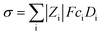Open Access Article
Open Access ArticleOrigin of the effects of PEG additives in electrolytes on the performance of quantum dot sensitized solar cells†
Yu Suna,
Guocan Jianga,
Mengsi Zhoua,
Zhenxiao Pan*b and
Xinhua Zhong *ab
*ab
aSchool of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: zhongxh@ecust.edu.cn
bCollege of Materials and Energy, South China Agricultural University, 483 Wushan Road, Guangzhou 510642, China. E-mail: zxpan@scau.edu.cn
First published on 24th August 2018
Abstract
It has been well established that polymer additives in electrolyte can impede the charge recombination processes at the photoanode/electrolyte interface, and improve performance, especially Voc, of the resulting sensitized solar cells. However, there are few reports about the effect of electrolyte additives on counter electrode (CE) performance. Herein, we systematically investigated the effect of polyethylene glycol (PEG) additives with various molecular weights (Mw from 300 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in polysulfide electrolyte on the performance of two representative CdSe and Zn–Cu–In–Se (ZCISe) quantum dot sensitized solar cells (QDSCs), and explored the mechanism of the observed effects. Electrochemical impedance spectroscopy measurements indicate that all PEG additives can improve the charge recombination resistance at the photoanode/electrolyte interface, therefore suppressing the unwanted charge recombination process, and enhancing the Voc of the resulting cell devices accordingly. On the CE side, with the increase of Mw of PEG additives, the initial effect of reducing the charge transfer resistance at the CE/electrolyte interface evolves into an increasing resistance; accordingly the initial positive effect on FF turns into negative one. Accordingly, low Mw PEG can improve efficiency for both CdSe (increasing from 6.81% to 7.60%) and ZCISe QDSCs (increasing from 9.26% to 10.20%). High Mw PEG is still effective for CdSe QDSCs with an efficiency of 7.38%, but falls flat on ZCISe QDSCs (with an efficiency of 9.11%).
000) in polysulfide electrolyte on the performance of two representative CdSe and Zn–Cu–In–Se (ZCISe) quantum dot sensitized solar cells (QDSCs), and explored the mechanism of the observed effects. Electrochemical impedance spectroscopy measurements indicate that all PEG additives can improve the charge recombination resistance at the photoanode/electrolyte interface, therefore suppressing the unwanted charge recombination process, and enhancing the Voc of the resulting cell devices accordingly. On the CE side, with the increase of Mw of PEG additives, the initial effect of reducing the charge transfer resistance at the CE/electrolyte interface evolves into an increasing resistance; accordingly the initial positive effect on FF turns into negative one. Accordingly, low Mw PEG can improve efficiency for both CdSe (increasing from 6.81% to 7.60%) and ZCISe QDSCs (increasing from 9.26% to 10.20%). High Mw PEG is still effective for CdSe QDSCs with an efficiency of 7.38%, but falls flat on ZCISe QDSCs (with an efficiency of 9.11%).
1. Introduction
Exploring photovoltaic cells with high efficiency and low cost is believed to be a promising way to solve the urgent energy and environment issues.1,2 Being a new type of photovoltaic cell, quantum dot sensitized solar cells (QDSCs) are attracting increasing research interest due to a variety of unique advantages of semiconductor QD light harvesting materials such as: high absorption coefficient, tunable light harvesting range, solution processability, high stability toward light, heat, and moisture, as well as high theoretical power conversion efficiency (PCE) due to multi-exciton generation possibilities.3–9 In the past several years, the reported highest PCE for QDSCs has been increased from less than 5% to over 12%.10–19 The enhancement of PCE for QDSCs has been achieved mainly through two approaches: (i) by exploring new types of counter electrode (CE), mainly Cu2S/brass and mesoporous carbon/titanium mesh CEs, to improve the fill factor (FF), and open-circuit voltage (Voc) of a cell device;10,11,20–22 (ii) by expanding the solar light harvesting range through the adoption of near infrared adsorption QD sensitizers, mainly Cd–Se–Te, and Cu–In–Se based QDs, and exploring effective QD sensitization methods to ensure high QD loading amount on TiO2 film electrodes to realize the full capture of solar light and the enhancement of short-circuit current (Jsc) from the aspect of photoanodes.12–19,23Compared to the extensively studied photoanodes and CEs, the other vital component redox couple electrolyte is significantly less concerned. In fact, electrolyte plays the crucial role of QD regeneration and charge transfer between photoanode and CE, which is critical in determining the photovoltaic performance of a cell device, especially Voc and FF values.24,25 Up to date, polysulfide/sulfide (Sn2−/S2−) redox couple electrolyte is the most commonly used one in QDSCs since this electrolyte media can stabilize the commonly adopted QD light-absorbers, and enables effectively scavenging photo-induced holes to neutralize and regenerate oxidized QDs and make circulatory cells practicable. However, due to the undesirable high redox potential of Sn2−/S2− redox couple, a high overpotential is required for QD regeneration. This results in a relatively low Voc value in the corresponding cell devices.10–19 In order to overcome this shortage, new redox couples, such as I−/I3−, Co2+/3+ complexes, and organic redox couples, have been exploited for QDSCs in recent years.26–32 Unfortunately, these redox couples failed in achieving better photovoltaic performance due to the incompatibility of QDs in these redox couple electrolyte media or the existence of severe unwanted charge recombination. Therefore, at current stage it is a wise strategy to modify electrochemical features of the conventional polysulfide electrolyte with introduction of suitable organic or inorganic additives. This strategy can usually bring forward three beneficial effects based on literature results: (i) improving Voc of the cells due to the adsorption of organic polymer on the surface of QD sensitized TiO2 film electrode and the formation of energetic barrier layer to impede the charge recombination processes occurring at photoanode/electrolyte interface;33–36 (ii) improving device stability due to the gelation effect by the strong water-absorbing capacity of the polymer additives;37–42 (iii) tuning the potential of redox couple, or even shifting the conduction band edge of the TiO2 substrate in the case of some small molecule additives.43–45 However, there are few reports about the effect of electrolyte additives on the performance of CE in sensitized solar cells.
