Open Access Article
Open Access ArticleNovel SrMg2La2W2O12:Mn4+ far-red phosphors with high quantum efficiency and thermal stability towards applications in indoor plant cultivation LEDs
Shaoying Wang,
Qi Sun,
Balaji Devakumar,
Liangling Sun,
Jia Liang and
Xiaoyong Huang *
*
Key Lab of Advanced Transducers and Intelligent Control System, Ministry of Education and Shanxi Province, College of Physics and Optoelectronics, Taiyuan University of Technology, Taiyuan 030024, P. R. China. E-mail: huangxy04@126.com
First published on 28th August 2018
Abstract
Novel Mn4+-activated far-red emitting SrMg2La2W2O12 (SMLW) phosphors were prepared by a conventional high-temperature solid-state reaction method. The SMLW:Mn4+ phosphors showed a broad excitation band peaking at around 344 nm and 469 nm in the range of 300–550 nm. Under 344 nm near-ultraviolet light or 469 nm blue light, the phosphors exhibited a far-red emission band in the 650–780 nm range centered at about 708 nm. The optimal Mn4+ doping concentration in the SMLW host was 0.2 mol% and the CIE chromaticity coordinates of SMLW:0.2% Mn4+ phosphors were calculated to be (0.7322, 0.2678). In addition, the influences of crystal field strength and nephelauxetic effect on the emission energy of Mn4+ ions were also investigated. Moreover, the internal quantum efficiency of SMLW:0.2% Mn4+ phosphors reached as high as 88% and they also possessed good thermal stability. Specifically, the emission intensity at 423 K still maintained about 57.5% of the initial value at 303 K. Finally, a far-red light-emitting diode (LED) lamp was fabricated by using a 365 nm near-ultraviolet emitting LED chip combined with the as-obtained SMLW:0.2% Mn4+ far-red phosphors.
1. Introduction
Nowadays, indoor plant cultivation has gained much attention and it is becoming a powerful solution to the global food problem for the reason that a controlled environment (e.g., filtered air, steady temperature, and special growth media) can allow plants to grow steadily without being affected by terrible weather such as drought, storms, hail, torrential rain, fog, and haze.1–5 An artificial light source for indoor plant cultivation has become an important condition for efficient production, because cultivating plants in an artificial lighting environment can make up for the deficiency of bad climate, regulate the growth cycle on demand, and improve the yield and quality of crops.6,7Solid-state phosphor-converted light-emitting diodes (LEDs) have been considered as a good kind of artificial light source for indoor plant cultivation owing to their outstanding advantages including small size, long working time, good stability, high photoelectric conversion efficiency, and environmental friendliness compared with traditional incandescent and fluorescent lamps.8–22 Besides, the light quality of the artificial light source is one of the essential factors affecting the development of plants. The absorption of light by plants is not full-wavelength but selective. Blue light around 450 nm (440–480 nm) is beneficial to the growth of stem and the morphogenesis of leaf; red light around 660 nm (620–690 nm) promotes the synthesis of plant carbohydrates, blossoming, and yielding fruits; while far-red light around 730 nm (700–740 nm) contributes to the photosynthesis.23–25 In addition, in the light reaction of plants, the primary light receptor is phytochrome, which is extremely sensitive to red light and far-red light, thus including PR and PFR, respectively.26 The effects of phytochrome on plant morphology include seed germination, de-etiolation, stem elongation, leaf expansion, and flowering induction. Therefore, it plays an important role in the whole process of plant growth and development from germination to maturity.27 Consequently, it is very urgent to develop far-red emitting phosphors that can be used in solid-state phosphor-converted LEDs as artificial light source for indoor plant cultivation.
Mn4+ ions doped red phosphors have some advantages such as suitable spectra, simple synthesis procedure, as well as the cheap and readily available raw materials compared with the traditional rare-earth ions (such as Eu3+/Eu2+) doped red phosphors.28 In detail, Mn4+ ions with a 3d3 electronic configuration belong to transition mental ions, while the phosphors doped with Mn4+ ions can be excited by near-ultraviolet (near-UV) and blue light, and show far-red emission ranging from 620 to 750 nm centered at about 660 nm owing to the 2Eg → 4A2g transition of Mn4+ ions.2,12,29–33 Besides, the crystal field environment is greatly important to the optical properties of Mn4+ ions and Mn4+ ions can occupy the cation sites of octahedrons in the host.34 So, finding a novel host material, which can provide octahedral sites for Mn4+ ions, is also very significant. Tungstates are good host materials used in phosphors because they have many merits including low price, good chemical stability, and outstanding optical properties.35,36 Recently, Mn4+ ions activated tungstates far-red emitting phosphors have been reported such as Ca3La2W2O12:Mn4+,3 Sr2ZnWO6:Mn4+,37 and NaLaMgWO6:Mn4+.38 In addition, some phosphors based on tungstate SrMg2La2W2O12 (SMLW) have been investigated previously, such as SMLW:Tb3+,Eu3+,39 SMLW:Dy3+,Tm3+,40 and SMLW:Tb3+,Sm3+,Tm3+,41 and SMLW including [WO6] and [MgO6] octahedrons can provide octahedral sites, which indicates that SMLW is a suitable host material for Mn4+ ions doped phosphors.
