Open Access Article
Open Access ArticleMicrowave-assisted delivery of an anticancer drug to cancer cells†
Sina Atrin Mazinaniab,
Jeffrey A. Stuartbc and
Hongbin Yan *ab
*ab
aDepartment of Chemistry, Brock University, 1812 Sir Isaac Brock Way, St. Catharines, Ontario, Canada L2S 3A1. E-mail: tyan@brocku.ca
bCentre for Biotechnology, Brock University, 1812 Sir Isaac Brock Way, St. Catharines, Ontario, Canada L2S 3A1
cDepartment of Biological Sciences, Brock University, 1812 Sir Isaac Brock Way, St. Catharines, Ontario, Canada L2S 3A1
First published on 7th September 2018
Abstract
Exposure of MCF-7 breast and PC-3 prostate cancer cells to 10 W microwaves at 2.45 GHz increased their uptake of the cancer drug doxorubicin from media by almost 100%, concomitantly increasing cell death, while microwave exposure alone had no cellular toxicity. Addition of inhibitors of endocytosis during the treatment of MCF-7 cells with doxorubicin and microwaves showed no impact on the uptake of the anticancer drug. Furthermore, the uptake of oligonucleotides by MCF-7 cells is not affected by the treatment with microwaves. These observations suggest that endocytosis is not involved in the uptake of doxorubicin while cells are exposed to microwave irradiation. Thus, targeted low power microwave irradiation could be a safe and effective means of promoting chemotoxin delivery to cancer cells, potentially reducing the dosages and side effects of anti-cancer drugs.
Introduction
In the last few decades, microwave irradiation has been recognized for its unique properties in transmitting energy as a heating source and passage of signals, however, there has been some controversy surrounding the safety of microwave exposure. Much of the recent investigation on microwaves as a heating source focuses on the existence of “microwave-specific effects”,1,2 that is, possible aspects of microwaves that are not associated with heating. We have explored several of these microwave-specific effects by studying the behaviours of biological systems exposed to microwaves while the temperature was carefully controlled through simultaneous cooling. Using this approach, we demonstrated that low power microwaves can affect enzyme activities. Trypsin, but not α-amylase or phosphatase, activity was significantly increased by exposure to 10 W microwaves at constant temperature.3,4 Low power microwaves also appear to slightly perturb membrane properties in PC-3 prostate cancer cells, without causing apoptosis or necrosis.5 Escherichia coli growth is also affected by exposure to non-lethal microwaves, though the effect is transient (unpublished results). Taken together, our findings suggest that exposure of cells to low power microwave irradiation at constant temperatures causes subtle perturbations to cells without toxicity.Based on our work with PC-3 human prostate cancer cells,5 we hypothesized that exposure of cells to non-lethal microwave irradiation at constant temperature transiently disrupts cell membrane permeability and/or stimulates endocytosis, promoting uptake of extracellular molecules. This scenario resembles electroporation, but with tissue penetration depth (2–4 cm)6 that is quite relevant for targeting tumors. It may therefore be possible to use microwave (or radiofrequency) to promote internalization of therapeutic agents into cells. In the present communication, we report significantly increased cellular uptake of the anticancer drug doxorubicin by MCF-7 human breast and PC-3 human prostate cancer cells, associated with reductions in cell viability.