Herein, we systematically investigated the effects of polyethylene glycol (PEG) additives with various molecular weights (Mw from 300 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in polysulfide electrolyte on the performance of QDSCs and explored the origin of the observed effects. Two representative QDSCs, CdSe, and Zn–Cu–In–Se (ZCISe), were used as the model device to evaluate these effects. It was found that all kinds of PEG additives have a positive effect on Voc and FF, negligible effect on Jsc value in CdSe QDSCs. In ZCISe QDSCs system, the positive effect on Voc by PEG additives was also realized, while with the increase of Mw of PEG, the positive or neutral effects on FF and Jsc turned into negative ones gradually. Relying on the electrochemical impedance measurements, the effects of PEG additives on the performance of photoanodes and CEs, and therefore the influence on photovoltaic parameters of Voc, Jsc, FF, and PEC were explored. Furthermore, a facile and general route for remarkably improving photovoltaic performance of QDSCs was offered with introduction of low Mw PEG additives into the polysulfide electrolyte.
000) in polysulfide electrolyte on the performance of QDSCs and explored the origin of the observed effects. Two representative QDSCs, CdSe, and Zn–Cu–In–Se (ZCISe), were used as the model device to evaluate these effects. It was found that all kinds of PEG additives have a positive effect on Voc and FF, negligible effect on Jsc value in CdSe QDSCs. In ZCISe QDSCs system, the positive effect on Voc by PEG additives was also realized, while with the increase of Mw of PEG, the positive or neutral effects on FF and Jsc turned into negative ones gradually. Relying on the electrochemical impedance measurements, the effects of PEG additives on the performance of photoanodes and CEs, and therefore the influence on photovoltaic parameters of Voc, Jsc, FF, and PEC were explored. Furthermore, a facile and general route for remarkably improving photovoltaic performance of QDSCs was offered with introduction of low Mw PEG additives into the polysulfide electrolyte.
2. Experimental section
QDs synthesis and cell device fabrication
The oleylamine-capped 5.2 nm CdSe QDs, and 4.1 nm ZCISe QDs were synthesized according to literature method.18,35,46 Water-soluble mercaptopropionic acid (MPA) capped QDs were obtained via a ligand exchange process with use of MPA as phase transfer reagent. The TiO2 mesoporous film electrodes were prepared according to standard literature method.47 The water-soluble QDs were tethered on the TiO2 mesoporous film through the capping ligand induced self-assembly route by dipping the QD solution on the film and staying for a certain period.21 Thereafter, ZnS, and SiO2 passivation layer were deposited on the QD sensitized TiO2 film electrodes and served as photoanodes. Sandwich structured cell devices were fabricated through assembling the photoanode, and Cu2S/brass electrode CEs, and filling with electrolyte aqueous solutions with composition of 2.0 M S and Na2S, 0.2 M KCl, and different Mw PEG additives (i.e. 20 wt% for both PEG-300, and PEG-1K, 15 wt% for PEG-4K and 8 wt% for PEG-20K). For the reference sample, plain polysulfide/sulfide electrolyte without the existence of any PEG additives. To ensure the reliability of the data, 5 pieces of cells under each condition were fabricated and measured.Characterization
The performance of QDSCs was tested using a Keithley 2400 source meter under the illumination of simulated AM1.5G solar light (Oriel, model no. 94022A) with intensity of 100 mW cm−2 (1 full solar light). The light intensity was calibrated by a NREL standard Si solar cell. The effective area of the cells was determined by a 0.235 cm2 black mask. The conductivity of the electrolyte solutions was tested by DDSJ-308A conductivity meter at 25 °C. Electrochemical impedance spectroscopy (EIS) measurements were carried out on a Zennium electrochemical workstation (Zahner). The EIS spectra for a full solar cell were obtained under dark conditions at different forward bias ranging from −0.35 to −0.65 V, applying a 20 mV AC sinusoidal signal over the constant applied bias with the frequency ranging from 1 MHz to 0.1 Hz. The EIS spectra and Tafel-polarization curves for symmetric dummy cells consisting of two identical Cu2S/brass electrode were measured by applying a 20 mV AC sinusoidal signal over the constant applied bias with the frequency range of 100 mHz to 100 kHz.3. Results and discussion
Influence of PEG molecular weight of on the cell performance
According to our previous results, high molecular weight (Mw) PEG modifying polysulfide electrolytes can improve the photovoltaic performance of CdSe QDSCs, but fails flat for ZCISe QDSCs.31 In this work, we systematically studied the influence of PEG additives with different Mw on the photovoltaic performance of the most commonly studied CdSe and ZCISe QDSCs. On the other hand, the selected ZCISe QDSCs exhibit best performance among all kind of QDSCs.18,19,35 Commercially available PEG with Mw of 300, 1000, 4000, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (noted as PEG-300, PEG-1K, PEG-4K, and PEG-20K, respectively henceforth) were selected and evaluated in this study. QDSC cell devices were constructed according to literature method by assembling QD sensitized photoanodes and Cu2S/brass CEs.15,35,47 Electrolyte solutions were derived from conventional polysulfide/sulfide aqueous solution electrolyte with addition of optimum weight fractions of different PEG additives. Detailed procedure for the fabrication and measurement of QDSCs can be found in the Experimental section. Under each condition, five cells were fabricated and measured in parallel, and the average values were used in the evaluation of the photovoltaic performance in order to ensure the validity of the reported results.
000 (noted as PEG-300, PEG-1K, PEG-4K, and PEG-20K, respectively henceforth) were selected and evaluated in this study. QDSC cell devices were constructed according to literature method by assembling QD sensitized photoanodes and Cu2S/brass CEs.15,35,47 Electrolyte solutions were derived from conventional polysulfide/sulfide aqueous solution electrolyte with addition of optimum weight fractions of different PEG additives. Detailed procedure for the fabrication and measurement of QDSCs can be found in the Experimental section. Under each condition, five cells were fabricated and measured in parallel, and the average values were used in the evaluation of the photovoltaic performance in order to ensure the validity of the reported results.