In this work, a series of novel Mn4+-activated SMLW phosphors were prepared by a conventional high-temperature solid-state reaction method. It was discovered that SMLW:Mn4+ phosphors can be excited at 344 nm or 469 nm and exhibited a far-red emission band in the 650–780 nm range centered at about 708 nm, which matched well with the absorption band of phytochrome PFR. The optimal Mn4+ doping concentration in the SMLW host was 0.2%. And the full width at half maximum (FWHM) of the far-red emission band was determined to be about 37 nm. Moreover, the IQE of SMLW:0.2% Mn4+ phosphors reached as high as 88% and they also possessed good thermal stability. Finally, a far-red LED device was fabricated by coating as-prepared SMLW:0.2% Mn4+ far-red phosphors on a 365 nm near-UV emitting LED chip. The results suggested that the far-red emitting SMLW:Mn4+ phosphors were potential luminescent materials that can be applied for indoor plant cultivation LEDs.
2. Experimental
A series of SrMg2La2W2(1−x)O12:xMn4+ (SMLW:xMn4+; x = 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) phosphors were successfully prepared by a conventional high-temperature solid-state reaction method. SrCO3 (analytical reagent, AR), MgO (AR), La2O3 (99.99%), (NH4)6H2W12O40 (AR), MnCO3 (AR) were used as raw materials. They were weighed on the basis of stoichiometric ratio and ground in an agate mortar to make them uniform. Then, these mixtures were put into the alumina crucibles and pre-fired at 600 °C for 4 h in the air, after that they were ground again and then sintered at 1200 °C for 6 h in the air. When the samples were cooled down to room temperature, they were ground again and gathered for further characterization.The phase purity of the phosphors was tested by X-ray diffraction (XRD) patterns recorded on a Bruker D8 Focus diffractometer with Cu Kα radiation. The morphology properties of the samples were obtained by using a field-emission scanning electron microscope (FE-SEM; TESCAN MAIA3). The room-temperature photoluminescence (PL) and photoluminescence excitation (PLE) spectra were measured by Edinburgh FS5 spectrometer equipped with a 150 W continued-wavelength xenon lamp. The decay times of phosphors were recorded on an Edinburgh FS5 spectrometer with a pulsed xenon lamp. Temperature-dependent PL spectra were also measured by using the Edinburgh FS5 spectrometer equipped with a temperature controller. The IQE was tested on an Edinburgh FS5 spectrometer equipped with an integrating sphere coated with BaSO4.
3. Results and discussion
The Rietveld refinement for the XRD patterns of SMLW:0.2% Mn4+ was analyzed to study its crystal structure and the site occupancy, as shown in Fig. 1(a). According to the refinement results, we can find that the crystal structure of SMLW:0.2% Mn4+ belongs to orthorhombic crystal system with the P222 space group, and the cell parameters were calculated to be a = 7.8465 Å, b = 7.8627 Å, c = 7.9014 Å, α = 90°, β = 90°, γ = 90°, and V = 487.47 Å3. The crystal structure of SMLW:0.2% Mn4+ included [MgO6] and [WO6] octahedrons formed by Mg and W atoms coordinated with six oxygen atoms around respectively, as can be seen in Fig. 1(b). As well-known, Mn4+ ions can occupy the cation sites of octahedrons.42 In this work, Mn4+ ions were more likely to occupy the site of W6+ because the radius of Mn4+ ion (0.53 Å) is much closer to that of W6+ ion (0.62 Å) than Mg2+ ion (0.72 Å).12,37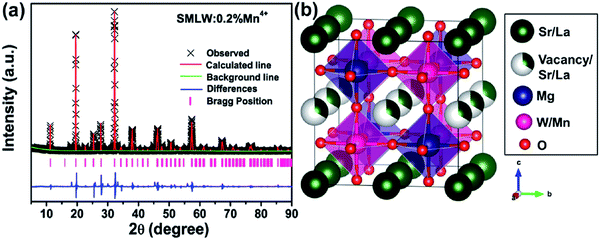 | ||
| Fig. 1 (a) The Rietveld refinement for the XRD patterns of SMLW:0.2% Mn4+ phosphors. (b) The crystal structure of SMLW:0.2% Mn4+ phosphors. | ||
In order to study the possibility that Mn4+ ions can substitute W6+ ions in the host, we can calculate the radius percentage difference between the doped ions Mn4+ and the substituted ions W6+ in SMLW host by using the following formula:43
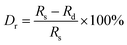 | (1) |
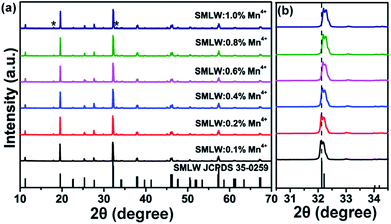
Fig. 2(a) shows the XRD patterns of the as-prepared SMLW:xMn4+ (x = 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) and the standard PDF card of SMLW (JCPDS # 35-0259). The XRD patterns of the samples matched well with the standard data of SMLW (JCPDS # 35-0259) except that there were two weak impurity peaks due to the SrWO4 (JCPDS # 08-0490). This result indicated that doping Mn4+ into SMLW did not make significant changes to the host crystal structure. According to the local XRD patterns in the 2θ range of 30.5–34.5 degree shown in Fig. 2(b), we can find that the XRD diffraction peaks slightly shifted to the larger angle in comparison with the standard data when the Mn4+ doping concentration was increased for the reason that the smaller ions Mn4+ (r = 0.53 Å) substituted larger ions W6+ (r = 0.62 Å) in the SMLW host which resulted in the expansion of the lattice on the basis of Bragg equation (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = λ, where d, θ, and λ refer to crystal surface spacing, diffraction angle, and X-ray wavelength, respectively). The results further confirmed the above conclusion that Mn4+ ions can occupy the sites of W6+ ions.
