Open Access Article
Open Access ArticleOne-pot synthesis of end-functionalised soluble star-shaped polymers by living ring-opening metathesis polymerisation using a molybdenum-alkylidene catalyst†
Zelin Sun and
Kotohiro Nomura *
*
Department of Chemistry, Faculty of Science, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo 192-0397, Japan
First published on 3rd August 2018
Abstract
Precise synthesis of soluble star-shaped polymers has been achieved by adopting living ring-opening metathesis polymerisation (ROMP) using a molybdenum-alkylidene catalyst with sequential addition of norbornene and cross-linking agent; the method provides efficient one-pot synthesis of high molecular weight end-functionalised star-shaped polymers (Mn = >1.37 × 105) with more arms (branching) with rather low PDI values (Mw/Mn = 1.17–1.37) under the optimised conditions.
Introduction
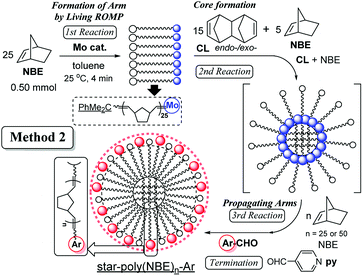
Star polymers containing multiple linear arms connected at a central branched core are one of the simplest nonlinear polymers,1–10 and development of precise synthesis methods by living polymerisation techniques attracts considerable attention.1–10 Reported examples for the synthesis by adopting living ring-opening metathesis polymerisation (ROMP)11–23 via arm (brush) first24–30 and core first (in–out)31–35 approaches by sequential addition of monomers/cross linkers (CLs) are known. Moreover, related examples for synthesis of cross-linked polymers (which are insoluble in common organic solvents) by ROMP are also known,36–39 especially in terms of application as monolith materials for separation. In particular, the approach by the sequential living ROMP of norbornene (NBE) and cross-linking reagent (1,4,4a,5,8,8a-hexahydro-1,4,5,8-exo-endo-dimethano-naphthalene, CL) using a Mo-alkylidene catalyst enables us to introduce functionality to the polymer chain ends (star surface) through cleavage of the polymer-metal double bonds with aldehyde via Wittig-type reaction.15,23,31–34,40–42 However, the previous reports31–34 adopting the so called Method 1 (shown in Scheme 1) only demonstrated synthesis of soluble polymers with a small number of arms under limited conditions (5 or 10 equiv. of CL,31–34 shown below in Table 1) due to the difficulty of molecular weight control, as also described below.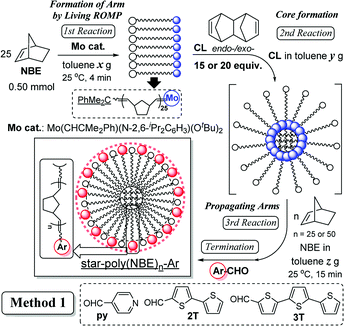 | ||
| Scheme 1 Synthesis of star-shaped ROMP polymers by sequential addition of norbornene (NBE) and cross-linker (CL) in the presence of molybdenum-alkylidene catalyst (Mo cat.). | ||
| Run | Tolueneb/g | 2nd | 3rd | Are | Mnf × 10−4 | Mw/Mnf | Yieldg/% | ||
|---|---|---|---|---|---|---|---|---|---|
| x/y/z | CLc/equiv. | Conc.d/×10−2 M | Time/min | NBEc/equiv. | |||||