First, optimum concentrations for all selected PEG additives in polysulfide electrolyte were determined, and the corresponding J–V curves are illustrated in Fig. S1 of the ESI.† Experimental results indicate that the optimum concentration range for low Mw PEG-300, and PEG-1K is located in 20–30 wt%. While for high Mw PEG-4K and PEG-20K, their optimum concentrations correspond to their corresponding highest solubility in this electrolyte solution, i.e. 15 wt% and 8 wt%, respectively. Correspondingly, in the following experiments, 25 wt% was chosen for PEG-300, and PEG-1K, while 15 wt% for PEG-4K, and 8 wt% for PEG-20K. After that, cell performances corresponding to polysulfide electrolyte containing different PEG additives under their optimum concentrations were measured together with reference sample without any PEG additives in polysulfide electrolyte. The detailed J–V curves and corresponding extracted main photovoltaic parameters (Voc, Jsc, FF, and PCE,) are available in Fig. S2, S3, Tables S1 and S2.†
Fig. 1 illustrates the dependence of main photovoltaic parameters on polysulfide electrolyte containing different PEG additives for both CdSe and ZCISe QDSC systems, and the detailed average photovoltaic parameters are summarized in Tables 1 and 2. From Fig. 1a, d, Tables 1 and 2, we can find that all kinds of PEG additives in polysulfide electrolyte can enhance Voc values for both CdSe and ZCISe cells in comparison with reference samples without any PEG additives (in CdSe cells, Voc improving from 0.65 V to 0.68–0.69 V; in ZCISe cells, Voc improving from 0.59 V to 0.62–0.63 V). In the case of Jsc parameter, the effects of PEG additives for CdSe and ZCISe QDSCs are different. All PEG additives have negligible effects on CdSe QDSCs (the Jsc value keeping nearly constant in a narrow range of 15.68–15.84 mA cm−2). While for ZCISe QDSCs, low Mw PEG (PEG-300, and PEG-1K) additives have insignificant effects on Jsc, but high molecular weight PEG (PEG-4K and PEG-20K) additives have slightly negative effects on it (decreasing from 26.55 to 26.02, and 25.55 mA cm−2, respectively). For the FF value, low Mw PEG additives (PEG-300 and PEG-1K) have positive effect for both CdSe and ZCISe QDSCs (in CdSe, improving from 66.0% to 68.8–69.1%; in ZCISe, improving from 59.0% to 61.1%, and 60.8%); high Mw PEG (PEG-4K and PEG-20K) additives still have positive effects for CdSe cells (improving to 68.3%, and 68.8%, respectively), but negative effects for ZCISe cells (decreasing the FF value to 58.5%, and 55.6%, respectively). Correspondingly, for PCE value, all PEG additives have positive effect without significant difference for CdSe QDSCs in comparison with reference samples (improving from 6.81% to 7.38–7.60%); for ZCISe QDSCs, with the increase of Mw of PEG additive, the extent of improving PEC value decreases systematically. In detail, low Mw PEG-300, and PEG-1K can remarkably improve the PEC of ZCISe QDSCs from 9.26% to 10.20%, and 10.14%, respectively, while PEG-4K can only slightly enhance the PEC value to 9.62%; inversely, PEG-20K even has negative effect and decrease the value to 9.11%. In summary, low Mw PEG-300 and PEG-1K can serve as a general photovoltaic performance enhancement additive for the representative CdSe and ZCISe QDSCs. High Mw PEG, especially PEG-20K, can only improve the performance of CdSe QDSCs, but fails flat in ZCISe cells due to the negative effects on FF and Jsc.
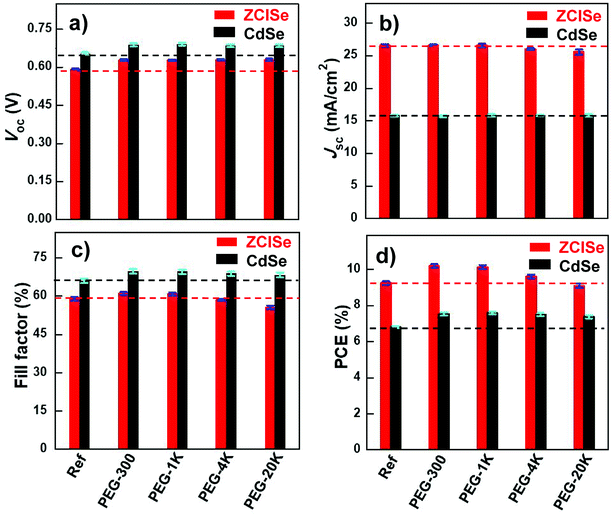 | ||
| Fig. 1 The dependence of photovoltaic parameters: (a) PCE, (b) Voc, (c) Jsc, and (d) FF, on PEG additives with different molecular weights in polysulfide electrolyte in ZCISe and CdSe QDSCs. | ||
| PEG additives | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Ref. | 15.81 ± 0.17 | 0.654 ± 0.005 | 66.0 ± 0.8 | 6.81 ± 0.11 |
| PEG-300 | 15.72 ± 0.15 | 0.688 ± 0.007 | 69.7 ± 1.1 | 7.54 ± 0.11 |
| PEG-1K | 15.8 ± 0.20 | 0.691 ± 0.005 | 69.6 ± 0.9 | 7.60 ± 0.05 |
| PEG-4K | 15.84 ± 0.12 | 0.685 ± 0.005 | 68.8 ± 0.6 | 7.50 ± 0.04 |
| PEG-20K | 15.83 ± 0.17 | 0.685 ± 0.006 | 68.3 ± 0.4 | 7.38 ± 0.07 |