θ = λ, where d, θ, and λ refer to crystal surface spacing, diffraction angle, and X-ray wavelength, respectively). The results further confirmed the above conclusion that Mn4+ ions can occupy the sites of W6+ ions.
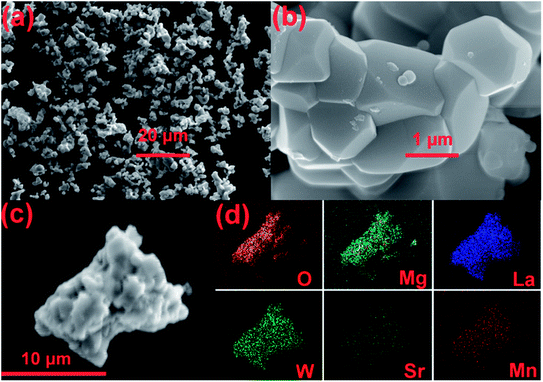
Fig. 3(a–c) shows the FE-SEM images of the SMLW:0.2% Mn4+ phosphors. The sample was made up of irregular microparticles with the particle size ranging from 1 to 5 μm. The elemental mapping was shown in Fig. 3(d), according to which we can see that all the elements (O, Mg, La, W, Sr, and Mn) were uniformly distributed over the whole particles, which indicated that the SMLW:Mn4+ phosphors were successfully synthesized.
Fig. 4(a) exhibits the PLE and PL spectra of SMLW:0.2% Mn4+ phosphors. The PLE spectrum monitored at 708 nm showed a broad absorption band in the near-UV and blue regions ranging from 300–550 nm centered at 344 nm and 469 nm, respectively, which can be Gaussian fitted into four bands peaking at around 326 nm, 350 nm, 402 nm, and 479 nm, corresponding to the Mn–O charge transfer band (CTB), the 4A2g → 4T1g, 4A2g → 2T2g, and 4A2g → 4T2g transitions of Mn4+ ions, respectively.44 The PL spectra excited at 344 nm and 469 nm with the similar profiles consisted of a narrow far-red emission band in the range of 650–780 nm peaking at around 708 nm, due to the 2Eg → 4A2g transition of Mn4+ ions.3 And the PL intensity of the SMLW:0.2% Mn4+ phosphors excited at 344 nm was much stronger than that excited at 469 nm. Furthermore, the emission spectrum of SMLW:0.2% Mn4+ and absorption spectrum of PFR were shown in Fig. 4(b), and we can see that the far-red emission band of SMLW:0.2% Mn4+ matched well with the absorption band of phytochrome PFR, indicating that the SMLW:Mn4+ phosphors were potential far-red emitting materials towards applications in LEDs for indoor plant cultivation. Besides, the full width at half maximum (FWHM) of the PL spectrum under the 344 nm excitation was about 37 nm.
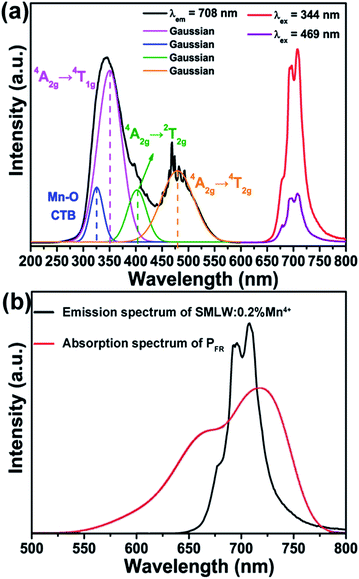 | ||
| Fig. 4 (a) PLE (λem = 708 nm) and PL (λex = 344 nm and 469 nm) spectra of SMLW:0.2% Mn4+ phosphors. (b) The emission spectrum of SMLW:0.2% Mn4+ and absorption spectrum of PFR. | ||
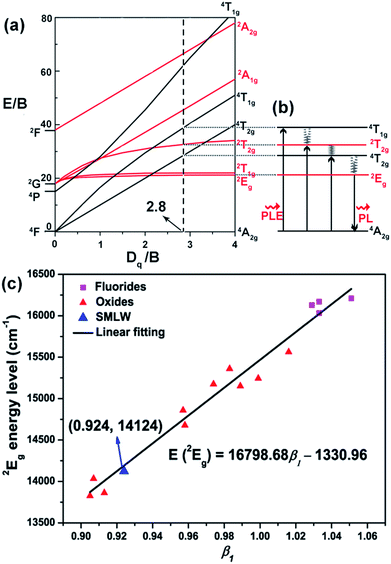
Fig. 5(a) shows the Tanabe–Sugano energy level diagram, which can illustrate the electron transitions of Mn4+ ions corresponding to different energy levels in an octahedral crystal field. There are two important free ion states including 2H excited level and 4F ground level. The 4F ground level can split into 4A2g, 4T2g, and 4T1g states, when excited at near-UV/blue light, the electrons at the ground state 4A2g transit to the excited states (4T1g, 2T2g(2G), and 4T2g) corresponding to the spin-allowed transitions 4A2g → 4T1g, 4A2g → 2T2g, and 4A2g → 4T2g respectively, then relax to the lowest excited state 2Eg in a way of nonradiative transition, and finally come back to the ground state 4A2g through radiative transition (2Eg → 4A2g), as can be described by a simple energy level diagram of Mn4+ ions in Fig. 5(b).