| a Conditions: toluene at 25 °C (detailed procedure, see Scheme 1).b Amount of toluene (in gram) in each step (shown in Scheme 1).c Equiv. to Mo.d Calculated concentration of NBE + CL charged (mmol L−1) at the second stage (core formation).e ArCHO employed for the termination (py = 4-pyridine carboxaldehyde; 2T = 2,2′-bithiophene-5-carboxaldehyde; 3T = 2,2′:5′,2′′-terthiophene-5-carboxaldehyde).f GPC data in THF vs. polystyrene standards (g mol−1).g Isolated yield (%) as MeOH insoluble fraction.h Bimodal (or multi-modal) molecular weight distributions observed on GPC trace. | |||||||||
| 1 | 3.0/4.0/4.0 | 10 | 6.36 | 50 | 25 | py | 8.9 | 1.18 | 95 |
| 2 | 3.0/4.0/4.0 | 10 | 6.36 | 50 | 25 | py | 8.8 | 1.19 | 96 |
| 3 | 3.0/4.0/4.0 | 10 | 6.36 | 50 | 25 | py | 8.8 | 1.19 | 96 |
| 4 | 3.0/4.0/4.0 | 15 | 7.27 | 50 | 25 | py | 13.4 | 1.30 | 96 |
| 5 | 3.0/4.0/4.0 | 15 | 7.27 | 70 | 25 | py | 20.8 | 2.88h | 94 |
| 6 | 3.0/4.0/4.0 | 15 | 7.27 | 90 | 25 | py | 21.1 | 1.98h | 92 |
| 7 | 3.0/4.0/4.0 | 15 | 7.27 | 120 | 25 | py | 23.5 | 2.09h | 94 |
| 8 | 3.0/4.0/4.0 | 15 | 7.27 | 50 | 50 | py | 39.4 | 3.09h | 93 |
| 9 | 3.0/4.0/4.0 | 15 | 7.27 | 50 | 50 | py | 39.1 | 3.37h | 90 |
| 10 | 3.0/4.0/4.0 | 15 | 7.27 | 70 | 50 | py | 42.3 | 3.13h | 91 |
| 11 | 3.0/4.0/4.0 | 15 | 7.27 | 70 | 50 | py | 41.4 | 3.42h | 92 |
| 12 | 5.0/4.0/6.0 | 15 | 5.33 | 50 | 25 | py | 13.7 | 1.44 | 97 |
| 13 | 5.0/4.0/6.0 | 15 | 5.33 | 70 | 25 | py | 14.4 | 1.46 | 99 |
| 14 | 5.0/4.0/6.0 | 15 | 5.33 | 90 | 25 | py | 15.7 | 1.47h | 98 |
| 15 | 5.0/4.0/6.0 | 15 | 5.33 | 120 | 25 | py | 16.9 | 1.38h | 98 |
| 16 | 5.0/4.0/6.0 | 15 | 5.33 | 50 | 50 | py | 15.5 | 1.50h | 93 |
| 17 | 5.0/4.0/6.0 | 15 | 5.33 | 70 | 50 | py | 16.4 | 1.60h | 95 |
| 18 | 5.0/4.0/6.0 | 15 | 5.33 | 90 | 50 | py | 17.8 | 1.92h | 95 |
| 19 | 5.0/4.0/6.0 | 15 | 5.33 | 120 | 50 | py | 19.0 | 1.86h | 94 |
| 20 | 11.0/4.0/5.0 | 15 | 4.00 | 50 | 25 | py | 13.7 | 1.22 | 90 |
| 21 | 11.0/4.0/5.0 | 15 | 4.00 | 70 | 25 | py | 14.9 | 1.37 | 94 |
| 22 | 11.0/4.0/5.0 | 15 | 4.00 | 50 | 25 | 2T | 15.5 | 1.33 | 88 |
| 23 | 11.0/4.0/5.0 | 15 | 4.00 | 50 | 25 | 3T | 15.2 | 1.29 | 81 |
| 24 | 11.0/4.0/5.0 | 15 | 4.00 | 50 | 50 | py | 15.6 | 1.17 | 96 |
| 25 | 11.0/4.0/5.0 | 15 | 4.00 | 70 | 50 | py | 16.4 | 1.28 | 94 |
| 26 | 3.0/4.0/4.0 | 20 | 8.18 | 50 | 25 | py | 28.6 | 2.51h | 95 |
| 27 | 3.0/4.0/4.0 | 20 | 8.18 | 50 | 25 | py | 30.7 | 2.37h | 95 |
| 28 | 3.0/4.0/4.0 | 20 | 8.18 | 70 | 25 | py | 34.9 | 4.31h | 98 |
| 29 | 3.0/4.0/4.0 | 20 | 8.18 | 90 | 25 | py | 28.6 | 2.19h | 90 |
| 30 | 3.0/4.0/4.0 | 20 | 8.18 | 120 | 25 | py | 32.7 | 2.12h | 95 |
| 31 | 5.0/4.0/6.0 | 20 | 6.00 | 50 | 25 | py | 19.4 | 1.99h | 92 |
| 32 | 5.0/4.0/6.0 | 20 | 6.00 | 70 | 25 | py | 21.1 | 2.65h | 94 |
| 33 | 5.0/4.0/6.0 | 20 | 6.0 | 90 | 25 | py | 24.1 | 2.05h | 98 |
| 34 | 5.0/4.0/6.0 | 20 | 6.0 | 120 | 25 | py | 23.6 | 2.58h | 98 |
| 35 | 11.0/4.0/5.0 | 20 | 4.50 | 50 | 25 | py | 14.9 | 1.44h | 90 |
| 36 | 11.0/4.0/5.0 | 20 | 4.50 | 70 | 25 | py | 17.8 | 1.54h | 91 |
We thus herein demonstrate that the protocols for synthesis of soluble high molecular weight end-functionalised star-shaped polymers with more arms (branching) have been developed by using the living ROMP technique under the carefully optimised conditions.43 The synthetic protocols should contribute to providing new (or improved) properties by placement of more functionalities on the star surface.