Results and discussions
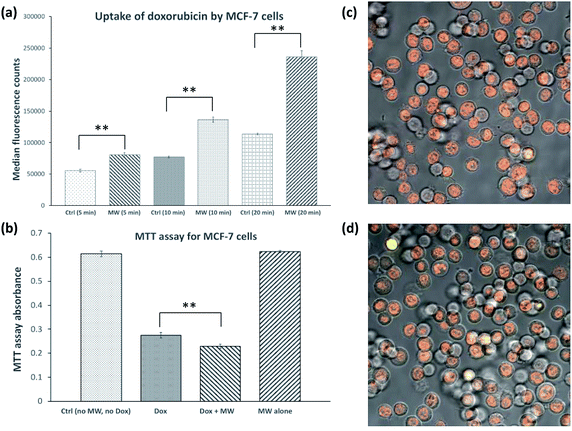
MCF-7 breast cancer cells were cultured in a CEM Coolmate microwave reactor at 37 °C in the presence or absence of 26 μM doxorubicin. Temperature was monitored with a fibre optic temperature probe and constant temperature was maintained through simultaneous cooling (see Fig. S1† for representative temperature and microwave power profiles for cultures in the microwave reactor). As demonstrated previously,3–5 we were able to maintain the temperature of the culture within ±1–2 °C of setpoint values.Two experiments were carried out at 37 °C in the presence of doxorubicin, one where MCF-7 cells were grown without microwave, while in the other MCF-7 cells were exposed to 10 W microwave. After 5, 10 and 20 minutes of exposure, the cultures were pelleted by centrifugation, washed and subsequently analyzed by flow cytometry to compare the level of doxorubicin internalization based on its inherent fluorescent property. As Fig. 1a shows, the uptake of doxorubicin was significantly increased by exposure to microwave in a time-dependent manner. The uptake of doxorubicin by MCF-7 cells treated with microwave for 20 minutes was 108% higher than that of control cells. Fig. 1c and d show fluorescence microscopy images of MCF-7 cells treated with doxorubicin alone and doxorubicin in the presence of microwave irradiation, respectively. Doxorubicin was clearly localized in the nucleus, consistent with literature observations.7
In order to demonstrate the toxicity of internalized doxorubicin, MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)) assays were conducted.8 In this experiment, MCF-7 cell cultures were incubated under four conditions, (1) in the absence of doxorubicin, (2) in the presence of 26 μM doxorubicin, and (3) in the presence of 26 μM doxorubicin and exposed to 10 W microwave, and (4) exposed to 10 W microwave in the absence of doxorubicin. After 20 minutes, microwave exposure was discontinued, and all four cultures were incubated for 24 h, followed by standard MTT assay quantification. Fig. 1b showed no effect of exposure to microwave for 20 minutes alone, while the MTT signal was reduced by 55 and 63% in cultures (2) and (3), respectively. This result indicates that doxorubicin killed and/or inhibited growth of MCF-7 cells and that this effect was magnified when microwave is administered together with doxorubicin.
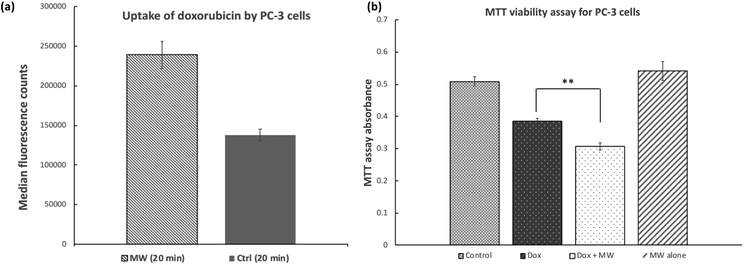
Similar results were obtained when PC-3 human prostate cancer cells were treated with doxorubicin while exposed to microwave irradiation (Fig. 2a). Under the same conditions as described above for MCF-7 cells, uptake of doxorubicin by PC-3 cells was increased by 73% in the presence of microwave for 20 minutes. Cell viability/growth as indicated by MTT signal was reduced by 25 and 40% for PC-3 cells treated with doxorubicin alone and doxorubicin in the presence of microwave, respectively (Fig. 2b). Again, treatment of PC-3 cells with microwave alone did not lead to significant reduction in cell viability as indicated by the MTT assay.
Doxorubicin is believed to enter cells by passive diffusion,9,10 however, there is some evidence that doxorubicin can be internalized via endocytosis, especially when it forms a complex or conjugate with another substance.11–13 To determine whether endocytosis is involved in the microwave-promoted uptake, inhibitors of this pathway were included during experiments. Thus, Pitstop2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and Dynasore15 which are known inhibitors of clathrin-independent and clathrin-dependent endocytosis, were added to cell cultures 30 minutes prior to treatment with doxorubicin. Doxorubicin uptake was subsequently measured by flow cytometry. As can be seen in Fig. 3, no effect of Pitstop2 or Dynasore on the uptake of doxorubicin in the absence of microwave was observed, indicating that endocytosis does not contribute to doxorubicin uptake under our cell culture conditions. Similarly, when MCF-7 cells were pre-treated with Pitstop2 and Dynasore for 30 minutes, followed by doxorubicin in the presence of microwave there was no decrease in doxorubicin uptake (Fig. 3). As a positive control, MCF-7 cells were treated with Pitstop2 and Dynasore under the same conditions, followed by addition of a human transferrin-dye conjugate (CF® Dye Conjugate). After incubation for 20 min uptake of CF® Dye Conjugate was quantified by flow cytometry. A 25% decrease in the median fluorescence intensity was seen in the cells pre-treated with endocytosis inhibitors. While this level of inhibition is not as drastic as shown in the literature for other cell lines,14,15 the inhibitory properties of Pitstop2 and Dynasore are obvious. These results suggest that the enhanced uptake of doxorubicin facilitated by microwave is unlikely via endocytosis.