| PEG additives | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Ref. | 26.55 ± 0.23 | 0.591 ± 0.004 | 58.96 ± 0.7 | 9.26 ± 0.10 |
| PEG-300 | 26.58 ± 0.18 | 0.629 ± 0.003 | 61.06 ± 0.7 | 10.20 ± 0.09 |
| PEG-1K | 26.56 ± 0.26 | 0.628 ± 0.003 | 60.8 ± 0.6 | 10.15 ± 0.04 |
| PEG-4K | 26.03 ± 0.12 | 0.630 ± 0.003 | 58.6 ± 0.4 | 9.62 ± 0.03 |
| PEG-20K | 25.55 ± 0.42 | 0.630 ± 0.004 | 56.6 ± 0.7 | 9.11 ± 0.12 |
Impedance spectroscopy of full cell devices
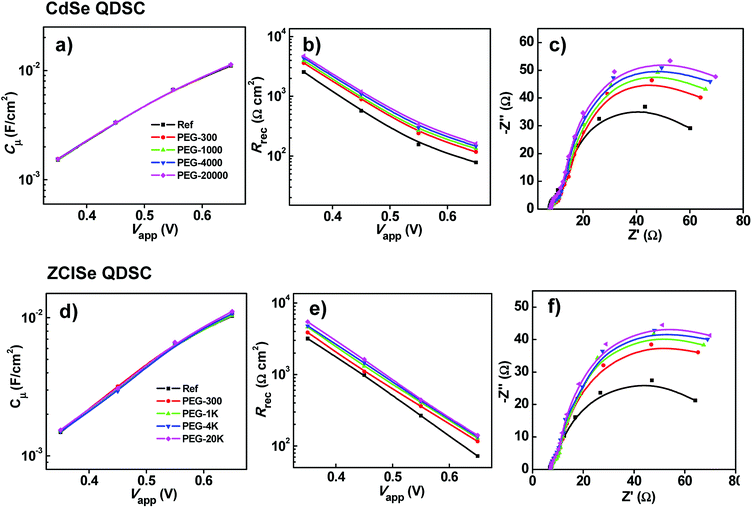
In order to unravel the intrinsic mechanism for the influence of different PEG additives in polysulfide/sulfide electrolyte on the photovoltaic performance of CdSe and ZCISe QDSCs, electrochemical impedance spectroscopy (EIS) for a full cell device was employed. This is because EIS is a well-established technique to distinguish the electrochemical features corresponding to different constituents in a cell device.48,49 EIS measurements were carried out under dark condition with forward bias voltage in a range from −0.35 to −0.65 V by applying a 20 mV AC sinusoidal signal in a frequency range of 100 kHz to 0.1 Hz. Nyquist curves for each QDSCs corresponding to different electrolyte systems under different bias are available in Fig. S4–S6.† Main EIS parameters including series resistance (Rs), chemical capacitance (Cμ) and charge recombination resistance (Rrec) were deduced from the EIS curves with use of a standard simulation circuit as reported in literature.47 It is noted that chemical capacitance (Cμ) stands for the change of electron density as a function of Fermi level, and the parameter charge recombination resistance (Rrec) corresponds to the charge recombination resistance at the photoanode/electrolyte interfaces.48,49 The applied bias (Vapp) dependent chemical capacitance, and charge recombination resistance values were illustrated in Fig. 2.From Fig. 2a and d, we can find that in both CdSe and ZCISe QDSCs, the extracted Cμ values exhibit a near linear relationship to the forward bias voltage in each electrolyte system, and all these curves are overlapped together. This means that cell devices with different electrolyte systems show ignorable variation in the obtained Cμ values. This indicates that the introduction of PEG additives in the initial polysulfide electrolyte solution does not alter the conduction band edge or the electron density of the states of TiO2 matrix.48–50 This result is also in accordance with previous reports,34,37,38 wherein other water-soluble polymers such as poly(vinylpyrrolidone) (PVP), sodium carboxymethylcellulose (CMC-Na), and sodium polyacrylate (PAAS) were used as additives to modify the polysulfide/sulfide electrolyte in the construction of QDSCs.34,37,38 However, from Fig. 2b and e it can be found that with the increase of Mw of PEG additives in electrolyte solutions, the obtained Rrec values enhance systematically, and the Rrec values exhibit a near linear relationship to the forward bias in each electrolyte system. For clarity, Nyquist plots of cell devices under forward bias near the Voc value (herein of −0.65 V) are shown in Fig. 2c and f. Accordingly, the extracted EIS parameters based on these plots are listed in Table 3. We can find that accompanying with the increase of Mw of PEG additives in the electrolyte, the diameter of the EIS semicircles increases systematically. This indicates that with the presence of PEG additives, the Rrec value at photoanode/electrolyte interface are improved accordingly, and the effect is strengthened systematically with the increase of Mw of PEG additives. As listed in Table 3, we can find that the Rrec value of the PEG modified electrolyte system is increased systematically in both CdSe and ZCISe QDSC systems (for CdSe, increasing from 78.2 to 160.6 Ω cm;2 for ZCISe, increasing from 72.4 to 141.8 Ω cm2). These results indicate that the charge recombination from photoanode to oxidation species (herein Sn2−) of electrolyte at photoanode/electrolyte interfaces is effectively suppressed by the PEG additives, and the extent is strengthened with the increase of PEG Mw. Therefore, an increase of Voc can be observed in the corresponding cell devices.