Results and discussion, experimental
As reported previously,31–34 the method consists of 4 steps by sequential addition of NBE, CL, NBE, and the termination by addition of aldehyde (Scheme 1). In order to obtain the star-shaped polymers with more arms (branching), two approaches especially at the core formation step (2nd reaction in Scheme 1), such as (i) the approach by increasing the CL under optimised conditions (Method 1, Scheme 1), (ii) the approach by reacting with CL in the co-presence of norbornene (NBE) (formation of different core size, Method 2, Scheme 2 shown below), have thus been considered. Mo(CHCMe2Ph)(N-2,6-iPr2C6H3)(OtBu)2 (Mo cat.) has also been chosen as the initiator due to its ability to prepare the multi-block ring-opened copolymers in a precise manner with moderate propagation as well as with complete conversion of monomers.15,23,40–42 This is also due to that the initiator shows markedly higher reactivity toward strained cyclic olefins (norbornene derivatives) than the internal olefins (in the ring-opened polymers).15,23,40–42,44 1,4,4a,5,8,8a-hexahydro-1,4,5,8-exo-endo-dimethanonaphtalene (CL, exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo = 0.15
endo = 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00)45 has also been chosen as the cross-linker, and the polymerisation was terminated with 4-pyridine carboxaldehyde.31,34 The conditions for the first and the third ROMPs with NBE (4 and 15 minutes, respectively) were unchanged, because the NBE consumption should be completed based on our previous study.31 The results are summarised in Table 1.
1.00)45 has also been chosen as the cross-linker, and the polymerisation was terminated with 4-pyridine carboxaldehyde.31,34 The conditions for the first and the third ROMPs with NBE (4 and 15 minutes, respectively) were unchanged, because the NBE consumption should be completed based on our previous study.31 The results are summarised in Table 1.
It turned out that, as reported previously,31–34 the sequential ROMP with 10 equiv. of CL afforded ring-opened star-shaped polymers with rather low PDI values (Mw/Mn = 1.18, 1.19, runs 1–3) and the results are thus reproducible.46 However, the PDI values in the resultant polymers eventually became broad if these polymerisations were conducted in the presence of 15 equiv. of CL under the same conditions (Mw/Mn = 1.30–2.88, runs 4–6); prolong the reaction time at the second (core formation) step led to increase in the Mn values with broad molecular weight distributions. Similarly, the PDI values in the resultant polymers became broad if the polymerisations were conducted in the presence of 20 equiv. of CL under the same conditions (Mw/Mn = 2.51, 2.37, runs 26,27); it becomes more difficult to control the molecular weights (as well as to obtain the reproducibility).