14 and Dynasore15 which are known inhibitors of clathrin-independent and clathrin-dependent endocytosis, were added to cell cultures 30 minutes prior to treatment with doxorubicin. Doxorubicin uptake was subsequently measured by flow cytometry. As can be seen in Fig. 3, no effect of Pitstop2 or Dynasore on the uptake of doxorubicin in the absence of microwave was observed, indicating that endocytosis does not contribute to doxorubicin uptake under our cell culture conditions. Similarly, when MCF-7 cells were pre-treated with Pitstop2 and Dynasore for 30 minutes, followed by doxorubicin in the presence of microwave there was no decrease in doxorubicin uptake (Fig. 3). As a positive control, MCF-7 cells were treated with Pitstop2 and Dynasore under the same conditions, followed by addition of a human transferrin-dye conjugate (CF® Dye Conjugate). After incubation for 20 min uptake of CF® Dye Conjugate was quantified by flow cytometry. A 25% decrease in the median fluorescence intensity was seen in the cells pre-treated with endocytosis inhibitors. While this level of inhibition is not as drastic as shown in the literature for other cell lines,14,15 the inhibitory properties of Pitstop2 and Dynasore are obvious. These results suggest that the enhanced uptake of doxorubicin facilitated by microwave is unlikely via endocytosis.
To further rule out the involvement of endocytosis in the microwave-assisted internalization of doxorubicin, we measured uptake of a fluorescently labeled oligodeoxyribonucleotide (FAM-5′-TTG GTG GAT AGT TAT TAG-3′, FAM: fluorescein) by MCF-7 cells in the presence and absence of microwave exposure. While the uptake of oligonucleotides by cells is complex, it is believed to involve endocytosis.16 Results indicate (Fig. 4) that microwave did not increase uptake of the oligonucleotide by MCF-7 cells. While previous work17 reported that treatment of cultured myoblasts with rather high power microwave (420 W in pulses, as opposed to 10 W used in the present study) led to enhanced delivery of 2′-O-methylphosphorothioate antisense oligonucleotides, cells exposed to such a significant microwave power are likely to experience notable alteration in cellular structures and functions; as such, no comparison can be drawn between the present work and the literature precedence.
Materials and methods
Media
Modified DMEM (Dulbecco's Modified Eagle Medium) contains 10% fetal bovine serum, 2% non-essential amino acids, and 1% penicillin/streptomycin.MCF-7 and PC-3 cell cultures
MCF-7 human breast cancer cells and PC-3 human prostate cancer cells (the American Type Culture Collection, ATCC) were cultured in modified DMEM in 10 cm cell culture dishes (Sarstedt) until a confluent monolayer of adherent cells was obtained. The cells were washed twice with PBS (4.0 ml, pH 7.4). Trypsin–EDTA solution (3 ml, 0.25%, Sigma, T4049) was then added and the plate was incubated at 37 °C for 3 min in a culture incubator (20% O2, 5% CO2, 37 °C). DMEM (4.0 ml) was then added to the plate and the cell suspension was transferred to a 15 ml Falcon tube and centrifuged at 970 g for 3 min to obtain a cell pellet, which was used for subsequent experiments.Microwave irradiation set up
A CEM Discovery Coolmate microwave system running at 2.45 GHz was used for the irradiation of MCF-7 and PC-3 cell cultures. The microwave reactor was controlled with Synergy software (CEM). MCF-7 and PC-3 cultures were incubated in CEM standard reaction vessels (catalogue number 168302), together with the CEM attenuator assembly (catalogue number 542476) that allows for the circulation of coolant around the culture tube to maintain the temperature of cultures. Solvay Solexis H Galden Zt 130 coolant was pre-cooled in the Coolmate coolant reservoir by liquid nitrogen. During experiments, the culture temperature was maintained at 37 ± 2 °C by adjusting the temperature of coolant and flow rates and monitored with a fibre optic temperature probe in real time. Cultures were mixed by stirring with a stirring bar (1 × 8 mm), with the stirring setting at low (540 revolution per minute as determined by an Omega HHT13 Tachometer).Fluorescence microscopy