| QDSCs | PEG additives | Rs (Ω cm2) | Rrec (Ω cm2) | Cμ (mF cm−2) |
|---|---|---|---|---|
| CdSe | Ref. | 7.6 | 78.2 | 11.0 |
| PEG-300 | 7.8 | 117.1 | 11.2 | |
| PEG-1K | 7.9 | 132.6 | 11.2 | |
| PEG-4K | 7.8 | 145.6 | 11.2 | |
| PEG-20K | 7.8 | 160.6 | 11.3 | |
| ZCISe | Ref. | 6.8 | 72.4 | 10.3 |
| PEG-300 | 7.1 | 116.2 | 10.7 | |
| PEG-1K | 7.4 | 128.9 | 10.4 | |
| PEG-4K | 7.2 | 135.3 | 10.7 | |
| PEG-20K | 7.4 | 141.8 | 11.1 |
The mechanism for the observed retarded charge recombination is due to the adherence of PEG molecules on photoanode surface. This is because the presence of terminal hydroxyl groups and ether bonding oxygen atoms in the PEG molecules, which have strong coordinating capacity to TiO2 substrate.33,51 The adsorbed PEG molecules act as a passivation layer and an insulating energetic barrier layer, which prevent the direct contact of electrons on the surface of photoanode and electrolyte in the aspect of stereoscopic space and energetic field, and therefore inhibit the charge recombination processes through the surface defects in photoanode or with oxidation species in electrolyte.16 With the increase of Mw of PEG additives, the insulating feature, as shown in Tables 1–4, become intensified. Simultaneously, stereoscopic shielding effect has also been improved with the increase of Mw. The increased insulating property combined with the improved stereoscopic shielding effect result in better barrier effect in isolating photogenerated electrons on photoanode surface with redox couple in the electrolyte, and therefore inhibiting the charge recombination process more effective. This can give a reasonable explanation for the enhancement of charge recombination resistance value with the increase of Mw of PEG additives in electrolyte solutions as observed in Fig. 2b, e and Table 3. The reason for the decrease of overall photovoltaic performance of cell devices with the increase of Mw of PEG additives can be ascribed to the decrease of electric conductivity of corresponding electrolyte systems as shown in Table 4. The decrease of conductivity is mainly derived from the lower diffusion coefficient for higher Mw PEG. The conductivity (σ) of an electrolyte solution is defined by the equation:
| PEG additives | Rs Ω cm2 | R1 Ω cm2 | Rct Ω cm2 | Conductivity mS cm−1 |
|---|---|---|---|---|
| Ref. | 0.33 | 0.84 | 4.42 | 82.5 |
| PEG-300 | 0.39 | 0.55 | 2.87 | 59.1 |
| PEG-1K | 0.38 | 0.55 | 3.03 | 58.4 |
| PEG-4K | 0.72 | 1.34 | 10.63 | 45.0 |
| PEG-20K | 1.02 | 1.54 | 12.30 | 39.8 |
Impedance spectroscopy of symmetric dummy cells
Since all kinds of PEG additives can suppress the charge recombination dynamics occurring at photoanode/electrolyte interface and bring forward an enhancement of Voc value in the resulting cell devices, the question of what causes the different effects on FF value for both CdSe and ZCISe QDSCs by PEG additives in electrolyte comes naturally. Generally, the FF in a sensitized solar cell is largely affected by the internal series resistance of the cell device, which includes various components for photoelectrochemical generation. Among these resistances, the charge transfer resistance Rct at the counter electrode/electrolyte interface seems more severe than the ohmic resistance of the charge collection electrode and the resistances associated with the charge carrier transport by the electrolyte and TiO2 matrix.10,48 Since identical charge collection electrodes and TiO2 matrix were used in different cell systems, there should be no resistance difference in these components. Therefore, the main difference of internal resistance in difference systems should be the charge transfer resistance at CE/electrolyte interface, which is derived from the different compositions of the electrolyte solutions. Therefore, in the investigated systems, FF is mainly determined by the Rct values.In order to explore the origin of different FF values in cell devices, EIS measurements were carried out using symmetric dummy cells consisting of two identical Cu2S/brass CEs placed face to face in a sandwich configuration and filling with polysulfide electrolyte solutions containing different PEG additives. The configuration of symmetric form can rule out the interference from photoanode and simply the measurement and analysis.52 The obtained Nyquist curves corresponding to different PEG additive systems are shown in Fig. 3a. As expected, Nyquist plots for each electrolyte system consist of two semicircles, which are related to the resistance and capacitance (R1 and C1) of the solid–solid contact between Cu2S catalytic materials and brass substrate, and charge transfer resistance and capacitance (Rct and C) at the CE/electrolyte interface, respectively.53–55 The high frequency intercept on the real axis corresponds to the series resistance (Rs). These three parameters are related to the contact of electrode substrate and catalytic material, the catalytic activity of CE catalytic materials, and conductivity of electrode and electrolyte. Standard equivalent circuit as reported in literature are used to extract the EIS data from the Nyquist plots,53 and key parameters including Rs, R1, and Rct are listed in Table 4. Owing to the outstanding conductivity of the brass substrate, the Rs values of all CE systems are small, but with the increase of Mw of PEG additives, the corresponding Rs values increase systematically (from 0.33 to 1.02 Ω cm2) in comparison with reference sample without the presence of any PEG additives. The observed increase of Rs value should be ascribed to the decrease of electrical conductivity with the introduction of insulating PEG additives, especially with the increase of Mw, as shown in Table 4.