In contrast, importantly, it turned out that the molecular weight distributions in the resultant polymers became unimodal when the polymerisation runs were conducted under diluted conditions (Mw/Mn = 1.22–1.46, runs 16–19, 20–23, Fig. 1). It should be noted that the polymerisation under high dilution (at the second step) afforded the high molecular weight star-shaped ROMP polymers with rather low PDI values [Mn = 1.37 × 105, Mw/Mn = 1.22 (run 20); Mn = 1.49 × 105, Mw/Mn = 1.37 (run 21), shown in Fig. 1]. Moreover, further increase of NBE (in the third step) also afforded the high molecular weight ROMP polymers with rather low PDI values [Mn = 1.56 × 105, Mw/Mn = 1.17 (run 24); Mn = 1.64 × 105, Mw/Mn = 1.28 (run 25)]. It seems that rather low PDI values could be maintained under high dilution conditions (runs 20,21,24,25), whereas the PDI values became rather large when the ROMPs were conducted with 50 equiv. of NBE in the third step under conditions conducted in runs 12 and 13 (Mw/Mn = 1.50–1.92, Table 1, runs 16). The results could thus probably suggest that further cross-linking (like star–star coupling upon increasing the reaction time at the second step) would be controlled under these diluted conditions (in the presence of 15 equiv. of CL), which means that the monomer concentration at the core formation step seems critical for obtainment of star-shaped ROMP polymers with low PDI values. The resultant polymers are soluble in toluene, tetrahydrofuran (THF), chloroform, dichloromethane (for measurement of NMR spectra and/or ordinary GPC analysis), but insoluble in methanol, ethanol, water, n-hexane etc. at room temperature.
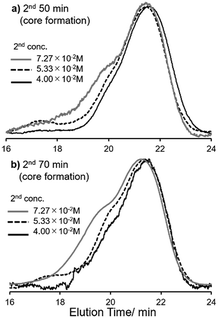 | ||
| Fig. 1 Selected GPC traces of star-shaped ROMP polymers conducted under different concentration conditions, different time in the 2nd reaction (core formation, Scheme 1). The reaction time (2nd step): (a) 50 min (runs 4, 12, 20, Table 1); (b) 70 min (runs 5, 13, 21, Table 1). The hights in the GPC traces were normalized. | ||
Upon presence of 20 equiv. of CL, the ROMPs under high dilution conditions only afforded the star-shaped polymers with rather low PDI values, and the Mn value increased upon increasing the reaction time at the second step with broadening the distribution [Mn = 1.49 × 105, Mw/Mn = 1.44 (run 35), Mn = 1.78 × 105, Mw/Mn = 1.54 (run 36)]. These results also suggest that certain optimisation (especially, concentration and the reaction time at the second step) should be necessary.
As shown in Table 1, the Mn values in the polymers (prepared under the optimised conditions) increased upon increasing the CL with rather low PDI values [Mn = 8.9 × 104 (run 1, CL = 10), 1.37 × 105 (run 20, CL = 15) or 1.49 × 105 (run 21, CL = 15), 1.78 × 105 (run 36, CL = 20)], clearly suggesting that number of arms (branching) should be increasing upon increasing the amount of CL. Moreover, the Mn values also increased upon increasing amount of NBE in the third step (after formation of core), and the values are apparently higher than those in the linear ROMP polymers. The results clearly suggest that the resultant polymers are star-shaped polymers consisting of the core and arms. It also turned out that the similar polymerisations terminated with 2,2′:5′,2′′-terthiophene-5-carboxaldehyde (3T-CHO), 2,2′-bithiophene-5-carboxaldehyde (2T-CHO) in place of 4-pyridine carboxaldehyde afforded the high molecular weight polymers with relatively low PDI values [Mn = 1.55 × 105, Mw/Mn = 1.33 (run 22, terminated with 2T-CHO); Mn = 1.52 × 105, Mw/Mn = 1.29 (run 23, terminated with 3T-CHO)]. The results also suggest that the method can be applied to synthesis of star-shaped ROMP polymers with different end-groups.