Fluorescence images of MCF-7 and PC-3 cells were obtained using a Carl Zeiss Axio Observer microscope. The microscope stage temperature was kept at 37 °C under 5% CO2. For doxorubicin assay, red fluorescence of doxorubicin was detected using a 545 ± 12.5 nm excitation and 605 ± 35 nm emission filter set. The intensity of fluorescence excitation by an X-Cite 120LED light source and camera exposure times were held constant across experiments.Flow cytometry
Median fluorescence intensity of stained MCF-7 and PC-3 cells were obtained using a BD ACCURI C6 flow cytometer. In doxorubicin assay, red fluorescence of doxorubicin was measured in Fl-3 channel (Em 610 ± 10 nm). Number of events recorded was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for all experiments and FSC-H threshold was set to 80
000 for all experiments and FSC-H threshold was set to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
Doxorubicin assay (MCF-7 and PC-3 cells)
Cell density was measured by a hemocytometer. By dilution, cell density was adjusted to ∼500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml prior to each experiment. To a 6 ml cell suspension in modified DMEM was added 10 μl of doxorubicin (1 mg ml−1) to give a final concentration of 26 μM. Two aliquots of 3 ml cell suspension were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment).
000 cells per ml prior to each experiment. To a 6 ml cell suspension in modified DMEM was added 10 μl of doxorubicin (1 mg ml−1) to give a final concentration of 26 μM. Two aliquots of 3 ml cell suspension were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment).
CEM reaction vessel parameters were adjusted to 37 °C, maximum power 10 W, and slow stirring (540 rpm). For control, the temperature of the water bath was pre-set at 37 °C and the stirring speed of hotplate was set to 540 rpm. Samples were treated for 20 minutes under each condition. At the end of each treatment, 4 samples of 0.75 ml were taken from each reaction vessel (MW and control) and were transferred to microcentrifuge tubes. The microcentrifuge tubes were spun at 2300 rcf for 3 min to obtain cell pellets. After aspirating the supernatant, the cell pellets were re-suspended in 1× PBS (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 0.75 ml 1× PBS. The samples were analyzed by a flow cytometer (BD Accuri) and the median fluorescence of each sample at FL-3 channel was recorded. Fluorescent images were obtained in channels mcherry (Ex: 560 ± 20 nm, Em: 630 ± 37.5 nm) and cy3 (Ex: 545 ± 12.5 nm, Em: 605 ± 35 nm).
MTT assay
Cell density was measured by a hemocytometer. By dilution, cell density was adjusted to ∼500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml prior to each experiment. To a 6 ml cell suspension in modified DMEM was added 10 μl of doxorubicin at 1 mg ml−1 to reach a final concentration of 26 μM. Cell suspensions (3 ml) were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment). As a negative control, to a 3 ml cell suspension at similar cell density (500
000 cells per ml prior to each experiment. To a 6 ml cell suspension in modified DMEM was added 10 μl of doxorubicin at 1 mg ml−1 to reach a final concentration of 26 μM. Cell suspensions (3 ml) were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment). As a negative control, to a 3 ml cell suspension at similar cell density (500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml) was added 5 μl DMSO which was transferred to a reaction vessel in water bath.
000 cells per ml) was added 5 μl DMSO which was transferred to a reaction vessel in water bath.
CEM reaction vessel parameters were adjusted to 37 °C, maximum power 10 W, and slow stirring (540 rpm). For control, the temperature of the water bath was previously set to 37 °C and the stirring speed of hotplate was set to 540 rpm. Samples were treated for 20 minutes under each condition. At the end of each treatment, samples of 1 ml from each reaction vessel (MW and control) were transferred to microcentrifuge tubes. The microcentrifuge tubes were spun at 2300 rcf for 3 min to obtain cell pellets. After aspirating the supernatant, the cell pellets were re-suspended in modified DMEM (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in modified DMEM (1 ml). The cell suspension was then transferred to counting plates (SARSTEDT, TC Dish, ref. 83.3901.002) containing 3 ml modified DMEM.