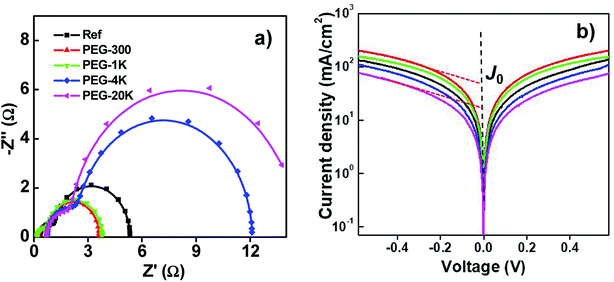 | ||
| Fig. 3 Electrochemical characterizations of symmetric dummy cells corresponding to electrolyte containing different PEG additives: (a) Nyquist curves, (b) Tafel polarization curves. | ||
With the introduction of low Mw PEG-300, and PEG-1K in the electrolyte solution, charge transfer resistance (Rct) at CE/electrolyte interface decreased remarkably (from 4.42 to 2.87, and 3.03 Ω cm2). In contrast, the introduction of high Mw PEG-4K, and PEG-20K led to a significant increase of Rct value (10.63, and 12.30 Ω cm2). Generally, Rct can significantly influence the cell performance from two aspects. First, Rct is considered to be a major hurdle in attaining a high FF value as stated in the above section. Second, Rct is directly related to the reaction barrier of redox couple reduction reaction at the CE/electrolyte interface and hence plays a significant effect on the electrocatalytic properties of the catalyst.10,48,54 High Rct value means slow charge transfer rate at the counter electrode. This leads to a high overpotential for the redox couple reduction reaction and creates a bottleneck for the electron flowing from CE to electrolyte, thereby promoting back electron transfer at the photoanode. These effects would result in a low Jsc and FF for the corresponding cell devices.10,11
Origin of the PEG additives effect difference between CdSe and ZCISe QDSCs
The origin of the decrease of the CE/electrolyte charge transfer resistance by the introduction of PEG additives should be ascribed to the surfactant feature of this kind of polymer, which wets the surface of the CE catalytic materials and favors the close contact between CE and electrolyte, and therefore facilitates the catalytic reduction reaction of the redox couple and promotes the charge transfer from CE to electrolyte. With the increase of Mw, the adherence of PEG on CE surface together with the electrical insulating feature of the PEG polymer impede the transfer of electron from CE to electrolyte and therefore increase the Rct value and deteriorate the photovoltaic performance, especially the FF and Jsc values as observed in the J–V measurement results.To further verify the effects of PEG additives in the polysulfide electrolyte on photovoltaic performance of cell devices, Tafel-polarization measurements were also employed in a dummy cell configuration identical to the one used in EIS measurements. The obtained Tafel curves with logarithmic current density (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J) as a function of the applied voltage are shown in Fig. 3b. We can find that with the introduction of low Mw PEG (PEG-300, and PEG-1K) additives into the electrolyte systems, both the cathodic and anodic branches of the Tafel curves exhibit a larger slope in comparison with those for reference sample, demonstrating a higher exchange current density (J0) on the corresponding CE surface. This also indicates that the catalytic reduction of Sn2− to S2− is accelerated at CE/electrolyte interface. While the presence of high Mw PEG (PEG-4K, and PEG-20K) additives in the polysulfide electrolyte plays an inverse effect, resulting in a lower J0 value, and therefore slowing the catalytic reduction of redox couples. Since J0 is inversely proportional to the Rct as defined by J0 = RT/nFRct,56 the observed variation trend for J0 obtained in Tafel polarization measurements is in agreement with that of Rct in EIS measurements. This gives a further support for the observed increase of FF, and Jsc for the resultant QDSCs corresponding to polysulfide electrolyte containing low Mw PEG additives.
J) as a function of the applied voltage are shown in Fig. 3b. We can find that with the introduction of low Mw PEG (PEG-300, and PEG-1K) additives into the electrolyte systems, both the cathodic and anodic branches of the Tafel curves exhibit a larger slope in comparison with those for reference sample, demonstrating a higher exchange current density (J0) on the corresponding CE surface. This also indicates that the catalytic reduction of Sn2− to S2− is accelerated at CE/electrolyte interface. While the presence of high Mw PEG (PEG-4K, and PEG-20K) additives in the polysulfide electrolyte plays an inverse effect, resulting in a lower J0 value, and therefore slowing the catalytic reduction of redox couples. Since J0 is inversely proportional to the Rct as defined by J0 = RT/nFRct,56 the observed variation trend for J0 obtained in Tafel polarization measurements is in agreement with that of Rct in EIS measurements. This gives a further support for the observed increase of FF, and Jsc for the resultant QDSCs corresponding to polysulfide electrolyte containing low Mw PEG additives.
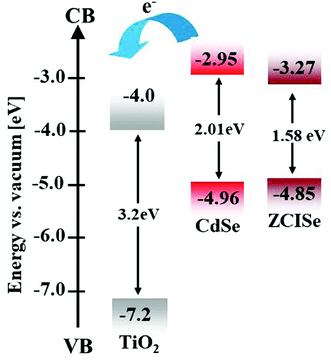
Based on the above data, we can understand that the positive effects for PEG-300, and PEG-1K additives, and the negative effect for PEG-20K additives on the photovoltaic performance of QDSCs. However, the results for PEG-20K additive on CdSe and ZCISe cells are different. What is the cause for this difference? Based on previous literature data,18,57 the conduction band edge energy (VCB) for the adopted 5.2 nm CdSe QDs is higher by 0.32 eV in comparison with the investigated 4.1 nm ZCISe QDs. For clarity, the energy level diagrams of investigated CdSe and ZCISe together with TiO2 substrate are schematically illustrated in Fig. 4.
It is well established that this energy difference between QD and TiO2 serves as the driving force to ensure the effective injection of photo-generated electron from light absorber QD to TiO2 substrate.13–15,58,59 In the construction of high efficiency QDSC, a higher VCB of QDs relative to that of TiO2 electron acceptor is a prerequisite for QD sensitizers. The fact that the higher energy difference is accompanied with a greater electron injection rate (Ket) from QD to TiO2 electron acceptor has been well studied in previous reports.15,18,58 Meanwhile, the greater Ket value can brings forward not only greater photocurrent, but also more effective transfer of photogenerated electrons through the photoanode film, and from external circuit to electrolyte via CE. This means that higher VCB in QD light absorber would favor Voc, and FF of the resulting QDSCs. Therefore, the relatively high VCB in CdSe QDs would offset the negative effects by high Mw PEG additive in electrolyte, and ensure high Voc and FF values in the resultant QDSCs. In fact, literature results have well demonstrated that with the enhancement of VCB via alloying process, the Voc and FF values in the Zn–Cu–In–S, and Zn–Cu–In–Se based QDSCs are remarkably higher than those of Cu–In–S, and Cu–In–Se QDSCs.18,60
4. Conclusions
In summary, the origin for the effects of different Mw PEG additives in polysulfide electrolyte on the performance of QDSCs has been explored. Furthermore, a facile and general route for remarkably improving photovoltaic performance of QDSCs is achieved with introduction of low Mw PEG additives into the polysulfide electrolyte. The energetic barrier effect due to the insulating feature together with the passivation effect on photoanode surface from PEG additives bring forward the suppression of charge recombination at photoanode/electrolyte interface, accompanied with the improvement of Voc of the resultant cell device. The wetting effect of the PEG surfactant reduces the charge transfer resistance at EC/electrolyte interface, and results in the improvement of FF and Jsc. The intensified insulating feature with the increase of Mw of PEG favors the Voc value, but has a negative effect on FF and Jsc for the resulting cell devices. Due to the relative high conduction band edge of CdSe light absorber, the negative effect on FF by high Mw PEG additive in ZCISe QDSCs is not realized in CdSe QDSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the National Natural Science Foundation of China (NFSC No. 51732004, 91433106, and 21703071)References
- K. W. J. Barnham, M. Mazzer and B. Clive, Nat. Mater., 2006, 5, 161–164 CrossRef.
