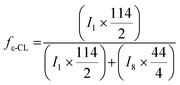Open Access Article
Open Access ArticleSynthesis and characterization of 4-arm star-shaped amphiphilic block copolymers consisting of poly(ethylene oxide) and poly(ε-caprolactone)
Adnan Murad Bhayo,
Rubina Abdul-Karim,
Syed Ghulam Musharraf and
Muhammad Imran Malik
and
Muhammad Imran Malik *
*
H. E. J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi 75270, Pakistan. E-mail: mimran.malik@iccs.edu
First published on 10th August 2018
Abstract
Star-shaped polymers exhibit lower hydrodynamic volume, glass transition temperature, critical micelles concentration (CMC), and higher viscosity and drug-loading capacity compared to their linear counterparts. In the present study, amphiphilic biodegradable 4-arm star-shaped block copolymers, based on poly(ethylene oxide) (PEO) as a hydrophilic part and poly(ε-caprolactone) (PCL) as a hydrophobic segment, are synthesized by ring-opening polymerization of ε-caprolactone employing pentaerythritol as an initiator and stannous octoate as a catalyst, followed by the coupling reaction with carboxyl-functionalized monomethoxy poly(ethylene oxide) (MeO-PEO-COOH). The structures of intermediates were deduced through 1H-NMR and FT-IR spectroscopy. Average chemical composition of the star block copolymer is determined by proton NMR. Information related to molar mass distribution of targeted products and their precursors is obtained by size exclusion chromatography (SEC). However, due to its inherent poor resolution SEC could not reveal whether all the parent homopolymers are coupled to each other or remained unattached to the other segment. In order to comprehensively characterize the synthesized star-block copolymers, liquid chromatography at critical conditions of both blocks is employed. The study allowed for separation of homopolymer precursors from targeted star-block copolymers. The study exposed heterogeneity of star block copolymers that was not possible by conventional techniques.
1. Introduction
Amphiphilic block copolymers have emerged as promising candidates for solubilization, stabilization, transportation and controlled release of hydrophobic drugs. Amphiphilic block copolymers when dropped in water form well-defined, nano-sized, core/shell type architectures called micelles. The hydrophobic part of the block copolymer forms the core whereas the hydrophilic segment self-assembles to make a protective shell for the non-polar block. The core of the micelles serves as a reservoir for hydrophobic drugs that are insoluble in water while the hydrophilic corona makes a protective shell around it.1,2 All the segments of the block copolymer have to be biodegradable/biocompatible for any potential application in the biomedical field. Block copolymers of poly(ε-caprolactone) and poly(ethylene oxide) can be classified under the heading of biodegradable polymers. Hence, these are being extensively employed in drug delivery applications due to their excellent properties of biodegradability, biocompatibility, non-immunogenicity and absence of systemic toxicity.3,4Poly(ε-caprolactone) (PCL) is a saturated aliphatic polyester composed of hexanoate repeating units. PCL exhibits several unique features including hydrophobicity, semi-crystallinity, biocompatibility, flexible mechanical properties, miscibility with other polymers and inherent biodegradability. Ester bond in poly(ε-caprolactone) is labile towards hydrolysis making it biodegradable.5 On the other hand, poly(ethylene oxide) (PEO) has been extensively utilized as hydrophilic segment in conjunction with hydrophobic block for biodegradable polymers. PEO is a non-ionic polymer exhibiting important properties such as biocompatibility, high chemical stability and flexibility, solubility in both aqueous and organic solvents, non-immunogenicity and toxicity, resistance to recognition by immune system and ease of excretion from body.6
In this context, minimum concentration of the polymer required for their self-assembly into micellar structures is called critical micelles concentration (CMC). Low CMC is one of very important prerequisite for drug delivery applications since effective concentration drops to large extent when micelles enter into blood stream. Therefore, the stability of micelles upon dilution is extremely important for successful delivery of drugs to the targeted site.7 It has been noticed that the self-assembly of block copolymer (micellization) is strongly influenced by architecture of the polymers such as linear, branched, cross-linked and so forth.8 Star-shaped block copolymers have low CMC and high drug-loading capacity compared to their linear counterparts.8,9
Star-shaped polymers are composed of many linear polymeric arms of comparable length that originates from a multifunctional central point. Star polymers have smaller hydrodynamics radius, lower melt viscosity, high density of end-functionality and improved physical processing compared to linear analogs of similar molar mass and composition. Enhanced encapsulation efficiency and low CMC of star block copolymers have made them a subject of immense interest in drug/gene delivery applications.10–12
Realizing the impact of star-shaped polymers, 4-arm star-shaped block copolymers consisting of poly(ethylene oxide) and poly(ε-caprolactone) are synthesized in this study by ring-opening polymerization of ε-caprolactone using pentaerythritol as an initiator and stannous octoate as a catalyst, followed by coupling reaction with carboxyl-functionalized methoxy poly(ethylene oxide). Size-exclusion chromatography (SEC) is frequently used technique for molecular characterization of polymers that yields information of relative molar mass and its distribution. SEC separates polymers according to their hydrodynamic volume that can be correlated to relative molar mass of the employed standards. SEC is often incapable of discriminating a mixture of homopolymers and block copolymers due to poor detector sensitivity and separation selectivity. Both precursors and star block copolymers are also analyzed for end-group modification and chemical composition by proton NMR. A significant amount of residual parent homopolymers can be present in block copolymers prepared by any modern or feasible polymerization technique, especially when coupling of two already synthesized polymers is involved. Therefore, rigorous characterization of such fascinating materials is crucial. Liquid chromatography at critical conditions is now an established method for such investigations.13–16 In this study, chromatographic critical conditions of both PEO and PCL were established on normal phase (NP) and reversed phase (RP) columns, respectively. The non-critical segment eluted in size exclusion mode in both cases. The developed method exposed any unattached parent homopolymers in the sample present alongside targeted 4-arm star-shaped block copolymers. Up to the best of our knowledge, LCCC have rarely been employed for analysis of star-block copolymers17 despite number of studies devoted to analysis of di- and tri-block copolymers.14
2. HPLC of polymers
Chromatographic separation of polymers using porous stationary phase can be divided into three different modes i.e., size exclusion chromatography (SEC), interaction chromatography (IC), and liquid chromatography at critical conditions (LCCC).13,14SEC is a standard technique for determination of molar mass distributions of polymers. It is an entropy controlled process where the polymers are separated according to their hydrodynamic volume i.e., extent to which polymers are excluded from the pores of the stationary phase. Molecules with a large hydrodynamic volume do not have access to small pores of the stationary phase and therefore elute earlier compared to smaller sized polymer chains that have more access to the pores of stationary phase.18,19 The resolving power of SEC is rather poor, however, it is a most widely employed and straight forward test of hydrodynamic size of macromolecules in dilute solution that can be correlated to their relative molar mass.