The plates were incubated for 24 hours in an incubator (5% CO2, 20% O2 and 37 °C). After 24 hour incubation, cells were trypsinized and then re-suspended in 1 ml DMEM buffer. To the cell suspension was added 100 μl of MTT (5 mg ml−1) in a 15 ml Falcon tube. After incubation at 37 °C for 30 minutes, the cell suspension was centrifuged at 4255 rcf for 3 min. The supernatant was discarded, and the cell pellet was dissolved in 0.5 ml DMSO, followed by incubation at 37 °C for 10 minutes. Aliquots of 100 μl of the solution were transferred to a 96-well microtiter plate and the absorbance was recorded at 540 nm by a plate reader (Bio-Tek PowerWave Microplate Spectrophotometer).
MTT assay for control to investigate the effect of microwave treatment on cells in the absence of doxorubicin
Same MTT assay protocol as described above was followed, except that instead of doxorubicin, 10 μl of filter-sterilized DMSO was used.Pitstop2 and Dynasore assay
To a 6 ml cell suspension of MCF-7 cells at a density of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml was added 20 μl of Pitstop2 (5 mg ml−1 in DMSO) and 12 μl of Dynasore hydrate (5 mg ml−1 in DMSO). As a negative control, a 3 ml cell suspension at a cell density of 500
000 cells per ml was added 20 μl of Pitstop2 (5 mg ml−1 in DMSO) and 12 μl of Dynasore hydrate (5 mg ml−1 in DMSO). As a negative control, a 3 ml cell suspension at a cell density of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml was used to which was added 16 μl of filter-sterilized DMSO. The cell suspensions were transferred to a water bath on hotplate and were treated for 30 minutes at 37 °C and 540 rpm. To the 6 ml cell suspension was then added 10 μl of doxorubicin (1 mg ml−1). Two aliquots of 3 ml cell suspension were transferred to a reaction vessel in CEM (MW) and a reaction vessel on hotplate (control). CEM microwave reaction vessel parameters were adjusted to 37 °C, maximum power 10 W, and slow stirring (540 rpm). For control, the temperature of the water bath was pre-set to 37 °C and the stirring speed of hotplate was set to 540 rpm. Samples were treated for 20 minutes under each condition. At the end of each treatment, two samples of 1 ml cell suspension were taken from each reaction vessel (MW and control) and were transferred to microcentrifuge tubes and spun at 2300 rcf for 3 min to obtain cell pellets. After the supernatant was removed by aspiration, the cell pellets were re-suspended in 1× PBS (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 1 ml 1× PBS. The samples were analyzed by a BD Accuri C6 flow cytometer and the median fluorescence of each sample at FL-3 channel was recorded. Fluorescent images were obtained by a Carl Zeiss Axio Observer microscope.
000 cells per ml was used to which was added 16 μl of filter-sterilized DMSO. The cell suspensions were transferred to a water bath on hotplate and were treated for 30 minutes at 37 °C and 540 rpm. To the 6 ml cell suspension was then added 10 μl of doxorubicin (1 mg ml−1). Two aliquots of 3 ml cell suspension were transferred to a reaction vessel in CEM (MW) and a reaction vessel on hotplate (control). CEM microwave reaction vessel parameters were adjusted to 37 °C, maximum power 10 W, and slow stirring (540 rpm). For control, the temperature of the water bath was pre-set to 37 °C and the stirring speed of hotplate was set to 540 rpm. Samples were treated for 20 minutes under each condition. At the end of each treatment, two samples of 1 ml cell suspension were taken from each reaction vessel (MW and control) and were transferred to microcentrifuge tubes and spun at 2300 rcf for 3 min to obtain cell pellets. After the supernatant was removed by aspiration, the cell pellets were re-suspended in 1× PBS (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 1 ml 1× PBS. The samples were analyzed by a BD Accuri C6 flow cytometer and the median fluorescence of each sample at FL-3 channel was recorded. Fluorescent images were obtained by a Carl Zeiss Axio Observer microscope.