- H. Tada, M. Fujishima and H. Kobayashi, Chem. Soc. Rev., 2011, 40, 4232–4243 RSC.
- I. Mora-Sero and J. Bisquert, J. Phys. Chem. Lett., 2010, 1, 3046–3052 CrossRef.
- J. Duan, H. Zhang, Q. Tang, B. He and L. Yu, J. Mater. Chem. A, 2015, 3, 17497–17510 RSC.
- R. Wang, Y. Shang, P. Kanjanaboos, W. Zhou, Z. Ning and E. H. Sargent, Energy Environ. Sci., 2016, 9, 1130–1143 RSC.
- P. V. Kamat, Acc. Chem. Res., 2012, 45, 1906–1915 CrossRef PubMed.
- G. H. Carey, A. L. Abdelhady, Z. Ning, S. M. Thon, O. M. Bakr and E. H. Sargent, Chem. Rev., 2015, 115, 12732–12763 CrossRef PubMed.
- A. J. Nozik, M. C. Beard, J. M. Luther, M. Law, R. J. Ellingson and J. C. Johnson, Chem. Rev., 2010, 110, 6873–6890 CrossRef PubMed.
- J. B. Sambur, T. Novet and B. A. Parkinson, Science, 2010, 330, 63–66 CrossRef PubMed.
- J. G. Radich, R. Dwyer and P. V. Kamat, J. Phys. Chem. Lett., 2011, 2, 2453–2460 CrossRef.
- S. Jiao, J. Du, Z. Du, D. Long, W. Jiang, Z. Pan, Y. Li and X. Zhong, J. Phys. Chem. Lett., 2017, 8, 559–564 CrossRef PubMed.
- Q. Zhang, X. Guo, X. Huang, S. Huang, D. Li, Y. Luo, Q. Shen, T. Toyoda and Q. Meng, Phys. Chem. Chem. Phys., 2011, 13, 4659–4667 RSC.
- P. K. Santra and P. V. Kamat, J. Am. Chem. Soc., 2012, 134, 2508–2511 CrossRef PubMed.
- J. Wang, I. Mora-Seró, Z. Pan, K. Zhao, H. Zhang, Y. Feng, G. Yang, X. Zhong and J. Bisquert, J. Am. Chem. Soc., 2013, 135, 15913–15922 CrossRef PubMed.
- Z. Pan, I. Mora-Seró, Q. Shen, H. Zhang, Y. Li, K. Zhao, J. Wang, X. Zhong and J. Bisquert, J. Am. Chem. Soc., 2014, 136, 9203–9210 CrossRef PubMed.
- K. Zhao, Z. Pan, I. Mora-Seró, E. Cánovas, H. Wang, Y. Song, X. Gong, J. Wang, M. Bonn, J. Bisquert and X. Zhong, J. Am. Chem. Soc., 2015, 137, 5602–5609 CrossRef PubMed.
- J. Y. Kim, J. Yang, J. H. Yu, W. Baek, C. H. Lee, H. J. Son, T. Hyeon and M. J. Ko, ACS Nano, 2015, 9, 11286–11295 CrossRef PubMed.
- J. Du, Z. Du, J. Hu, Z. Pan, Q. Shen, J. Sun, D. Long, H. Dong, L. Sun, X. Zhong and L. Wan, J. Am. Chem. Soc., 2016, 138, 4201–4209 CrossRef PubMed.
- W. Wang, W. Feng, J. Du, W. Xue, L. Zhang, L. Zhao, Y. Li and X. Zhong, Adv. Mater., 2018, 30, 1705746 CrossRef PubMed.
- I. Mora-Seró, S. Giménez, T. Moehl, F. Fabregat-Santiago, T. Lana-Villareal, R. Gómez and J. Bisquert, Nanotechnology, 2008, 19, 424007 CrossRef PubMed.
- Y. Li, L. Zhao, Z. Du, J. Du, W. Wang, Y. Wang, L. Zhao, X. Cao and X. Zhong, J. Mater. Chem. A, 2018, 6, 2129 RSC.
- L. Zhao, L. Zhao, W. Xue, W. Fang, Y. Wang and Y. Li, Sol. Energy, 2018, 169, 505 CrossRef.
- W. Li and X. Zhong, J. Phys. Chem. Lett., 2015, 6, 796–806 CrossRef PubMed.
- H. Zhu, N. Song and T. Lian, J. Am. Chem. Soc., 2013, 135, 11461–11464 CrossRef PubMed.
- J. Y. Cong, X. C. Yang, L. Kloo and L. C. Sun, Energy Environ. Sci., 2012, 5, 9180–9194 RSC.
- C. H. Chang and Y. L. Lee, Appl. Phys. Lett., 2007, 91, 053503 CrossRef.
- H. Lee, M. K. Wang, P. Chen, D. R. Gamelin, S. M. Zakeeruddin, M. Gratzel and M. K. Nazeeruddin, Nano Lett., 2009, 9, 4221–4227 CrossRef PubMed.
- S. Y. Chae, Y. J. Hwang and O. S. Joo, RSC Adv., 2014, 4, 26907–26911 RSC.
- Z. Ning, C. Yuan, H. Tian, Y. Fu, L. Li, L. Sun and H. Agren, J. Mater. Chem., 2012, 22, 6032–6037 RSC.