On the other hand, interaction chromatography (IC) is enthalpy-driven process where polymeric chains interact with stationary phase. Longer chains elute later since they have more interacting groups compared to shorter chains. IC is employed for separation of polymers with respect to end-group functionality and chemical composition.14,20,21 This mode can also be applied for oligomeric separation of low molar mass polymers.14,22,23
Liquid chromatography at critical conditions exists at the transition point of SEC and IC, where the entropic effect due to exclusion and enthalpic effect due to interaction are compensated. At LCCC one block of block copolymers becomes ‘chromatographically invisible’ i.e., it does not contribute to retention. At this point, so-called chromatographic critical point, block copolymer is assumed to be eluted exclusively with respect to the functionality/end-group20,21,24,25 or other block in block copolymer.26–34 A fair estimation of non-critical block length and quantifications of critical homopolymers is possible by appropriately applying LCCC.14,28,35
3. Experimental
3.1. Materials
ε-Caprolactone (ε-CL, TCI, Japan) was dried over CaH2 and distilled under reduced pressure prior to use. Methoxy poly(ethylene oxide) (MeO-PEO, Aldrich, Germany, Mn = 550, 2000 and 5000 g mol−1) were dried by azeotropic distillation with toluene. Maleic anhydride (Aldrich, Germany) was recrystallized from toluene and dried in vacuum for 24 h. Methylene chloride was dried by refluxing over CaH2 followed by distillation. Toluene was dried by refluxing over sodium-benzophenone complex. Stannous octoate [Sn(Oct)2, Aldrich, Germany], pentaerythritol (Aldrich, Germany), 4-dimethylaminopyridine (Across Organics, USA), trimethylamine (Daijung, Korea) were used without further purification. The solvents – dichloromethane (DCM), chloroform, acetonitrile (ACN), tetrahydrofuran (THF), dimethylformamide (DMF) – all HPLC grade, were used as received.3.2. Synthetic part
| Products | Polymerization protocols | Size exclusion chromatography | |||||
|---|---|---|---|---|---|---|---|
| Pentaerythritol, g (mmoles) | Stannous octoate, g (mmoles) | ε-Caprolactone, g (mmoles) | Mn (g mol−1) | Mw (g mol−1) | Mp (g mol−1) | Đ (Mw/Mn) | |
| 4-Arm star-PCL2k | 0.33 (2.43) | 0.005 (0.012) | 5.0 (44) | 2200 | 3200 | 3000 | 1.45 |
| 4-Arm star-PCL5k | 0.13 (0.95) | 0.005 (0.012) | 5.0 (44) | 6700 | 7900 | 7600 | 1.18 |
| 4-Arm star-PCL10k | 0.06 (0.44) | 0.005 (0.012) | 5.0 (44) | 9700 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.41 |
• SBC-1: 4-arm star-shaped PCL10k-b-PEO2k-OMe copolymer
• SBC-2: 4-arm star-shaped PCL2k-b-PEO550-OMe copolymer
• SBC-3: 4-arm star-shaped PCL5k-b-PEO550-OMe copolymer
• SBC-4: 4-arm star-shaped PCL10k-b-PEO5k-OMe copolymer
3.3. Liquid chromatography
SEC measurements were carried out on an Agilent 1200 series HPLC system that was equipped with an isocratic pump, a manual injector, a column thermostat, and a refractive index detector. A set of three PLgel columns (300 mm × 7.5 mm, 5 μm mixed D) that were connected in front with a PLgel guard column (50 mm × 7.5 mm) was employed for SEC analysis. Temperature of the columns was maintained at 30 °C. Chloroform was used as eluent at a flow rate of 1.0 mL min−1. Hundred microliter of analyte solution in chloroform having concentration between 1.0–5.0 mg mL−1, were injected. Data acquisition and processing were performed using Agilent Open LAB ChemStation software. Instrument was calibrated with polystyrene standards (Mn = 165 g mol−1 to 3 × 106 g mol−1), purchased from Polymer Standards Services (PSS), Germany.LCCC analyses were carried out on an Agilent technologies chromatograph (infinity 1200 series). System comprised of a binary pump, an auto-sampler, column oven and an evaporative light scattering detector (ELSD). Nitrogen was used as carrier gas for ELSD and its pressure at nebulizer was set to 55 psi. The temperature of drift tube was maintained at 60 °C. Flow rate of mobile phase was 0.5 mL min−1. Mobile phase consisted of binary mixture that were mixed by volume and vacuum degassed. Samples were prepared in the same solvent mixture as mobile phase and concentrations were kept between 3–10 mg mL−1, and 20 μL sample solution was injected. The data were acquired using Agilent Open LAB ChemStation for LC system. Following columns were used in LCCC analysis
Normal phase: PerfectSil (MZ Analysentechnik, Germany); 250 × 4.6 mm; pore size: 300 Å; particle size: 5 μm.
Mobile phase used for LCCC on NP was composed of a binary mixture tetrahydrofuran (THF) and dimethylformamide (DMF).
Reversed phase: Jupiter RP-C18 (Phenomenex, USA); 250 × 4.6 mm; pore size: 300 Å; particle size: 5 μm.
Mobile phase used for LCCC at RP was composed of a ternary mixture of acetonitrile (ACN), tetrahydrofuran (THF) and triethylamine (TEA).