Human transferrin, Pitstop2 and Dynasore hydrate assay
To a 6 ml cell suspension of MCF-7 cells at a density of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml was added 20 μl of Pitstop2 (5 mg ml−1 in DMSO) and 12 μl of Dynasore hydrate (5 mg ml−1 in DMSO). As a negative control, a 6 ml cell suspension at a cell density of 500
000 cells per ml was added 20 μl of Pitstop2 (5 mg ml−1 in DMSO) and 12 μl of Dynasore hydrate (5 mg ml−1 in DMSO). As a negative control, a 6 ml cell suspension at a cell density of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml was used to which was added 32 μl of filter-sterilized DMSO. Each 6 ml cell suspension was divided to three aliquots of 2 ml in three reaction vessels. The 2 ml cell suspensions were transferred to a water bath on hotplate and were treated for 30 minutes at 37 °C and 540 rpm. To each 2 ml cell suspension was then added 25 μl of Transferrin (human) CF® Dye Conjugates (1 mg ml−1, Biotium, cat. #00081). The reaction vessels were incubated in the water bath for 20 minutes, the temperature of the water bath was kept at 37 °C and the stirring speed of hotplate was kept at 540 rpm. At the end of the treatment, one sample of 1 ml cell suspension was taken from each reaction vessel and was transferred to microcentrifuge tubes and spun at 2300 rcf for 3 min to obtain cell pellets. After the supernatant was removed by aspiration, the cell pellets were re-suspended in 1× PBS (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 1 ml 1× PBS. The samples were analyzed by a BD Accuri C6 flow cytometer and the median fluorescence of each sample at FL-1 channel was recorded. Fluorescent images were obtained by a Carl Zeiss Axio Observer microscope.
000 cells per ml was used to which was added 32 μl of filter-sterilized DMSO. Each 6 ml cell suspension was divided to three aliquots of 2 ml in three reaction vessels. The 2 ml cell suspensions were transferred to a water bath on hotplate and were treated for 30 minutes at 37 °C and 540 rpm. To each 2 ml cell suspension was then added 25 μl of Transferrin (human) CF® Dye Conjugates (1 mg ml−1, Biotium, cat. #00081). The reaction vessels were incubated in the water bath for 20 minutes, the temperature of the water bath was kept at 37 °C and the stirring speed of hotplate was kept at 540 rpm. At the end of the treatment, one sample of 1 ml cell suspension was taken from each reaction vessel and was transferred to microcentrifuge tubes and spun at 2300 rcf for 3 min to obtain cell pellets. After the supernatant was removed by aspiration, the cell pellets were re-suspended in 1× PBS (1 ml) and were centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 1 ml 1× PBS. The samples were analyzed by a BD Accuri C6 flow cytometer and the median fluorescence of each sample at FL-1 channel was recorded. Fluorescent images were obtained by a Carl Zeiss Axio Observer microscope.
Oligonucleotide uptake assay
To a 6 ml cell suspension at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml in modified DMEM was added 15 μl of FAM-labelled 18 nt oligodeoxynucleotide (FAM-5′-TTG GTG GAT AGT TAT TAG-3′, FAM: fluorescein. IDT) at 1 mg ml−1. Two aliquots of 3 ml cell suspension were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment). As a negative control, a 3 ml cell suspension at a cell density of 500
000 cells per ml in modified DMEM was added 15 μl of FAM-labelled 18 nt oligodeoxynucleotide (FAM-5′-TTG GTG GAT AGT TAT TAG-3′, FAM: fluorescein. IDT) at 1 mg ml−1. Two aliquots of 3 ml cell suspension were transferred to the reaction vessel in CEM (for microwave treatment) and a reaction vessel in a water bath on hotplate (control treatment). As a negative control, a 3 ml cell suspension at a cell density of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per ml was used to which was added 7.5 μl of nuclease-free dH2O.
000 cells per ml was used to which was added 7.5 μl of nuclease-free dH2O.
CEM reaction vessel parameters were adjusted to 37 °C, maximum power 10 W, and slow stirring (540 rpm). For control, the temperature of the water bath was pre-set to 37 °C and the stirring speed of hotplate was set to 540 rpm. Samples were treated for 20 minutes under each condition. At the end of each treatment, two samples of 1 ml cell suspension were taken from each reaction vessel (MW and control) and were transferred to microcentrifuge tubes and spun at 2300 rcf for 3 min to obtain cell pellets. After the supernatant was removed by aspiration, the cell pellets were re-suspended in 1× PBS (1 ml) and centrifuged for 3 min at 2300 rcf. The supernatant was discarded, and the cell pellet was re-suspended in 1 ml 1× PBS. The samples were analyzed by a BD Accuri C6 flow cytometer and the median fluorescence of each sample in FL-1 channel (Em 533 ± 15 nm) was recorded. Fluorescent images were obtained by a Carl Zeiss Axio Observer microscope at EGFP channel.