In order to change the core size for obtainment of the star-shaped ROMP polymers with more arms (branching), the reaction with CL was conducted in the co-presence of NBE (5.0 equiv.) under the optimised conditions (conducted in Table 1 under high dilution). The results (by Method 2, Scheme 2) are summarised in Table 2.
| Run | 2nd | 3rd | Mnc × 10−4 | Mw/Mnc | Yieldd/% | ||
|---|---|---|---|---|---|---|---|
| CLb/equiv. | NBEb/equiv. | Time/min | NBEb equiv. | ||||
| a Conditions: toluene (total 20 g, shown in runs 20–25) at 25 °C, 4-pyridine carboxaldehyde was used for the termination (detailed procedure, see Scheme 2).b Equiv. to Mo.c GPC data in THF vs. polystyrene standards (g mol−1).d Isolated yield (%) as MeOH insoluble fraction. | |||||||
| 20 | 15 | — | 50 | 25 | 13.7 | 1.22 | 90 |
| 21 | 15 | — | 70 | 25 | 14.9 | 1.37 | 94 |
| 37 | 15 | 5.0 | 50 | 25 | 14.5 | 1.28 | 91 |
| 38 | 15 | 5.0 | 70 | 25 | 15.3 | 1.39 | 96 |
| 24 | 15 | — | 50 | 50 | 15.6 | 1.17 | 96 |
| 25 | 15 | — | 70 | 50 | 16.4 | 1.28 | 94 |
| 39 | 15 | 5.0 | 50 | 50 | 19.1 | 1.36 | 98 |
| 40 | 15 | 5.0 | 70 | 50 | 20.2 | 1.45 | 99 |
It seems that the Mn values increased upon co-presence of NBE [ex. 1.49 × 105 (run 21) vs. 1.53 × 105 (run 38)], and the difference became more significant upon addition of 50 equiv. of NBE after the core formation [third step, 1.64 × 105 (run 25) vs. 2.02 × 105 (run 40)], suggesting that the resultant polymers prepared in Method 2 possess more arm numbers compared to those in Method 1. The resultant polymers possessed relatively low PDI values (Mw/Mn = 1.28–1.45), therefore, the method can also be applied to controlled synthesis of star-shaped ROMP polymers with more branching.47
We have shown that protocols for facile and efficient synthesis of high molecular weight “soluble” star-shaped polymers with more branching (arms) have been developed by adopting the living ROMP with sequential addition of NBE and cross-linker using the molybdenum-alkylidene initiator under the optimised conditions (high dilution). The methods should provide a controlled synthesis of highly branched star-shaped ROMP polymers with different end groups (modification of the star surface), and interesting properties such as unique emission by interaction of the end group with the initiating fragment,33 supported concerted catalysts34 (by placement of different end groups/ligand or catalyst precursor) etc. can be thus highly expected. We are now exploring the possibility by introduction of various end groups into the star shaped ROMP polymers by adopting the present methods.
Experimental
1. General procedure
All experiments were carried out under a nitrogen atmosphere in a Vacuum Atmospheres dry-box or using standard Schlenk techniques. All chemicals used were of reagent grades and were purified by the standard purification procedures. Anhydrous grade toluene (Kanto Chemical Co., Inc.) was transferred into a bottle containing molecular sieves (mixture of 3A 1/16, 4A 1/8 and 13X 1/16) in the drybox, and was stored over sodium/potassium alloy, and was used after passing through an alumina short column under nitrogen flow prior to use. Mo(CHCMe2Ph)(N-2,6-iPr2C6H3)(OtBu)2 (ref. 48) and 1,4,4a,5,8,8a-hexahydro-1,4,5,8-exo-endo-dimethanonaphtalene (CL, exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo = 0.15
endo = 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00)45 were prepared according to the literature. 4-Pyridinecarboxaldehyde and 2,2′:5′,2′′-terthiophene-5-carboxaldehyde (3T-CHO) and 2,2′-bithiophene-5-carboxaldehyde (2T-CHO) were used in the dry-box as received (Aldrich Chemical Co.) without further purification.
