and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 3233–3238 CrossRef.
- C. Zhao, H. Luo, F. Chen, P. Zhang, L. Yi and K. You, Energy Environ. Sci., 2014, 7, 1700–1707 RSC.
- D. P. Jaihindh, C.-C. Chen and Y.-P. Fu, RSC Adv., 2018, 8, 6488–6501 RSC.
- R. Trofimovaite, C. M. A. Parlett, S. Kumar, L. Frattini, M. A. Isaacs, K. Wilson, L. Olivi, B. Coulson, J. Debgupta, R. E. Douthwaite and A. F. Lee, Appl. Catal., B, 2018, 232, 501–511 CrossRef.
- N. Singh, J. Prakash, M. Misra, A. Sharma and R. K. Gupta, ACS Appl. Mater. Interfaces, 2017, 9, 28495–28507 CrossRef PubMed.
- Q. Wang, J. Huang, H. Sun, K.-Q. Zhang and Y. Lai, Nanoscale, 2017, 9, 16046–16058 RSC.
- A. Biswas, A. Chakraborty and N. R. Jana, ACS Appl. Mater. Interfaces, 2018, 10, 1976–1986 CrossRef PubMed.
- M. Wang, J. Han, Y. Hu and R. Guo, RSC Adv., 2017, 7, 15513–15520 RSC.
- X. Wang, L. Bai, H. Liu, X. Yu, Y. Yin and C. Gao, Adv. Funct. Mater., 2018, 28, 1704208 CrossRef.
- E. Arcadipane, R. Sanz, G. Amiard, S. Boninelli, G. Impellizzeri, V. Privitera, J. Bonkerud, C. Bhoodoo, L. Vines and B. G. Svensson, RSC Adv., 2016, 6, 55490–55498 RSC.
- J. Zhang, Y. B. Cai, X. Hou, H. Zhou, H. Qiao and Q. Wei, J. Phys. Chem. C, 2018, 122, 8946–8953 CrossRef.
- M. Krbal, H. Sopha, D. Pohl, L. Benes, C. Damm, B. Rellinghaus, J. Kupčík, P. Bezdička, J. Šubrt and J. M. Macak, Electrochim. Acta, 2018, 264, 393–399 CrossRef.
- W.-C. Chen, M.-H. Yeh, L.-Y. Lin, R. Vittal and K.-C. Ho, ACS Sustainable Chem. Eng., 2018, 6, 3907–3915 CrossRef.
- Y. Jiang, H. Ning, C. Tian, B. Jiang, Q. Li, H. Yan, X. Zhang, J. Wang, L. Jing and H. Fu, Appl. Catal., B, 2018, 229, 1–7 CrossRef.
- X. Yu, Z. Zhao, J. Zhang, W. Guo, L. Li, H. Liu and Z. L. Wang, CrystEngComm, 2017, 19, 129–136 RSC.
- K. Lan, Y. Liu, W. Zhang, Y. Liu, A. Elzatahry, R. Wang, Y. Xia, D. Al-Dhayan, N. Zheng and D. Zhao, J. Am. Chem. Soc., 2018, 140, 4135–4143 CrossRef PubMed.
- S. L. Wang, X. Luo, X. Zhou, Y. Zhu, X. Chi, W. Chen, K. Wu, Z. Liu, S. Y. Quek and G. Q. Xu, J. Am. Chem. Soc., 2017, 139, 15414–15419 CrossRef PubMed.
- C. Sun, Q. Xu, Y. Xie, Y. Ling and Y. Hou, J. Mater. Chem. A, 2018, 6, 8289–8298 RSC.
- K. Natarajan, H. C. Bajaj and R. J. Tayade, Mater. Chem. Front., 2018, 2, 741–751 RSC.
- H. Han, F. Riboni, F. Karlicky, S. Kment, A. Goswami, P. Sudhagar, J. Yoo, L. Wang, O. Tomanec and M. Petr, Nanoscale, 2017, 9, 134–142 RSC.
- J. Rashid, S. Saleem, S. U. Awan, A. Iqbal, R. Kumar, M. Barakat, M. Arshad, M. Zaheer, M. RRafique and M. Awad, RSC Adv., 2018, 8, 11935–11945 RSC.
- B. Chen, Y. Meng, J. Sha, C. Zhong, W. Hu and N. Zhao, Nanoscale, 2018, 10, 34–68 RSC.
- M. P. Suryawanshi, U. V. Ghorpade, S. W. Shin, M. G. Gang, X. Wang, H. Park, S. H. Kang and J. H. Kim, ACS Catal., 2017, 7, 8077–8089 CrossRef.