The crystal field strength (Dq) of Mn4+ can be calculated by the peak energy (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 876 cm−1) of 4A2g → 4T2g transition according to the following equation:45–47
876 cm−1) of 4A2g → 4T2g transition according to the following equation:45–47
| Dq = E(4A2g–4T2g)/10 | (2) |
The Racah parameter B can be roughly estimated on the basis of the energy difference (7695 cm−1) between 4A2g → 4T2g and 4A2g → 4T1g transitions of Mn4+ ions using the formula as follows:
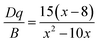 | (3) |
And the parameter x can be evaluated by the following formula:
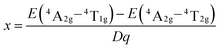 | (4) |
Besides, the Racah parameter C can be estimated according to the peak energy (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 cm−1) of the 2Eg → 4A2g transition of Mn4+ ions by the equation:
124 cm−1) of the 2Eg → 4A2g transition of Mn4+ ions by the equation:
| E(2Eg–4A2g)/B = 3.05C/B + 7.9 − 1.8B/Dq | (5) |
On the basis of the above equations, the value of the parameter Dq, B, and C were calculated to be around 2088, 746, and 2856 cm−1, respectively. Thus the value of Dq/B was determined to be about 2.8, which was higher than 2.2, indicating that Mn4+ ions occupied a strong crystal field in the SMLW host.48
According to the Tanabe–Sugano energy level diagram of Mn4+ ions, we can see that the energy of 2Eg level has no relation with the crystal field strength.49 Therefore, the emission wavelength of 2Eg → 4A2g transition of Mn4+ ions does not vary with the crystal field strength, but depends on the nephelauxetic effect, which is attributed to the covalence between the Mn4+ ions and ligand.27 In order to predict the emission wavelength of Mn4+ in different host, Brik et al. set up a dimensionless linear correlation by introducing a parameter of the nephelauxetic ratio (β1):15
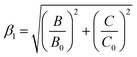 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 cm−1, and when the coordinates (0.924, 14
124 cm−1, and when the coordinates (0.924, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124) of SMLW:Mn4+ were put into Fig. 5(c), they were well consistent with the fitted linear relation.
124) of SMLW:Mn4+ were put into Fig. 5(c), they were well consistent with the fitted linear relation.
| Host | Dq/cm−1 | B/cm−1 | C/cm−1 | β1 | E(2Eg)/cm−1 | Ref. |
|---|---|---|---|---|---|---|
| Na2SiF6 | 2174 | 775 | 3475 | 1.051 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
63 |
| Na2SnF6 | 2101 | 589 | 3873 | 1.033 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171 171 |
64 |
| K2MnF6 | 2183 | 604 | 3821 | 1.029 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 129 129 |
65 |
| Cs2GeF6 | 2063 | 490 | 4056 | 1.033 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 032 032 |
66 |
| Y2Sn2O7 | 2100 | 700 | 3515 | 1.016 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 563 |
67 |
| CaAl2O19 | 2132 | 807 | 3088 | 0.999 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 244 |
68 |
| SrMgAl10O17 | 2237 | 791 | 3084 | 0.989 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 152 |
69 |
| Sr4Al14O25 | 2222 | 680 | 3397 | 0.983 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
70 |
| Ba2LaNbO6 | 1780 | 670 | 3290 | 0.958 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679 679 |
71 |
| Mg14Ge5O24 | 2375 | 709 | 3263 | 0.974 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175 |
72 |
| LaAlO3 | 2123 | 695 | 2941 | 0.907 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 034 034 |
73 |
| BaTiO3 | 1780 | 738 | 2820 | 0.913 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862 862 |
74 |
| SrTiO3 | 1818 | 719 | 2839 | 0.905 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 827 827 |
75 |