Statistical analysis
Non-paired two-tailed t-tests were carried out in Excel. Experiments with P values not greater than 0.05 are considered as statistically significant.Conclusions
Taken together, results from both MCF-7 and PC-3 cancer cell culture experiments support the notion that uptake of anticancer drugs such as doxorubicin by cells is promoted by exposure to non-lethal microwave. It is of interest to note that the promotion of doxorubicin internalization by microwave is dependent on the duration of exposure. Also, exposure to microwave alone does not lead to changes in cell viability or growth as evidenced by the MTT assay results. These observations are in agreement with our previous findings5 that suggested disturbance of cell membranes by non-lethal microwave. Thus, it is likely that exposure of cells to non-lethal microwave irradiation perturbs cell membranes, allowing for more efficient internalization of doxorubicin, perhaps in the same manner as electroporation. This work strongly supports a novel microwave (radiofrequency)-assisted drug delivery platform to improve the efficacy of chemotherapy, with potential to reduce side effects. As side effects and efficacy are the major challenges in cancer chemotherapy, strategies that can address both are valuable. By targeting tumors directly with microwave radiation to stimulate drug uptake it may be possible to lower the systemic drug dose, thus limiting side effects. No adverse effects associated with low power microwave have ever been identified, and higher power microwave is already used clinically for tissue ablation.18 Therefore, there seems to be limited risk associated with microwave and thus an opportunity for rapid clinical uptake of this approach. Future work will examine molecular mechanisms of the microwave-promoted uptake. In addition, optimal frequency, power, and duration of microwave will also be profiled.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada.Notes and references
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- G. B. Dudley, A. E. Stiegman and M. R. Rosana, Angew. Chem., Int. Ed., 2013, 52, 7918 CrossRef PubMed.
- S. A. Mazinani, B. DeLong and H. Yan, Tetrahedron Lett., 2015, 56, 5804 CrossRef.
- S. A. Mazinani and H. Yan, Tetrahedron Lett., 2016, 57, 1589 CrossRef.
- S. A. Mazinani, F. Moradi, J. A. Stuart and H. Yan, ChemistrySelect, 2017, 2, 7983 CrossRef.
- C. L. Brace, Critical Reviews in Biomedical Engineering, 2010, 38, 65 CrossRef PubMed.
- Z. Farhane, F. Bonnier and H. J. Byrne, Anal. Bioanal. Chem., 2017, 409, 1333 CrossRef PubMed.
- T. F. Slater, B. Sawyer and U. Strauli, Biochim. Biophys. Acta, 1963, 77, 383 CrossRef.
- M. Dalmark and H. H. Storm, J. Gen. Physiol., 1981, 78, 349 CrossRef PubMed.
- O. Tacar, P. Sriamornsak and C. R. Dass, J. Pharm. Pharmacol., 2013, 65, 157 CrossRef PubMed.
- S. Cai, A. A. Alhowyan, Q. Yang, W. C. Forrest, Y. Shnayder and M. L. Forrest, J. Drug Targeting, 2014, 22, 648 CrossRef PubMed.
- S. Majumdar, B. A. Tejo, A. H. Badawi, D. Moore, J. P. Krise and T. J. Siahaan, Mol. Pharm., 2009, 6, 396 CrossRef PubMed.
- S. K. Saha, Y. Yin, K. Kim, G. M. Yang, A. A. Dayem, H. Y. Choi and S. G. Cho, Int. J. Mol. Sci., 2017, 18, E1048 CrossRef PubMed.
- D. Dutta, C. D. Williamson, N. B. Cole and J. G. Donaldson, PLoS One, 2012, 7, e45799 CrossRef PubMed.
- E. Macia, M. Ehrlich, R. Massol, E. Boucrot, C. Brunner and T. Kirchhausen, Dev. Cell, 2006, 10, 839 CrossRef PubMed.
- R. L. Juliano and K. Carver, Adv. Drug Delivery Rev., 2015, 87, 35 CrossRef PubMed.
- T. J. Doran, P. J. Lu, G. S. Vanier, M. J. Collins, B. Wu and Q. L. Lu, Gene Ther., 2009, 16, 119 CrossRef PubMed.
- M. G. Lubner, C. L. Brace, J. L. Hinshaw and F. T. Lee, J. Vasc. Interv. Radiol., 2010, 21, S192–S203 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05605f |
| This journal is © The Royal Society of Chemistry 2018 |