- G. Odling and N. Robertson, ChemPhysChem, 2016, 17, 2872–2880 CrossRef PubMed.
- W. Wang, Y. Liu, J. Qu, Y. Chen, M. O. Tadé and Z. Shao, ChemPhotoChem, 2017, 1, 35–45 CrossRef.
- W. Chen, S. Yu, Y. Zhong, X.-B. Fan, L.-Z. Wu and Y. Zhou, New J. Chem., 2018, 42, 4811–4817 RSC.
- J. Cao, B. Luo, H. Lin, B. Xu and S. Chen, Appl. Catal., B, 2012, 111, 288–296 CrossRef.
- M. Yan, Y. Wu, F. Zhu, Y. Hua and W. Shi, Phys. Chem. Chem. Phys., 2016, 18, 3308–3315 RSC.
- T. Varga, H. Haspel, A. Kormányos, C. Janáky, Á. Kukovecz and Z. Kónya, Electrochim. Acta, 2017, 256, 299–306 CrossRef.
- M. Ahmed and G. Xinxin, Inorg. Chem. Front., 2016, 3, 578–590 RSC.
- X. Sun, Z. Liu and H. Cao, Thin Solid Films, 2011, 519, 3032–3036 CrossRef.
- S. Cwik, A. P. Milanov, V. Gwildies, T. Thiede, V. Vidyarthi, A. Savan, R. Meyer, H.-W. Becker, D. Rogalla and A. Ludwig, ECS Trans., 2010, 28, 159–165 Search PubMed.
- O. Kartachova, A. M. Glushenkov, Y. Chen, H. Zhang, X. J. Dai and Y. Chen, J. Power Sources, 2012, 220, 298–305 CrossRef.
- M. Yu, Y. Han, X. Cheng, L. Hu, Y. Zeng, M. Chen, F. Cheng, X. Lu and Y. Tong, Adv. Mater., 2015, 27, 3085–3091 CrossRef PubMed.
- L. M. Martínez Tejada, A. Muñoz, M. Centeno and J. A. Odriozola, J. Raman Spectrosc., 2016, 47, 189–197 CrossRef.
- A. S. Kshirsagar, A. Gautam and P. K. Khanna, J. Photochem. Photobiol., A, 2017, 349, 73–90 CrossRef.
- N. C. Saha and H. G. Tompkins, J. Appl. Phys., 1992, 72, 3072–3079 CrossRef.
- E. Hernández-Rodríguez, A. Márquez-Herrera, E. Zaleta-Alejandre, M. Meléndez-Lira, W. de la Cruz and M. Zapata-Torres, J. Phys. D: Appl. Phys., 2012, 46, 045103 CrossRef.
- Y. Hunge, A. Yadav, M. Mahadik, R. Bulakhe, J. Shim, V. Mathe and C. Bhosale, Opt. Mater., 2018, 76, 260–270 CrossRef.
- Z. Yang, L. Chen, Y. Yang, J. Wang, Y. Huang, X. Liu and S. Yang, Semicond. Sci. Technol., 2017, 32, 065008 CrossRef.
- Y. H. Ahmad, K. Eid, K. A. Mahmoud and S. Y. AlQaradawi, New J. Chem., 2018, 42, 14239–14245 RSC.
- K. Maeda, M. Higashi, D. Lu, R. Abe and K. Domen, J. Am. Chem. Soc., 2010, 132, 5858–5868 CrossRef PubMed.
- M. Higashi, R. Abe, T. Takata and K. Domen, Chem. Mater., 2009, 21, 1543–1549 CrossRef.
- S. Mohamed and E. Shaaban, Mater. Chem. Phys., 2010, 121, 249–253 CrossRef.
- X. Wang, G. Liu, L. Wang, J. Pan, G. Q. M. Lu and H.-M. Cheng, J. Mater. Chem., 2011, 21, 869–873 RSC.
- N. L. Reddy, S. Emin, M. Valant and M. Shankar, Int. J. Hydrogen Energy, 2017, 42, 6627–6636 CrossRef.
- H. Cheng, J. Hou, O. Takeda, X.-M. Guo and H. Zhu, J. Mater. Chem. A, 2015, 3, 11006–11013 RSC.
- D. Wang, L. Guo, Y. Zhen, L. Yue, G. Xue and F. Fu, J. Mater. Chem. A, 2014, 2, 11716–11727 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06477f |
| This journal is © The Royal Society of Chemistry 2018 |