| SrMg2La2W2O12 | 2088 | 746 | 2856 | 0.924 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 124 |
This work |
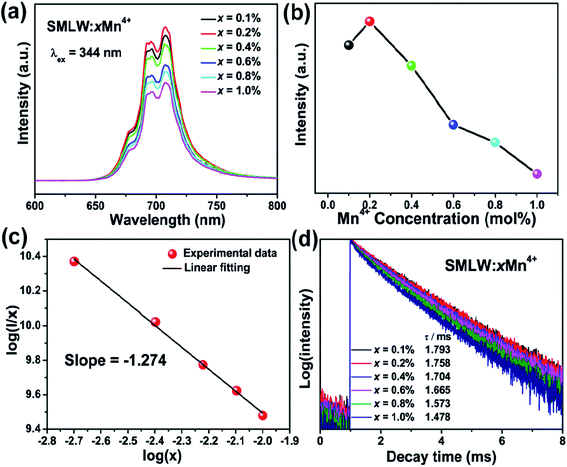
Fig. 6(a) presents the PL spectra of SMLW:xMn4+ (x = 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) phosphors under the 344 nm excitation. With the increasing concentration of Mn4+ ions, the PL intensity first increased to the maximum value and then gradually decreased because of the concentration quenching, which was attributed to the nonradiative energy transfer between Mn4+ ions.50,51 Fig. 6(b) shows the emission intensity of SMLW:xMn4+ as a function of Mn4+ ions concentration at the wavelength 708 nm. It is intuitive to see that the optimal doping concentration of Mn4+ ions in the SMLW:xMn4+ was 0.2%. In addition, we can approximately calculate the critical distance Rc by using the following equation to estimate the nonradiative energy transfer mechanism between Mn4+ ions:
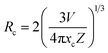 | (7) |
To further study the specific interaction in energy transfer mechanism, the relation between the log(I/x) and log(x) can be estimated by the formula as follows:53
log(I/x) = A − (θ/3)log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x
| (8) |
Fig. 6(d) shows the lifetime decay curves of SMLW:xMn4+ (x = 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) phosphors excited at 344 nm and monitored at 708 nm. All the curves can be well fitted to second-exponential formula as follows:28
I = I0 + A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (9) |
| τ = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (10) |
The average decay times of the SMLW:xMn4+ (x =0.1%, 0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) phosphors were calculated to be 1.793, 1.758, 1.704, 1.665, 1.573, and 1.478 ms, respectively. It was clear to see that the decay times decreased gradually with the increasing concentration of Mn4+ ions for the reason that the energy transfer among Mn4+ ions became more frequent owing to the closer distance between Mn4+ ions with the increasing concentration.58
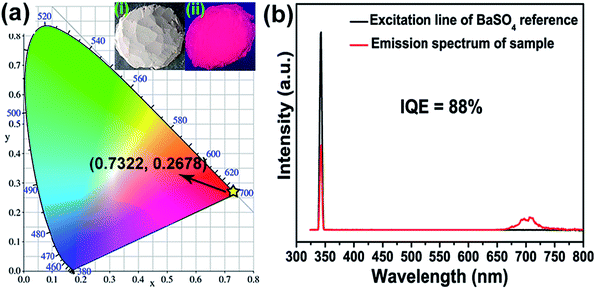
The CIE chromaticity coordinates diagram of SMLW:0.2% Mn4+ phosphors were shown in Fig. 7(a). The CIE chromaticity coordinates were calculated to be (0.7322, 0.2678) and it located in far-red region. Inset (i) and (ii) represent the photographs of the SMLW:0.2% Mn4+ sample under daylight and 365 nm near-UV light, respectively. It was clear to see that the as-prepared sample emitted bright red light excited at 365 nm. What's more, the excitation line of BaSO4 reference and emission spectrum of SMLW:0.2% Mn4+ sample were shown in Fig. 7(b), and the IQE of this sample can be calculated according to the equation as follows:59
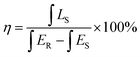 | (11) |
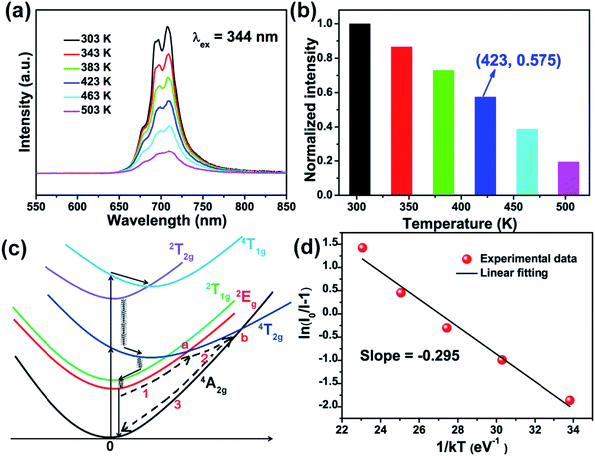
Fig. 8(a) shows the emission spectra of SMLW:0.2% Mn4+ phosphors under the 344 nm excitation at the temperature range from 303 to 503 K. There were no big changes in the profiles of the emission spectra at different temperature, but the PL intensity decreased gradually with the increasing of temperature owing to the thermal quenching effect for the reason that the lattice relaxation of the luminescence center increased and the non-radiation transition became more possible with increasing the temperature.39 Fig. 8(b) shows the normalized PL intensities of SMLW:0.2% Mn4+ phosphors at different temperature from 303 to 503 K. The emission intensity at 423 K still kept 57.5% of the initial value at 303 K, which was higher than that of some previously reported Mn4+-activated phosphors, such as CaYAlO4:Mn4+ (50%),61 La(MgTi)1/2O3:Mn4+ (53%),27 and Gd2ZnTiO6:Mn4+ (27.2%),29 indicating that SMLW:0.2% Mn4+ phosphors possessed good thermal stability.