1.00)45 were prepared according to the literature. 4-Pyridinecarboxaldehyde and 2,2′:5′,2′′-terthiophene-5-carboxaldehyde (3T-CHO) and 2,2′-bithiophene-5-carboxaldehyde (2T-CHO) were used in the dry-box as received (Aldrich Chemical Co.) without further purification.
Molecular weights and the molecular weight distributions of the resultant polymers were measured by gel-permeation chromatography (GPC). HPLC grade THF (Wako Pure Chemical Ind., Inc.) was used for GPC and was degassed prior to use. GPC were performed at 40 °C on a Shimadzu SCL-10A using a RID-10A detector (Shimadzu Co. Ltd.) in THF (containing 0.03 wt% 2,6-di-tert-butyl-p-cresol, flow rate 1.0 mL min−1). GPC columns (ShimPAC GPC-806, 804 and 802, 30 cm × 8.0 mm ϕ) were calibrated versus polystyrene standard samples. All 1H and 13C NMR spectra were recorded on a Bruker AV500 spectrometer (1H, 500.13 MHz; 13C, 125.77 MHz), and all chemical shifts are quoted in ppm and are referenced to SiMe4. Obvious multiplicities and routine coupling constants are usually not listed, and all spectra were obtained in the solvent indicated at 25 °C.
2. General polymerisation procedure
Typical polymerisation procedure (run 20, Method 1, Table 1) is as follows. A toluene solution (1.0 g) containing Mo(CHCMe2Ph)(N-2,6-iPr2C6H3)(OtBu)2 (2.00 × 10−5 mol) was added in one portion to a rapidly stirred toluene solution (10.0 g) containing the norbornene (25 equiv. to Mo) at room temperature (25 °C), and the solution was stirred for 4 min. A toluene solution (4.0 g) containing 1,4,4a,5,8,8a-hexahydro-1,4,5,8-exo-endo-dimethanonaphtalene (CL, 15 equiv. to Mo) was then added into the solution, and the mixture was stirred for prescribed time (50 min). Then a toluene solution (5.0 g) containing norbornene (25 equiv. to Mo) was then added in one portion to the mixture, and the reaction solution was further stirred for 15 min. The polymerisation was quenched by adding 4-pyridine carboxaldehyde (ca. >10 mg, excess), and the solution was stirred for 1 h for completion. The mixture was then removed in vacuo until it was dissolved in the minimum amount of toluene. The solution was poured dropwise into methanol to afford pale white precipitates. The polymer was then collected by filtration and dried in vacuo. In Method 2, the basic procedure was the same except that a toluene solution containing CL and norbornene (5.0 equiv. to Mo) was added in the second step.1H NMR (in CDCl3 at 25 °C): δ 5.35 and 5.21 (br.s, 2H olefinic), 2.79 and 2.43 (br.s, 2H), 1.85 and 1.03 (m, 2H), 1.78 and 1.36 (m, 4H) ppm. Moreover, resonances ascribed to pyridine end group [8.54 and 8.50 (d) ppm] or oligo(thiophene) (2T, 3T, 7.00–7.40) were also observed. 13C NMR (in CDCl3 at 25 °C): δ 133.9, 133.0, 128.2, 43.1, 38.6, 33.2, 32.2 ppm. Selected NMR spectra are shown in the ESI.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is supported by Grant-in-Aid for Challenging Exploratory Research (24656491, 15K14225), Japan Society for the Promotion of Science (JSPS). S. Z. acknowledges the Tokyo Metropolitan government (Tokyo Human Resources Fund for City Diplomacy) for pre-doctoral fellowship. The authors express their thanks to Profs. S. Komiya, K. Tsutsumi, and A. Inagaki (Tokyo Metropolitan Univ.) for helpful discussions.References
- For example, Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications, ed. K. Matyjaszewski, Y. Gnanou and L. Leibler, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed.