- L. Li, X. Yang, J. Gao, H. Tian, J. Zhao, A. Hagfeldt and L. Sun, J. Am. Chem. Soc., 2011, 133, 8458–8460 CrossRef PubMed.
- T. Shu, X. Li, Z. L. Ku, S. Wang, S. Wu, X. H. Jin and C. D. Hu, Electrochim. Acta, 2014, 137, 700–704 CrossRef.
- V. Jovanovski, V. González-Pedro, S. Giménez, E. Azaceta, G. Cabañero, H. Grande, R. Tena-Zaera, I. Mora-Seró and J. Bisquert, J. Am. Chem. Soc., 2011, 133, 20156–20159 CrossRef PubMed.
- J. Du, X. Meng, K. Zhao, Y. Li and X. Zhong, J. Mater. Chem. A, 2015, 3, 17091–17097 RSC.
- G. Jiang, Z. Pan, Z. Ren, J. Du, C. Yang, W. Wang and X. Zhong, J. Mater. Chem. A, 2016, 4, 11416–11421 RSC.
- J. Yu, W. Wang, Z. Pan, J. Du, Z. Ren, W. Xue and X. Zhong, J. Mater. Chem. A, 2017, 5, 14124–14133 RSC.
- H. Wei, G. Wang, J. Shi, H. Wu, Y. Luo, D. Li and Q. Meng, J. Mater. Chem. A, 2016, 4, 14194–14203 RSC.
- W. Feng, Y. Li, J. Du, W. Wang and X. Zhong, J. Mater. Chem. A, 2016, 4, 1461–1468 RSC.
- W. Feng, L. Zhao, J. Du, Y. Li and X. Zhong, J. Mater. Chem. A, 2016, 4, 14849–14856 RSC.
- H. Kim, I. Hwang and K. Yong, ACS Appl. Mater. Interfaces, 2014, 6, 11245–11253 CrossRef PubMed.
- Z. Yu, Q. Zhang, D. Qin, Y. Luo, D. Li, Q. Shen, T. Toyoda and Q. Meng, Electrochem. Commun., 2010, 12, 1776–1779 CrossRef.
- H. Chen, L. Lin, X. Yu, K. Qiu, X. Lu, D. Kuang and C. Su, Electrochim. Acta, 2013, 92, 117–123 CrossRef.
- M. C. Sekhar, K. Santhosh, J. P. Kumar, N. Mondal and S. Soumya, J. Phys. Chem. C, 2014, 118, 18481–18487 CrossRef.
- M. Shalom, S. Ruhle, I. Hod, S. Yahav and A. Zaban, J. Am. Chem. Soc., 2009, 131, 9876–9877 CrossRef PubMed.
- E. M. Barea, M. Shalom, S. Giménez, I. Hod, I. Mora-Seró, A. Zaban and J. Bisquert, J. Am. Chem. Soc., 2010, 132, 6834–6839 CrossRef PubMed.
- S. R. Raga, E. M. Barea and F. Fabregat-Santiago, J. Phys. Chem. Lett., 2012, 3, 1629–1634 CrossRef PubMed.
- X. Zhong, Y. Feng and Y. Zhang, J. Phys. Chem. C, 2007, 111, 526–531 CrossRef.
- Z. Du, H. Zhang, H. Bao and X. Zhong, J. Mater. Chem. A, 2014, 2, 13033–13040 RSC.
- I. Mora-Seró, S. Gimenez, F. Fabregat-Santiago, R. Gomez, Q. Shen, T. Toyoda and J. Bisquert, Acc. Chem. Res., 2009, 42, 1848–1857 CrossRef PubMed.
- F. Fabregat-Santiago, G. Garcia-Belmonte, I. Mora-Seró and J. Bisquert, Phys. Chem. Chem. Phys., 2011, 13, 9083–9118 RSC.
- V. González-Pedro, X. Xu, I. Mora-Seró and J. Bisquert, ACS Nano, 2010, 4, 5783–5790 CrossRef PubMed.
- B. Ballou, B. C. Lagerholm, L. A. Ernst, M. P. Bruchez and A. S. Waggoner, Bioconjugate Chem., 2004, 15, 79–86 CrossRef PubMed.
- W. Kwon, J. M. Kim and S. W. Rhee, J. Mater. Chem. A, 2013, 1, 3202–3215 RSC.
- Y. Yang, L. Zhu, H. Sun, X. Huang, Y. Luo, D. Li and Q. Meng, ACS Appl. Mater. Interfaces, 2012, 4, 6162–6168 CrossRef PubMed.
- J. Bisquert, D. Cahen, G. Hodes, S. Rühle and A. Zaban, J. Phys. Chem. B, 2004, 108, 8106–8118 CrossRef.
- T. N. Murakami, S. Ito, Q. Wang, M. K. Nazeeruddin, T. Bessho, I. Cesar, P. Liska, R. Humphry-Baker, P. Comte, P. Péchy and M. Graetzel, J. Electrochem. Soc., 2006, 153, A2255 CrossRef.
- I. Hwang and K. Yong, ChemElectroChem, 2015, 2, 634–653 CrossRef.
- R. W. Meulenberg, J. R. I. Lee, A. Wolcott, J. Z. Zhang, L. J. Terminello and T. van Buuren, ACS Nano, 2009, 3, 325–330 CrossRef PubMed.
- I. Robel, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2007, 129, 4136–4137 CrossRef PubMed.
- J. H. Bang and P. V. Kamat, ACS Nano, 2009, 3, 1467–1476 CrossRef PubMed.
- L. Yue, H. S. Rao, J. Du, Z. X. Pan, J. Yu and X. H. Zhong, RSC Adv., 2018, 8, 3637–3645 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed J–V curves, photovoltaic performance parameters, and additional EIS Nyquist curves under different forward bias for each QDSCs under electrolyte solutions containing different PEG additives. See DOI: 10.1039/c8ra05794j |
| This journal is © The Royal Society of Chemistry 2018 |