In addition, we can illustrate the mechanism of thermal quenching behavior by using a simple configuration diagram of Mn4+ ions shown in Fig. 8(c). Normally, when excited at near-UV/blue light, the electrons at the ground state 4A2g can transit to the excited states (4T1g, 2T2g, and 4T2g), then relax to the lowest excited state 2Eg which is a process of nonradiative transition, and finally return to the ground state 4A2g through radiative transition accompanied with emitting far-red light. With increasing the temperature, part of electrons at excited state 2Eg can be excited to the crossover point a and b, then return back to the ground state 4A2g which can be described through paths 1, 2, and 3, leading to lower probability of radiative transition 2Eg → 4A2g, and thus emission intensity of Mn4+ ions decreases with increasing the temperature.23,27
Furthermore, the value of activation energy (Ea) for thermal quenching can be calculated by using the following Arrhenius equation:62
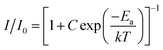 | (12) |
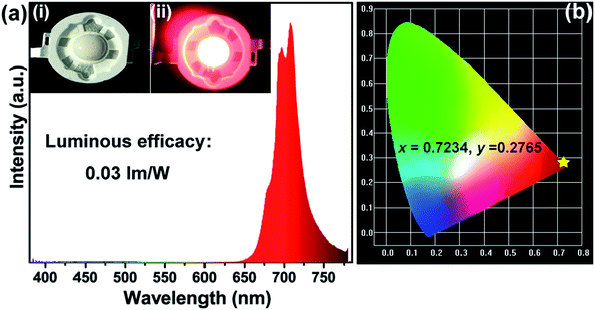
Moreover, a far-red emitting LED lamp was fabricated by using a 365 nm near-UV LED chip combined with the as-synthesized SMLW:0.2% Mn4+ far-red phosphors. Fig. 9(a) shows the electroluminescence (EL) spectrum of the fabricated red-emitting LED driven by 60 mA current. Inset (ii) and inset (i) were the photographs of the far-red LED lamp with and without current, respectively, and it exhibited bright far-red light under the 60 mA current. In addition, we can see that the spectrum excited at 365 nm consisted of a narrow far-red emission band peaking at around 708 nm ranging from 650–780 nm due to the 2Eg → 4A2g transition of Mn4+ ions, which matched well with the PL spectrum of SMLW:0.2Mn4+ phosphors under the 344 nm excitation mentioned above. The CIE chromaticity coordinates based on the EL spectrum of the fabricated far-red emitting LED were calculated to be (0.7234, 0.2765), which located in far-red region as shown in Fig. 9(b). The luminous efficacy of this lamp, which can represent the sensitivity of human eyes to light, was determined to be 0.03 lm W−1. In this work, the fabricated far-red emitting LED used for indoor plant cultivation emitted far-red light (∼708 nm) and the perception ability of human eyes to far-red light is relatively weak, indicating that the low luminous efficacy of this lamp is reasonable. These results suggested that the SMLW:Mn4+ phosphors were promising far-red emitting luminescent materials applied in LEDs for indoor plant growth.
4. Conclusions
Overall, novel Mn4+-activated SMLW phosphors were prepared by a traditional high-temperature solid-state reaction method. The as-synthesized SMLW:Mn4+ phosphors can be excited at 344 nm or 469 nm and exhibited a far-red emission band in the 650–780 nm range centered at about 708 nm, which matched well with the absorption band of phytochrome PFR. And the FWHM of the far-red emission band was about 37 nm. The optimal Mn4+ concentration in SMLW:xMn4+ was x = 0.2%, and the CIE chromaticity coordinates of SMLW:0.2% Mn4+ phosphors were calculated to be (0.7322, 0.2678). Besides, the crystal field strength Dq, Racah parameters B and C, as well as the nephelauxetic ratio β1 of SMLW:Mn4+ phosphors were estimated to analyze the influences of crystal field strength and nephelauxetic effect on the emission energy of Mn4+ ions. Moreover, the IQE of SMLW:0.2% Mn4+ phosphors reached as high as 88%. Importantly, the emission intensity at 423 K was still 57.5% of the initial value at 303 K, indicating that the SMLW:0.2% Mn4+ phosphors possessed good thermal stability. Finally, a far-red LED lamp was fabricated by using a 365 nm near-UV emitting LED chip combined with the SMLW:0.2% Mn4+ far-red phosphors. Consequently, the as-synthesized SMLW:0.2% Mn4+ sample was promising far-red phosphors applied in LEDs as artificial light source for indoor plant growth.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51502190), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology, No. 2017-skllmd-01).References
- R. Cao, Y. Ye, Q. Peng, G. Zheng, H. Ao, J. Fu, Y. Guo and B. Guo, Dyes Pigm., 2017, 146, 14–19 CrossRef.
- R. Cao, Z. Shi, G. Quan, T. Chen, S. Guo, Z. Hu and P. Liu, J. Lumin., 2017, 188, 577–581 CrossRef.
- X. Huang and H. Guo, Dyes Pigm., 2018, 152, 36–42 CrossRef.
- C. Yang, Z. Zhang, G. Hu, R. Cao, X. Liang and W. Xiang, J. Alloys Compd., 2017, 694, 1201–1208 CrossRef.