- For example (ref. 2–10), N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747–3792 CrossRef PubMed.
- A. Hirao, M. Hayashi, S. Loykulnant, K. Sugiyama, S. W. Ryu, N. Haraguchi, A. Matsuo and T. Higashihara, Prog. Polym. Sci., 2005, 30, 111–182 CrossRef.
- N. Hadjichristidis, H. Iatrou, M. Pitsikalis and J. Mays, Prog. Polym. Sci., 2006, 31, 1068–1132 CrossRef.
- M. Ouchi, T. Terashima and M. Sawamoto, Acc. Chem. Res., 2008, 41, 1120–1132 CrossRef PubMed.
- H. Gao and K. S. Matyjaszewski, Prog. Polym. Sci., 2009, 34, 317–350 CrossRef.
- K. Matyjaszewski and N. V. Tsarevsky, Nat. Chem., 2009, 1, 276–288 CrossRef PubMed.
- K. Khana, S. Varshney and A. Kakkar, Polym. Chem., 2010, 1, 1171–1185 RSC.
- K. Matyjaszewski and N. V. Tsarevsky, J. Am. Chem. Soc., 2014, 136, 6513–6533 CrossRef PubMed.
- J. M. Ren, T. G. McKenzie, Q. Fu, E. H. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer and G. G. Qiao, Chem. Rev., 2016, 116, 6743–6836 CrossRef PubMed.
- Examples for metathesis polymerisation (ref. 11–23), M. R. Buchmeiser, Chem. Rev., 2000, 100, 1565–1604 CrossRef PubMed.
- Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, Germany, 2003, vol. 3 Search PubMed.
- Metathesis Polymerisation, ed. M. Buchmeiser, Springer-Verlag, Berlin Heidelberg, Germany, 2005 Search PubMed.
- S. Hilf and A. F. M. Kilbinger, Nat. Chem., 2009, 1, 537–546 CrossRef PubMed.
- K. Nomura and M. M. Abdellatif, Polymer, 2010, 51, 1861–1881 CrossRef.
- A. Leitgeb, J. Wappel and C. Slugovc, Polymer, 2010, 51, 2927–2946 CrossRef.
- M. R. Buchmeiser, Macromol. Symp., 2010, 298, 17–24 CrossRef.
- H. Mutlu, L. M. de Espinosa and M. A. R. Meier, Chem. Soc. Rev., 2011, 40, 1404–1445 RSC.
- P. Atallah, K. B. Wagener and M. D. Schulz, Macromolecules, 2013, 46, 4735–4741 CrossRef.
- M. D. Schulz and K. B. Wagener, Macromol. Chem. Phys., 2014, 215, 1936–1945 CrossRef.
- Handbook of Metathesis, ed. R. H. Grubbs and E. Khosravi, Wiley-VCH, Weinheim, Germany, 2nd edn, 2015, vol. 3 Search PubMed.
- D. J. Lunn, E. H. Discekici, J. R. de Alaniz, W. R. Gutekunst and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2903–2914 CrossRef.
- Y. Chen, M. M. Abdellatif and K. Nomura, Tetrahedron, 2018, 74, 619–643 CrossRef.
- R. S. Saunders, R. E. Cohen, S. J. Wong and R. R. Schrock, Arm (brush) first approach by addition of cross-linked reagent(s) at the final stage, Macromolecules, 1992, 25, 2055–2057 CrossRef , ref. 24–30.
- J. Liu, A. O. Burts, Y. Li, A. V. Zhukhovitskiy, M. F. Ottaviani, N. J. Turro and J. A. Johnson, J. Am. Chem. Soc., 2012, 134, 16337–16344 CrossRef PubMed.
- L. Liao, J. Liu, E. C. Dreaden, S. W. Morton, K. E. Shopsowitz, P. T. Hammond and J. A. Johnson, J. Am. Chem. Soc., 2014, 136, 5896–5899 CrossRef PubMed.