3.4. 1H-NMR
1H-NMR spectra were recorded on a Bruker AVANCE 400 MHz spectrometer using CDCL3 as solvent and trimethylsilane as reference material.3.5. FTIR
FTIR spectra were obtained by using FTIR Bruker Vector 22 spectrometer (Germany). Samples were analyzed through KBr pellet method and measurements were conducted in mid IR region (400–4000 cm−1).4. Results and discussion
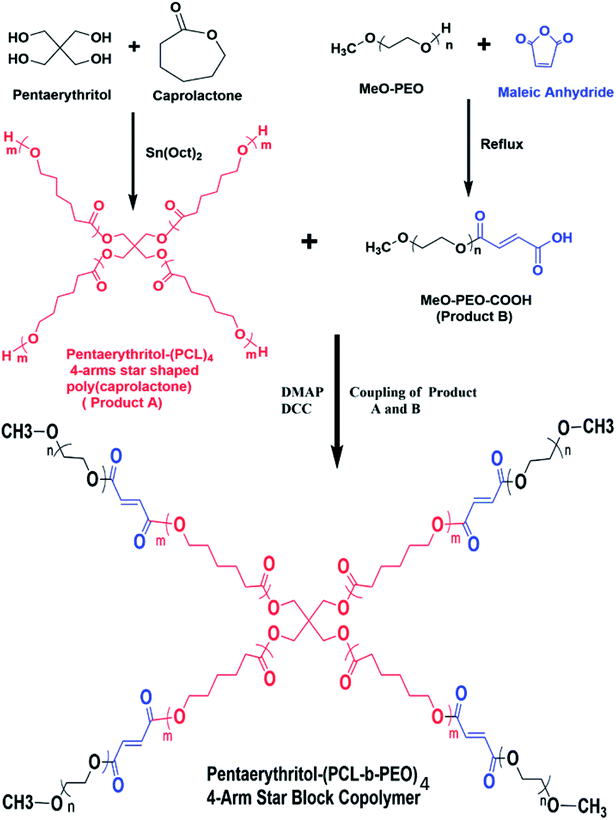
In this study, 4-arm star-shaped block copolymers, comprising of poly(ethylene oxide) as a hydrophilic part and poly(ε-caprolactone) as a hydrophobic segment are synthesized by employing divergent synthetic methodologies i.e., ring-opening polymerization (ROP) followed by coupling reaction, as demonstrated in Scheme 1. Briefly, 4-arm star-shaped poly(ε-caprolactone) having hydroxyl end-functionality are synthesized by ROP of ε-caprolactone in presence of tetrafunctional pentaerythritol as initiator. Three different products of star-PCL homopolymers with varying chain lengths, i.e., 2000 g mol−1 (2k), 5000 g mol−1 (5k) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (10k) are synthesized by adjusting molar feed ratio of monomer-to-initiator (M/I), see Table 1. The synthesized products are characterized by size exclusion chromatography (SEC) for augmentation in molar mass that serves as a preliminary technique for the confirmation of synthesis of the polymers. Comparison of SEC chromatograms of three star-PCL of varying molar masses is illustrated in Fig. 1. SEC separates polymers on the basis of hydrodynamic volume in dilute solution and a clear trend of decrease in retention volume with increasing molar mass is observed. Furthermore, molar masses of the products were in good agreement with expected values based on initial monomer to initiator ratio. It must be mentioned here that molar mass values as suggested by SEC are not real but relative to linear polystyrene standards. Average molar mass distribution and polydispersity indices (Đ) of products are summarized in Table 1.
000 g mol−1 (10k) are synthesized by adjusting molar feed ratio of monomer-to-initiator (M/I), see Table 1. The synthesized products are characterized by size exclusion chromatography (SEC) for augmentation in molar mass that serves as a preliminary technique for the confirmation of synthesis of the polymers. Comparison of SEC chromatograms of three star-PCL of varying molar masses is illustrated in Fig. 1. SEC separates polymers on the basis of hydrodynamic volume in dilute solution and a clear trend of decrease in retention volume with increasing molar mass is observed. Furthermore, molar masses of the products were in good agreement with expected values based on initial monomer to initiator ratio. It must be mentioned here that molar mass values as suggested by SEC are not real but relative to linear polystyrene standards. Average molar mass distribution and polydispersity indices (Đ) of products are summarized in Table 1.
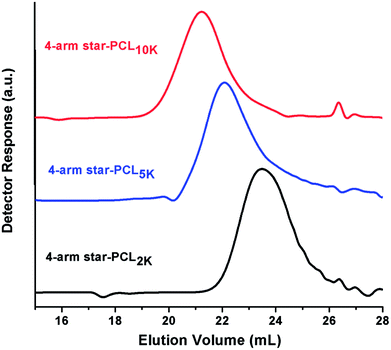 | ||
| Fig. 1 SEC chromatograms of 4-arm star-shaped poly(ε-caprolactone) of different molar mass, on a set of three PLgel columns (300 × 7.5 mm, 5 μm, mixed D) in CHCl3 at a flow rate of 1.0 mL min−1. | ||
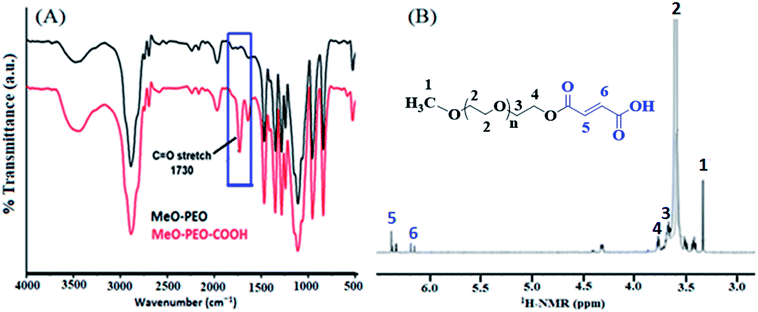
Commercially available methoxy poly(ethylene oxide) have hydroxyl end-group. The hydroxyl group of methoxy poly(ethylene oxide) is modified into carboxyl group by reaction with maleic anhydride. FTIR and 1H-NMR spectroscopic techniques were carried out to deduce structures of carboxyl-functionalized MeO-PEO (MeO-PEO-COOH), Fig. 2.