- R. Cao, J. Zhang, W. Wang, Z. Hu, T. Chen, Y. Ye and X. Yu, Mater. Res. Bull., 2017, 87, 109–113 CrossRef.
- J. Chen, N. Zhang, C. Guo, F. Pan, X. Zhou, H. Suo, X. Zhao and E. M. Goldys, ACS Appl. Mater. Interfaces, 2016, 8, 20856–20864 CrossRef PubMed.
- L. Li, Y. Pan, Z. Chen, S. Huang and M. Wu, RSC Adv., 2017, 7, 14868–14875 RSC.
- X. Huang, S. Wang, B. Li, Q. Sun and H. Guo, Opt. Lett., 2018, 43, 1307–1310 CrossRef PubMed.
- X. Huang, H. Guo and B. Li, J. Alloys Compd., 2017, 720, 29–38 CrossRef.
- X. Huang, B. Li, H. Guo and D. Chen, Dyes Pigm., 2017, 143, 86–94 CrossRef.
- X. Huang, B. Li and H. Guo, J. Alloys Compd., 2017, 695, 2773–2780 CrossRef.
- R. Cao, Z. Shi, G. Quan, Z. Luo, P. Tang, H. Ao and X. Yu, Opt. Mater., 2016, 57, 212–216 CrossRef.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef.
- H. Guo, B. Devakumar, B. Li and X. Huang, Dyes Pigm., 2018, 151, 81–88 CrossRef.
- B. Li and X. Huang, Ceram. Int., 2018, 44, 4915–4923 CrossRef.
- B. Li, X. Huang, H. Guo and Y. Zeng, Dyes Pigm., 2018, 150, 67–72 CrossRef.
- M. Zhao, Z. Xia, M. S. Molokeev, L. Ning and Q. Liu, Chem. Mater., 2017, 29, 6552–6559 CrossRef.
- J. Qiao, Z. Xia, Z. Zhang, B. Hu and Q. Liu, Sci. China Mater., 2018, 61, 985–992 CrossRef.
- J. Qiao, L. Ning, M. S. Molokeev, Y. C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 CrossRef PubMed.
- P. Du, L. Luo, X. Huang and J. S. Yu, J. Colloid Interface Sci., 2018, 514, 172–181 CrossRef PubMed.
- X. Huang, J. Alloys Compd., 2017, 690, 356–359 CrossRef.
- P. Du, X. Huang and J. S. Yu, Inorg. Chem. Front., 2017, 4, 1987–1995 RSC.
- A. Fu, L. Zhou, S. Wang and Y. Li, Dyes Pigm., 2018, 148, 9–15 CrossRef.
- L. Qin, S. Bi, P. Cai, C. Chen, J. Wang, S. I. Kim, Y. Huang and H. J. Seo, J. Alloys Compd., 2018, 755, 61–66 CrossRef.
- J. Xiang, J. Chen, N. Zhang, H. Yao and C. Guo, Dyes Pigm., 2018, 154, 257–262 CrossRef.
- Y. Zheng, H. Zhang, H. Zhang, Z. Xia, Y. Liu, M. S. Molokeev and B. Lei, J. Mater. Chem. C, 2018, 6, 4217–4224 RSC.
- Z. Zhou, J. Zheng, R. Shi, N. Zhang, J. Chen, R. Zhang, H. Suo, E. M. Goldys and C. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 6177–6185 CrossRef PubMed.
- S. S. Liang, M. M. Shang, H. Z. Lian, K. Li, Y. Zhang and J. Lin, J. Mater. Chem. C, 2016, 4, 6409–6416 RSC.
- H. Chen, H. Lin, Q. Huang, F. Huang, J. Xu, B. Wang, Z. Lin, J. Zhou and Y. Wang, J. Mater. Chem. C, 2016, 4, 2374–2381 RSC.
- Q. Sun, B. Li, S. Wang, H. Guo and X. Huang, J. Mater. Sci.: Mater. Electron., 2018, 29, 12972–12977 CrossRef.
- J. Liang, L. Sun, B. Devakumar, S. Wang, Q. Sun, H. Guo, B. Li and X. Huang, RSC Adv., 2018, 8, 27144–27151 RSC.
- Q. Sun, S. Wang, B. Li, H. Guo and X. Huang, J. Lumin., 2018, 203, 371–375 CrossRef.
- Q. Sun, S. Wang, B. Devakumar, B. Li, L. Sun, J. Liang and X. Huang, RSC Adv., 2018, 8, 28538–28545 RSC.
- G. Blasse, J. Solid State Chem., 1975, 14, 181–184 CrossRef.
- X. Huang, B. Li, P. Du, H. Guo, R. Cao, J. S. Yu, K. Wang and X. W. Sun, Dyes Pigm., 2018, 151, 202–210 CrossRef.
- P. Du, L. Krishna Bharat and J. S. Yu, J. Alloys Compd., 2015, 633, 37–41 CrossRef.
- R. Cao, X. Ceng, J. Huang, X. Xia, S. Guo and J. Fu, Ceram. Int., 2016, 42, 16817–16821 CrossRef.
- X. Huang, J. Liang, B. Li, L. Sun and J. Lin, Opt. Lett., 2018, 43, 3305–3308 CrossRef PubMed.