- A. O. Burts, A. X. Gao and J. A. Johnson, Macromol. Rapid Commun., 2014, 35, 168–173 CrossRef PubMed.
- J. C. Barnes, P. M. Bruno, H. V.-T. Nguyen, L. Liao, J. Liu, M. T. Hemann and J. A. Johnson, J. Am. Chem. Soc., 2016, 138, 12494–12501 CrossRef PubMed.
- H. V.-T. Nguyen, Q. Chen, J. T. Paletta, P. Harvey, Y. Jiang, H. Zhang, M. D. Boska, M. F. Ottaviani, A. Jasanoff, A. Rajca and J. A. Johnson, ACS Cent. Sci., 2017, 3, 800–811 CrossRef PubMed.
- Y. Shibuya, H. V.-T. Nguyen and J. A. Johnson, ACS Macro Lett., 2017, 6, 963–968 CrossRef.
- Core first (in and out) approach (ref. 31–34): K. Nomura, Y. Watanabe, S. Fujita, M. Fujiki and H. Otani, Macromolecules, 2009, 42, 899–901 CrossRef.
- H. Otani, S. Fujita, Y. Watanabe, M. Fujiki and K. Nomura, Macromol. Symp., 2010, 293, 53–57 CrossRef.
- K. Takamizu and K. Nomura, J. Am. Chem. Soc., 2012, 134, 7892–7895 CrossRef PubMed.
- K. Nomura, K. Tanaka and S. Fujita, Organometallics, 2012, 31, 5074–5080 CrossRef.
- J. A. Kalow and T. M. Swager, ACS Macro Lett., 2015, 4, 1229–1233 CrossRef PubMed.
- (reviews, ref. 36–39), S. Lubbad and M. R. Buchmeiser, Macromol. Rapid Commun., 2003, 24, 580–584 CrossRef.
- M. Mayr, D. Wang, R. Kröll, N. Schuler, S. Prühs, A. Fürstner and M. R. Buchmeiser, Adv. Synth. Catal., 2005, 347, 484–492 CrossRef.
- M. R. Buchmeiser, in Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, Germany, 2003, vol. 3, p. 226 Search PubMed.
- M. R. Buchmeiser, Chem. Rev., 2009, 109, 303–321 CrossRef PubMed.
- For example (ref. 40–42), R. R. Schrock, in Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, 2003, vol. 1, p. 8 Search PubMed.
- R. R. Schrock, in Metathesis Polymerisation of Olefins and Polymerisation of Alkynes, ed. Y. Imamoglu, NATO ASI Series, Kluwer Academic Publishers, 1998, pp. 1–27 and 357–380 Search PubMed.
- R. R. Schrock, Chem. Rev., 2009, 109, 3211–3226 CrossRef PubMed.
- The results were partly introduced in the Asian Polyolefin Workshop 2017 (APO2017), Tianjin, China, October, 2017 and the 8th International Conference on Polymer Chemistry (PC2018), Changchun, China, June, 2018.
- For example, J. J. Murphy, H. Furusho, R. M. Paton and K. Nomura, Chem.–Eur. J., 2007, 13, 8985–8997 CrossRef PubMed.
- J. K. Stille and D. A. Frey, J. Am. Chem. Soc., 1959, 81, 4273–4275 CrossRef.
- Selected NMR spectra in the resultant polymers are shown in the ESI.†.
- It should be noted that the polymerisation with 2 step additions [CL + NBE (15 + 5.0 equiv.) and CL (5.0 equiv.)] in the second step afforded the ROMP polymers with broad molecular weight distributions. Some of the resultant polymers became hardly soluble in toluene, tetrahydrofuran (probably due to highly cross-linking).
- R. R. Schrock, J. S. Murdzek, G. C. Bazan, J. Robbins, M. Dimare and M. B. O'Regan, J. Am. Chem. Soc., 1990, 112, 3875–3886 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05229h |
| This journal is © The Royal Society of Chemistry 2018 |