FTIR spectra of MeO-PEO and corresponding MeO-PEO-COOH are depicted in Fig. 2A. Existence of ether linkages in MeO-PEO is characterized by its asymmetrical stretching vibration at 1106 cm−1. The band observed at 2886 cm−1 is attributed to stretching vibration of methylene group. Presence of hydroxyl functionality is supported by a band at 3440 cm−1. After introducing carboxyl group onto MeO-PEO terminal, a new discrete sharp absorption band emerged at 1730 cm−1 that is assigned to stretching vibration of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The emergence of this band supports the success of carboxylation reaction.
O. The emergence of this band supports the success of carboxylation reaction.
1H-NMR analysis further substantiate the conversion of hydroxyl end-functionality of MeO-PEO into carboxylic group, Fig. 2B. The signals of the protons of repeating unit (–CH2–CH2–O–) appeared at δ 3.62 ppm while methoxy protons are attributed to a signal at δ 3.36 ppm. Signals at δ 3.35 ppm and δ 3.62 ppm are designated to terminal –CH3 and ether protons of MeO-PEO-COOH, respectively. Two distinct signals at δ 6.1 ppm and 6.3 ppm are assigned to vinyl protons in the terminal group. These signals endorse the FTIR results for successful incorporation of maleic anhydride on MeO-PEO. The resonance signal of proton of end unit (–OH) is not detectable due to intra-molecular hydrogen bonding.
Thereafter, hydroxyl terminated poly(ε-caprolactone) and carboxyl functionalized poly(ethylene oxide) are coupled to synthesize 4-arm star-shaped PCL-b-PEO copolymers.
In following text, we shall refer 4-arm star-shaped poly(ε-caprolactone) as ‘star-PCL’ and carboxyl terminated methoxy poly(ethylene oxide) (MeO-PEO-COOH) as ‘MeO-PEO’. Furthermore, star block copolymers are denoted as SBC-1, SBC-2, SBC-3 and SBC-4 (details in Experimental section).
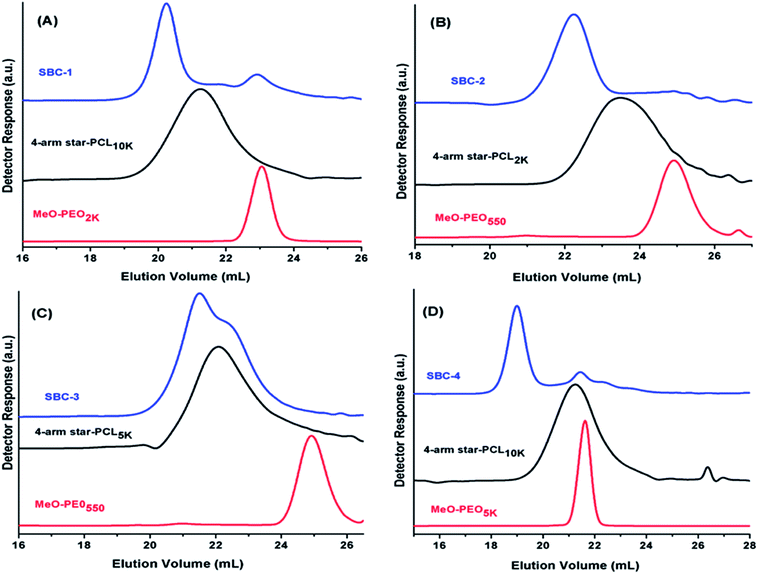
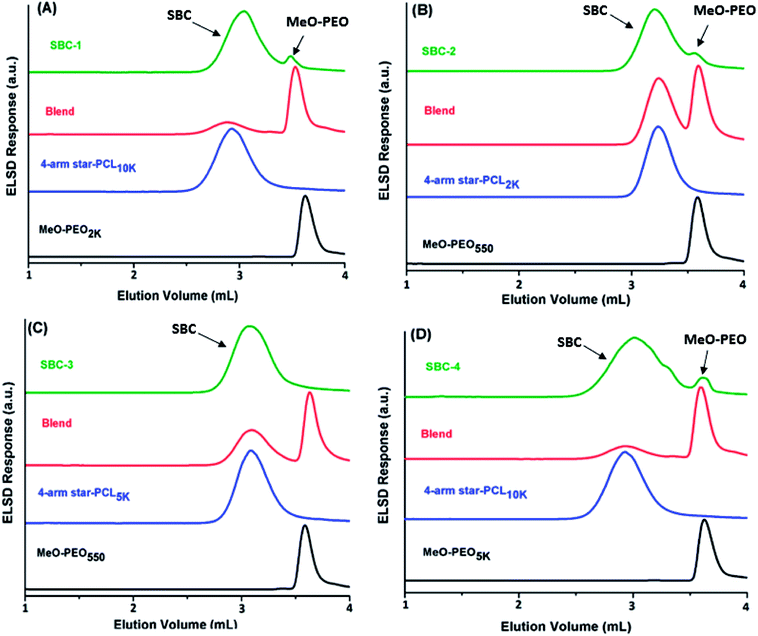
SEC chromatograms of four different coupled products along with their precursors are shown in Fig. 3. Peak of coupled products is shifted toward lower retention volume compared to their precursors that indicates the successful coupling reaction. The product SBC-1 was prepared by coupling of star PCL10k and MeO-PEO2k, the precursors have quite different molar mass hence elute one after other as sharp peaks. The elution volume of coupled product decreased considerably confirming the success of coupling reaction. However, small peak at the elution volume of MeO-PEO2k indicates presence of some amount of unreacted precursor. The same is true for SBC-2, synthesized by coupling of four arm star PCL2k and MeO-PEO550 (Fig. 3B). SBC-1 and SBC-2 are rather simple examples where it is not difficult to conclude about presence or absence of unwanted parent materials in the sample, though the detection limit might be highly dependent upon dn/dc of the analytes. On the contrary, SBC-3 and SBC-4 are rather complex samples with overlapping peaks of parent materials and final product. SBC-3 was synthesized by coupling of four arm star PCL5k and MeO-PEO550. The presence of MeO-PEO550 should easily be detected if present. However, the peaks of star PCL5k and final product are overlapping. The peak of SBC-3 has a high elution volume shoulder that might be due to presence of some unreacted star PCL5k. However, it is difficult to estimate the quantity of unwanted star PCL in the sample (Fig. 3C). SBC-4 is synthesized by coupling of star PCL10k and MeO-PEO5k. In this case peaks of both parent materials overlap. The coupled star block copolymer eluted earlier, nonetheless, a peak around elution volume of both the precursors is rather difficult to interpret (Fig. 3D). Hence, it is not always possible to conclude about the presence of parent materials in the sample because of poor resolution of SEC. The results clearly manifest limitations of SEC. Therefore, a more systematic study for determination of true chemical composition of star block copolymers is imperative.
As a next step, 1H-NMR analyses of precursors (MeO-PEO-COOH, 4-arm star-PCL) and star block copolymers were carried out to deduce their structures. Lowest molar mass of both precursors are shown for better visualization of end-group modifications. Fig. 4A depicts 1H-NMR of MeO-PEO550-COOH that was prepared by reaction of MeO-PEO550 with maleic anhydride. Six different signals appeared in the spectrum with respect to their chemical shift. Two doublet signals at δ 6.19 ppm and δ 6.39 ppm are ascribed to vinyl protons (H5 and H6) in the end-group of MeO-PEO550-COOH. Signals at δ 3.35 ppm and δ 3.62 ppm are attributed to terminal –CH3 (H1) and ether protons (H2) of MeO-PEO550-COOH, respectively. Signal at δ 3.70 ppm is assigned to methylene proton (H3) near to ether linkages and signal at δ 3.80 ppm is assigned to methylene protons (H4) near to the carboxylic end-group of MeO-PEO550-COOH. The conversion ratio of –OH group to –COOH group can be calculated by using integration ratio of signals at δ 6.19 (H5) ppm and δ 6.39 (H6) ppm to the signal at δ 3.35 (H1) ppm. The ratio of integration of above-mentioned signals is equal to unity, indicating almost complete conversion of –OH groups into –COOH groups.
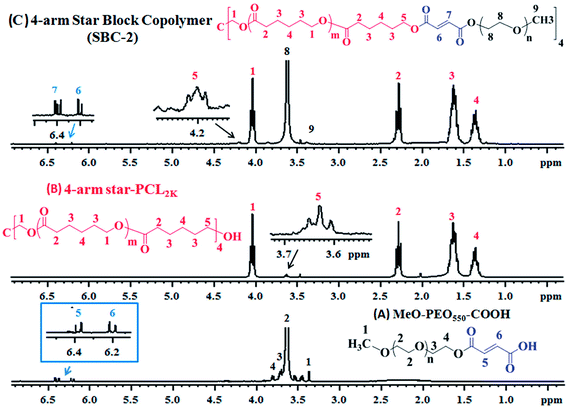 | ||
| Fig. 4 1H-NMR spectrum of (A) MeO-PEO550-COOH; (B) 4-arm star PCL2k; (C) 4-arm star-shaped PCL2k-b-PEO550-OMe copolymer. | ||
Proton NMR spectrum of 4-arm star-PCL2k is demonstrated in Fig. 4B. Five different signals observed in spectrum indicate the successful polymerization of ε-caprolactone on pentaerythritol. The signal of methylene protons (H1) adjacent to oxygen of ester group appears at δ 4.03 ppm whereas signal of terminal methylene proton (H5) near to –OH group appears at δ 3.63 ppm. The signal of methylene proton (H2) adjacent to carbonyl carbon of ester group is assigned at δ 2.28 ppm. The signals at δ 1.35 ppm and δ 1.62 ppm are due to protons of methylene group at position 3 and 4, respectively.
The 1H-NMR spectrum of 4-arm star-shaped PCL2k-b-PEO550-OMe copolymer (SBC-2) is presented in Fig. 4C. The signals at δ 1.36 (H4) ppm, δ 1.62 (H3) ppm, δ 2.28 (H2) ppm, δ 4.03 (H1) ppm are ascribed to methylene protons of ε-caprolactone units in 4-arm star-PCL2k. The signals at δ 3.35 (H9) ppm and δ 3.62 (H8) ppm are attributed to terminal methyl group and ethylene proton of ether units of MeO-PEO550 in block copolymers. Two doublet signals at δ 6.20 (H6) ppm and δ 6.40 (H7) ppm are observed due to vinyl protons in maleic anhydride units whereas signal at δ 4.20 ppm (H5) belongs to the methylene proton of 4-arm star-PCL2k adjacent to the maleic anhydride units. The presence of these signals indicate successful synthesis of 4-arm star shaped block copolymer.36–38
Chemical composition of 4-arm star shaped block copolymers can be calculated by using integration values of particular signals of 1H-NMR in equation (Fig. 4C).
Liquid chromatography at critical conditions (LCCC) has been explored as an attractive method for the characterization of individual block in block copolymers. LCCC allows efficient separation of block copolymers from corresponding homopolymers alongside revealing a reasonable estimate of chain length of non-critical block.14,28,35
Critical conditions corresponding to both homopolymer precursors are required for clearly concluding about their presence and quantification in a SBC sample. Several important aspects need to be considered critically while developing critical conditions for a particular polymer that includes solubility of the critical and non-critical segments of the polymer to be analyzed, expected elution behavior of the non-critical segment at critical conditions depending upon polarity, pore diameter of the column for better resolution of the non-critical segment and so forth. In this particular case obviously PEO is polar than PCL. Hence at chromatographic critical point of PEO on a NP column, PCL should elute in size exclusion mode earlier than the critical PEO. Similarly, at critical point of PCL on RP column PEO should be excluded.13,14
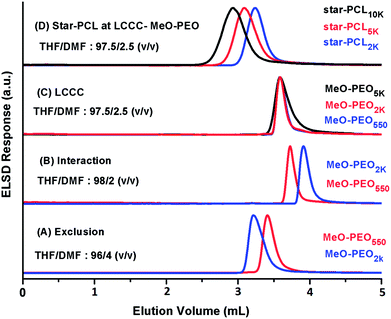
The first target in this context is the establishment of critical conditions for PEO. These conditions should ideally suppress the effect of addition of four MeO-PEO to star-PCL, rendering elution with regard to the molar mass of the star-PCL. Obviously, a polar stationary phase must be selected to ensure exclusion of PCL at the chromatographic critical point of MeO-PEO. Critical conditions corresponding to PEO has been documented in several articles.26,28 In this study, critical conditions of MeO-PEO were established on a normal phase plain silica column using a binary mixture of THF and DMF as a mobile phase. THF promotes retention of MeO-PEO onto stationary phase while DMF strongly promotes elution. Critical conditions corresponding to MeO-PEO were identified by varying ratio of retention and elution promoting solvents. Elution behavior of MeO-PEO standards of different molar masses by using various compositions of THF and DMF as mobile phase are depicted in Fig. 5. At eluent composition THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF: 96
DMF: 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v), typical SEC mode is observed, MeO-PEO2k eluted earlier than MeO-PEO550 (Fig. 5A). While at eluent composition THF
4 (v/v), typical SEC mode is observed, MeO-PEO2k eluted earlier than MeO-PEO550 (Fig. 5A). While at eluent composition THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF: 98
DMF: 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v), the retention behavior is reversed, MeO-PEO550 eluted earlier than MeO-PEO2k (Fig. 5B). This behavior can be explained by considering increased interactions between polymer molecules with the stationary phase by increasing content of THF in mobile phase. Chromatographic critical point of MeO-PEO was found at eluent composition THF
2 (v/v), the retention behavior is reversed, MeO-PEO550 eluted earlier than MeO-PEO2k (Fig. 5B). This behavior can be explained by considering increased interactions between polymer molecules with the stationary phase by increasing content of THF in mobile phase. Chromatographic critical point of MeO-PEO was found at eluent composition THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF: 97.5
DMF: 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (v/v), all MeO-PEO standards eluted at same elution volume irrespective of their molar mass (Fig. 5C). At critical point of MeO-PEO, star-PCL eluted in SEC regime as per expectation (Fig. 5D). The established chromatographic critical point of PEO is suitable not only for the separation of star block copolymers from MeO-PEO homopolymers but also for estimation of molar mass of star-PCL (non-critical block).
2.5 (v/v), all MeO-PEO standards eluted at same elution volume irrespective of their molar mass (Fig. 5C). At critical point of MeO-PEO, star-PCL eluted in SEC regime as per expectation (Fig. 5D). The established chromatographic critical point of PEO is suitable not only for the separation of star block copolymers from MeO-PEO homopolymers but also for estimation of molar mass of star-PCL (non-critical block).
Elution behavior of coupled products, parent homopolymers and their blends are monitored at critical point for MeO-PEO, Fig. 6. The conditions are critical for MeO-PEO therefore SBCs should elute with regard to the molar mass of non-critical star-PCL allowing separation of parent MeO-PEO from SBC. The elution volume of star block copolymers reflects the molar mass of star-PCL. Two distinctly different peaks are detected in chromatograms of SBC-1, SBC-2 and SBC-4 as shown in Fig. 6 A, B and D. Major and early eluting peak is assigned as SBC whereas minor fraction eluting at the critical point can be undoubtedly designated as unreacted MeO-PEO homopolymers. It is important to mention here that small amount of unreacted MeO-PEO was not detected by SEC analysis particularly in case of SBC-2 (Fig. 3B). Similarly it was not possible to conclusively deduce about the type of unreacted homopolymer for SBC-4 by SEC (Fig. 3D). LCCC analysis at critical conditions of PEO confirmed the presence of MeO-PEO homopolymers in the sample. SBC-3 does not contain unreacted MeO-PEO homopolymer that confirms the complete consumption of MeO-PEO in coupling reaction with star-PCL (Fig. 6C). However, LCCC of PEO cannot provide any information with regard presence or absence of free star-PCL in the sample. For this purpose, critical conditions of PCL are required. A slight late elution of SBC compared to parent star-PCL in case of SBC-1 and SBC-4 is attributed to effect of interactive block on the elution of SBC.31,39,40
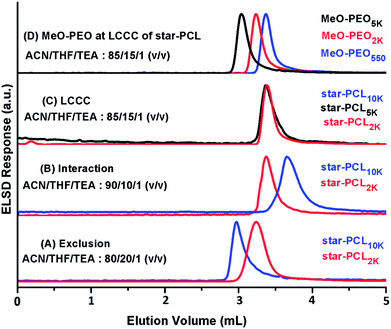
A logical next step would be to exploit critical conditions corresponding to star-PCL for separation of residual star-PCL homopolymers from coupled products. In our recent article, we reported critical conditions for linear-PCL on reversed phase octadecyl column using ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEA as the mobile phase.41 This mobile phase system extended the molar mass range of PCL compared to already reported critical conditions of PCL in aqueous mobile phase due to better solubility of higher molar mass PCL and higher pore size of the stationary phase.42 Same system is followed for establishment of critical conditions of star-PCL. A series of star-PCL homopolymers of varying molar mass (2000, 5000, 10
TEA as the mobile phase.41 This mobile phase system extended the molar mass range of PCL compared to already reported critical conditions of PCL in aqueous mobile phase due to better solubility of higher molar mass PCL and higher pore size of the stationary phase.42 Same system is followed for establishment of critical conditions of star-PCL. A series of star-PCL homopolymers of varying molar mass (2000, 5000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were utilized for establishment of chromatographic critical point of star-PCL, Fig. 7. Critical conditions for star-PCL are found at eluent composition ACN
000 g mol−1) were utilized for establishment of chromatographic critical point of star-PCL, Fig. 7. Critical conditions for star-PCL are found at eluent composition ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEA: 85
TEA: 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v. Star-PCL of varying molar masses eluted at same retention volume irrespective of their degree of polymerization (Fig. 7C). Exclusion behavior was observed while using mobile phase containing more content of THF and interaction while using less content of THF compared to critical point (Fig. 7A and B). MeO-PEO should be excluded from the pores of stationary phase at the chromatographic critical point of star PCL on RP column due to relatively higher polarity compared to PCL (Fig. 7D). The established conditions would allow separation of block copolymer with respect to the molar mass of PEO segment while allowing for separation of any unreacted star PCL homopolymers form the sample.
1, v/v. Star-PCL of varying molar masses eluted at same retention volume irrespective of their degree of polymerization (Fig. 7C). Exclusion behavior was observed while using mobile phase containing more content of THF and interaction while using less content of THF compared to critical point (Fig. 7A and B). MeO-PEO should be excluded from the pores of stationary phase at the chromatographic critical point of star PCL on RP column due to relatively higher polarity compared to PCL (Fig. 7D). The established conditions would allow separation of block copolymer with respect to the molar mass of PEO segment while allowing for separation of any unreacted star PCL homopolymers form the sample.
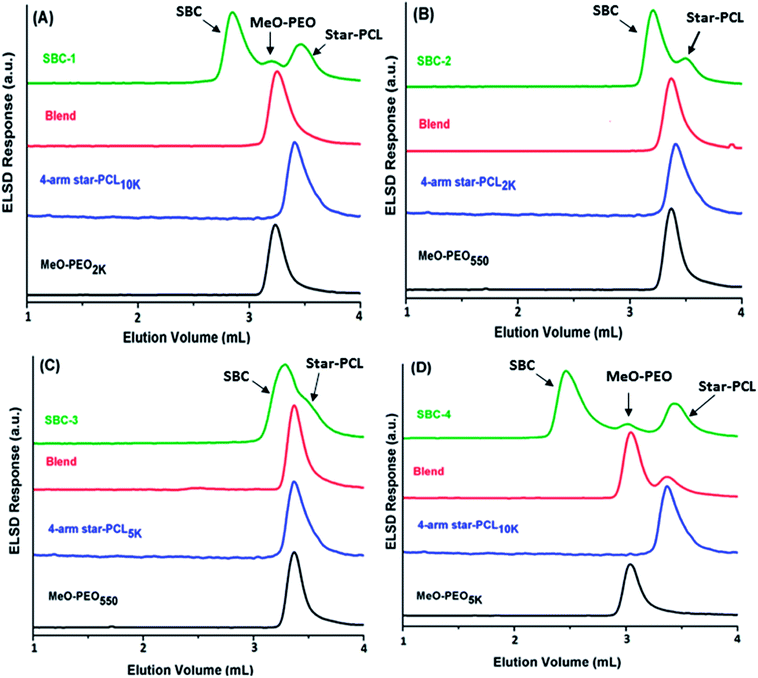
As a next step, SBCs are analyzed at the chromatographic critical point of star-PCL. Star-PCL should be separated from the star block copolymer at its critical point. Another important and fascinating aspect of this analysis is that four parent MeO-PEO are attached to one star-PCL making star block copolymer, hence, SBCs should elute earlier than parent MeO-PEO in size exclusion mode. The exclusion effect should be more pronounced in the SBCs containing MeO-PEO of higher molar mass, SBC-1 (Fig. 8A) and SBC-4 (Fig. 8D). The analysis allowed for separation of both parent homopolymers from SBC. The analysis of SBC-1 and SBC-4 (Fig. 8A and D) under these conditions clearly show separation of SBC from both critical (star PCL) and non-critical (MeO-PEO) parent materials in a single run. The chromatograms of individual and blends of parent materials confirm their elution volume. Obviously, the early eluting peak that is absent in above-mentioned elugrams of parent materials corresponds to SBC. Furthermore, SBC-3 (Fig. 8C) displayed shoulder at higher retention volume that may be assigned to residual star-PCL since analysis of this product at LCCC-PEO revealed the absence of unreacted MeO-PEO homopolymers. Broadness of SBC peak especially the high elution volume shoulder might be attributed to presence of small amounts of linear or tri-arm SBC containing two and three MeO-PEO precursors, respectively. However, it is not possible to unambiguously conclude this aspect by current analysis protocol.
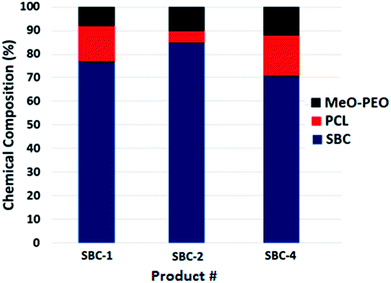
The analysis of SBCs by SEC, and LCCC of both segments reveal that products contain several unwanted impurities that are not easy to track out by any single technique. Nonetheless, LCCC of star-PCL seems to be most informative analysis approach compared to SEC and LCCC-PEO. All these analyses confirmed heterogeneity of products. The potential sources for presence of parent homopolymers in star block copolymers could be, (1) incomplete carboxylation of MeO-PEO (2) molar mass of MeO-PEO and star-PCL are not absolute but average, hence, require tedious experimentation for having fairly equal molar ratios. Furthermore, the composition of the products with regard to presence of precursors and targeted star block copolymer as obtained by current analysis is presented in Fig. 9. For quantification of precursors and targeted SBC, detector response for the precursors is evaluated as a function of injected amount under established conditions. The detector response for the precursors in SBC sample is employed for estimation of amount of unreacted precursors in the samples, while rest being the star block copolymers. The heterogeneity of the complex star block copolymers is exposed by combination of different chromatographic approaches that was not possible by any single technique. Quantification of SBC-3 was not possible due to insufficient separation. For such samples, columns with smaller pore diameter are required to achieve better resolution. The evaluation and comparison of the synthesized SBCs with regard to micellization behavior, and drug-loading and delivery applications with their linear counterparts will be subject of next publications of this series.
5. Conclusion
In this study, a comparison of different analysis methods for star block copolymers is presented. It is demonstrated that SEC cannot completely discriminate homopolymers impurities from star block copolymers. NMR is a useful tool for structure elucidation of intermediates especially for confirmation of end-group modification. LCCC of both the segments proved to be very useful for analysis of the presence of unwanted parent homopolymers in the SBC samples. Especially LCCC of star-PCL on RP column allowed for separation of both parent homopolymers from the targeted SBCs in a single run, in most of the cases. However, in some cases analysis by all three modes is required for complete insight into the product composition. All products contain considerable amount of homopolymer precursors that can really effect the performance properties. Comprehensive analysis is imperative for development of real structure–property correlations of these fascinating materials. The proposed separation protocol helps to obtain precursor free star block copolymer for any further application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research is supported by Higher Education Commission of Pakistan under “National Research Program for Universities” against project # 5754.References
- G. Gaucher, M.-H. Dufresne, V. P. Sant, N. Kang, D. Maysinger and J.-C. Leroux, J. Controlled Release, 2005, 109, 169–188 CrossRef PubMed.
- K. Kataoka, A. Harada and Y. Nagasaki, Adv. Drug Delivery Rev., 2001, 47, 113–131 CrossRef PubMed.
- S. Zhou, X. Deng and H. Yang, Biomaterials, 2003, 24, 3563–3570 CrossRef PubMed.
- W. Zhang, Y. Li, L. Liu, Q. Sun, X. Shuai, W. Zhu and Y. Chen, Biomacromolecules, 2010, 11, 1331–1338 CrossRef PubMed.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef.
- J. M. Harris, Poly(ethylene glycol) chemistry: biotechnical and biomedical applications, Springer Science & Business Media, New York, 2013 Search PubMed.
- S. C. Owen, D. P. Chan and M. S. Shoichet, Nano Today, 2012, 7, 53–65 CrossRef.
- E. Zhulina and O. Borisov, ACS Macro Lett., 2013, 2, 292–295 CrossRef.
- L. Y. Qiu and Y. H. Bae, Pharm. Res., 2006, 23, 1–30 CrossRef PubMed.
- J. M. Ren, T. G. McKenzie, Q. Fu, E. H. Wong, J. Xu, Z. An, S. Shanmugam, T. P. Davis, C. Boyer and G. G. Qiao, Chem. Rev., 2016, 116, 6743–6836 CrossRef PubMed.
- D. J. Cameron and M. P. Shaver, Chem. Soc. Rev., 2011, 40, 1761–1776 RSC.
- X. Pang, L. Zhao, M. Akinc, J. K. Kim and Z. Lin, Macromolecules, 2011, 44, 3746–3752 CrossRef.
- H. Pasch and B. Trathnigg, Multidimensional HPLC of polymers, Springer-Verlag, Berlin, Heidelberg, 2013 Search PubMed.
- M. I. Malik and H. Pasch, Prog. Polym. Sci., 2014, 39, 87–123 CrossRef.
- T. Macko and D. Hunkeler, Adv. Polym. Sci., 2003, 163, 61–136 CrossRef.
- A. Baumgaertel, E. Altuntaş and U. S. Schubert, J. Chromatogr. A, 2012, 1240, 1–20 CrossRef PubMed.
- H. Gao, K. Min and K. Matyjaszewski, Macromol. Chem. Phys., 2006, 207, 1709–1717 CrossRef.
- B. Trathnigg, Angew. Chem., Int. Ed., 2004, 43, 5117 CrossRef.
- A. Striegel, W. W. Yau, J. J. Kirkland and D. D. Bly, Modern size-exclusion liquid chromatography: practice of gel permeation and gel filtration chromatography, John Wiley & Sons, New York, 2009 Search PubMed.
- M. Irfan, J. Oh, S. G. Musharraf, M. R. Shah, S. Ahmed and M. I. Malik, RSC Adv., 2016, 6, 98117–98127 RSC.
- M. I. Malik, B. Trathnigg and C. O. Kappe, Eur. Polym. J., 2008, 44, 144–154 CrossRef.
- T. Chang, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 1591–1607 CrossRef.
- G. Glöckner, Gradient HPLC of copolymers and chromatographic cross-fractionation, Springer Science & Business Media, 2012 Search PubMed.
- M. Adler, F. Rittig, S. Becker and H. Pasch, Macromol. Chem. Phys., 2005, 206, 2269–2277 CrossRef.
- I. Park, S. Park, D. Cho, T. Chang, E. Kim, K. Lee and Y. J. Kim, Macromolecules, 2003, 36, 8539–8543 CrossRef.
- R. Abdul-Karim, S. G. Musharraf and M. I. Malik, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 1887–1893 CrossRef.
- M. I. Malik, G. W. Harding, M. E. Grabowsky and H. Pasch, J. Chromatogr. A, 2012, 1244, 77–87 CrossRef PubMed.
- M. K. Tufail, R. Abdul-Karim, S. Rahim, S. G. Musharraf and M. I. Malik, RSC Adv., 2017, 7, 41693–41704 RSC.
- W. Jiang, S. Khan and Y. Wang, Macromolecules, 2005, 38, 7514–7520 CrossRef.
- K. Baran, S. Laugier and H. Cramail, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 753, 139–149 CrossRef.
- S. Lee, H. Lee, L. Thieu, Y. Jeong, T. Chang, C. Fu, Y. Zhu and Y. Wang, Macromolecules, 2013, 46, 9114–9121 CrossRef.
- M. I. Malik, B. Trathnigg and R. Saf, J. Chromatogr. A, 2009, 1216, 6627–6635 CrossRef PubMed.
- M. I. Malik, G. W. Harding and H. Pasch, Anal. Bioanal. Chem., 2012, 403, 601–611 CrossRef PubMed.
- M. I. Malik, T. Mahboob and S. Ahmed, Anal. Bioanal. Chem., 2014, 406, 6311–6317 CrossRef PubMed.
- M. I. Malik, P. Sinha, G. M. Bayley, P. E. Mallon and H. Pasch, Macromol. Chem. Phys., 2011, 212, 1221–1228 CrossRef.
- M. R. Nabid, S. J. T. Rezaei, R. Sedghi, H. Niknejad, A. A. Entezami, H. A. Oskooie and M. M. Heravi, Polymer, 2011, 52, 2799–2809 CrossRef.
- G. Maglio, G. Nese, M. Nuzzo and R. Palumbo, Macromol. Rapid Commun., 2004, 25, 1139–1144 CrossRef.
- H. J. Lim, H. Lee, K. H. Kim, J. Huh, C.-H. Ahn and J. W. Kim, Colloid Polym. Sci., 2013, 291, 1817–1827 CrossRef.
- W. Lee, D. Cho, T. Chang, K. J. Hanley and T. P. Lodge, Macromolecules, 2001, 34, 2353–2358 CrossRef.
- W. Lee, S. Park and T. Chang, Anal. Chem., 2001, 73, 3884–3889 CrossRef PubMed.
- H. Ahmed and B. Trathnigg, J. Sep. Sci., 2009, 32, 1390–1400 CrossRef PubMed.
- M. Irfan, A. M. Bhayo, S. G. Musharraf and M. I. Malik, J. Sep. Sci., 2018, 41(17) DOI:10.1002/jssc.201800465.
| This journal is © The Royal Society of Chemistry 2018 |