- K. Pavani, J. Suresh Kumar and L. Rama Moorthy, J. Alloys Compd., 2014, 586, 722–729 CrossRef.
- K. Pavani, J. Suresh Kumar, L. Rama Moorthy and A. Srivastava, J. Am. Ceram. Soc., 2014, 97, 1481–1488 CrossRef.
- K. Pavani, J. Suresh Kumar and L. Rama Moorthy, Mater. Res. Express, 2014, 1, 016201 CrossRef.
- R. Cao, X. Ceng, J. Huang, H. Ao, G. Zheng, X. Yu and X. Zhang, Opt. Mater., 2016, 62, 706–710 CrossRef.
- K. Li, H. Lian and R. V. Deun, J. Lumin., 2018, 198, 155–162 CrossRef.
- A. Fu, C. Zhou, Q. Chen, Z. Lu, T. Huang, H. Wang and L. Zhou, Ceram. Int., 2017, 43, 6353–6362 CrossRef.
- S. Zhang, Y. Hu, H. Duan, Y. Fu and M. He, J. Alloys Compd., 2017, 693, 315–325 CrossRef.
- S. J. Kim, H. S. Jang, S. Unithrattil, Y. H. Kim and W. B. Im, J. Lumin., 2016, 172, 99–104 CrossRef.
- F. Baur and T. Jüstel, J. Lumin., 2016, 177, 354–360 CrossRef.
- M. G. Brik, S. J. Camardello and A. M. Srivastava, ECS J. Solid State Sci. Technol., 2014, 4, R39–R43 CrossRef.
- Y. Jin, Y. Hu, H. Wu, H. Duan, L. Chen, Y. Fu, G. Ju, Z. Mu and M. He, Chem. Eng. J., 2016, 288, 596–607 CrossRef.
- S. Wang, Q. Sun, B. Li, H. Guo and X. Huang, Dyes Pigm., 2018, 157, 314–320 CrossRef.
- J. Liang, P. Du, H. Guo, L. Sun, B. Li and X. Huang, Dyes Pigm., 2018, 157, 40–46 CrossRef.
- H. Guo and X. Huang, J. Alloys Compd., 2018, 764, 809–814 CrossRef.
- P. Du and J. S. Yu, Dyes Pigm., 2017, 147, 16–23 CrossRef.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang and J. Jiang, RSC Adv., 2018, 8, 12035–12042 RSC.
- H. Deng, Z. Gao, N. Xue, J. H. Jeong and R. Yu, J. Lumin., 2017, 192, 684–689 CrossRef.
- R. Yu, H. M. Noh, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, S. S. Yi and J. K. Jang, J. Alloys Compd., 2013, 576, 236–241 CrossRef.
- H. Li, R. Zhao, Y. Jia, W. Sun, J. Fu, L. Jiang, S. Zhang, R. Pang and C. Li, ACS Appl. Mater. Interfaces, 2014, 6, 3163–3169 CrossRef PubMed.
- K. Li, H. Lian and R. V. Deun, Dalton Trans., 2017, 47, 2501–2505 RSC.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef.
- R. Cao, Z. Shi, G. Quan, T. Chen, S. Guo, Z. Hu and P. Liu, J. Lumin., 2017, 188, 577–581 CrossRef.
- Y. Chen, M. Wang, J. Wang, M. Wu and C. Wang, J. Solid State Light., 2014, 1, 15 CrossRef.
- B. Li, S. Wang, Q. Sun, C. Lu, H. Guo and X. Huang, Dyes Pigm., 2018, 154, 252–256 CrossRef.
- Y. Xu and S. Adachi, J. Appl. Phys., 2009, 105, 013525 CrossRef.
- Y. Arai and S. Adachi, J. Lumin., 2011, 131, 2652–2660 CrossRef.
- R. Kasa, Y. Arai, T. Takahashi and S. Adachi, J. Appl. Phys., 2010, 108, 113503 CrossRef.
- E. Lifshitz and A. H. Francis, Chem. Phys., 1988, 127, 297–304 CrossRef.
- M. G. Brik, A. M. Srivastava and N. M. Avram, Opt. Mater., 2011, 33, 1671–1676 CrossRef.
- T. Murata, T. Tanoue, M. Iwasaki, K. Morinaga and T. Hase, J. Lumin., 2005, 114, 207–212 CrossRef.
- L. Meng, L. Liang and Y. Wen, J. Mater. Sci.: Mater. Electron., 2014, 25, 2676–2681 CrossRef.
- Y. D. Xu, D. Wang, L. Wang, N. Ding, M. Shi, J. G. Zhong and S. Qi, J. Alloys Compd., 2013, 550, 226–230 CrossRef.
- A. M. Srivastava and M. G. Brik, J. Lumin., 2012, 132, 579–584 CrossRef.
- T. M. Chen and J. T. Luo, US Pat. no. 7, 2010, vol. 846, p. 350.
- A. M. Srivastava and M. G. Brik, Opt. Mater., 2013, 35, 1544–1548 CrossRef.
- X. Wu, W. Fang, W. Feng and W. Zheng, Pramana, 2009, 72, 569–575 CrossRef.
- Z. Bryknar, V. Trepakov, Z. Potucek and L. Jastrabík, J. Lumin., 2000, 87–89, 605–607 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |