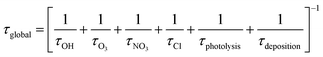Open Access Article
Open Access ArticleAtmospheric sink of β-ocimene and camphene initiated by Cl atoms: kinetics and products at NOx free-air†
Elizabeth Gaona-Colmána,
María B. Blanco*a,
Ian Barnesb,
Peter Wiesenb and
Mariano A. Teruel *a
*a
aInstituto de Investigaciones en Fisicoquímicas de Córdoba (INFIQC), Dpto. de Fisicoquímica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mteruel@fcq.unc.edu.ar; mblanco@fcq.unc.edu.ar; Fax: +54-351-4334180; Tel: +54-351-5353866 int. 53526
bPhysikalische Chemie/FBC, Bergische Universitaet Wuppertal, 42119 Wuppertal, Germany
First published on 31st July 2018
Abstract
Rate coefficients for the gas-phase reactions of Cl atoms with β-ocimene and camphene were determined to be (in units of 10−10 cm3 per molecule per s) 5.5 ± 0.7 and 3.3 ± 0.4, respectively. The experiments were performed by the relative technique in an environmental chamber with FTIR detection of the reactants at 298 K and 760 torr. Product identification experiments were carried out by gas chromatography with mass spectrometry detection (GC-MS) using the solid-phase microextraction (SPME) method employing on-fiber carbonyl compound derivatization with o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride. An analysis of the available rates of addition of Cl atoms and OH radicals to the double bond of alkenes and cyclic and acyclic terpenes with a conjugated double bond at 298 K is presented. The atmospheric persistence of these compounds was calculated taking into account the measured rate coefficients. In addition, tropospheric chemical mechanisms for the title reactions are postulated.
1. Introduction
The interaction between the biosphere and atmosphere is essential for all living systems and organisms. Hundreds of Volatile Organic Compounds (VOCs) are released from biogenic sources. The first biogenic volatile organic compound (BVOC) specifically identified to be important for atmospheric composition was isoprene.1 The biogenic VOCs include isoprenoids (isoprene and terpenes), alkanes, alkenes, carbonyls, alcohols, esters, ethers and acids, where the predominant species is isoprene. VOCs emitted from biogenic sources are more reactive than VOCs with anthropogenic origin.2Atmospheric concentrations of BVOCs range from few ppt to several ppb and their reactivity is high as reflected by their chemical lifetimes which range from minutes to hours.1 Many species of biogenic VOCs are rapidly oxidized by O3, OH radicals and/or NO3 radicals and it is also possible through reaction with chlorine (Cl) atoms. This later reaction has generally only been considered to be of importance in coastal and marine air environments.3–8 However, in field studies evidence has been presented for Cl chemistry in continental regions far removed from coastal and marine regions where observations of nitryl chloride (ClNO2), a gaseous photolytic Cl atom precursor, were made9–12 and in a recent study, different chlorine species have been detected in particles in urban air.13
Atmospheric degradation of BVOCs leads to the production of secondary chemical species which can enhance concentrations of tropospheric ozone and other oxidants in areas rich in nitrogen oxides and monoterpenes.2 On the other hand, the oxidation of BVOCs under low concentrations of NOx generates peroxides and acids.14 Acids as well as some other BVOC oxidation products may undergo gas-to-particle conversion leading to the growth of secondary organic aerosols (SOA).15
Monoterpene compounds as β-ocimene and camphene are emitted especially by conifers such as pine, spruce, fir, mint or citrus families16–18 and are known to constitute the main fraction of terpenic or essential oils produced in plant secretory organs.1 Monoterpene emissions are mostly dependent on ambient temperature, which typically increases their emissions. Some species (e.g. oaks) also show light-dependence of emissions suggesting link between monoterpene formation and photosynthetic process. Therefore, weather extreme conditions (such as high temperature) have large implications for the fluxes of matter between plants and the atmosphere that will also impact on the atmospheric chemistry.19,20
In order to assess the impact of these species on air quality, kinetic and mechanistic information on their tropospheric degradation is therefore needed.
In this work, we report room temperature relative kinetic determinations of the rate coefficients for the reactions of Cl atoms with β-ocimene and camphene performed in a long path photoreactor coupled to an FTIR spectrometer at room temperature and atmospheric pressure of nitrogen:
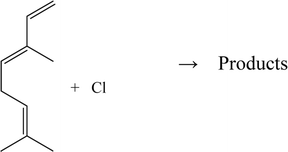 | (1) |
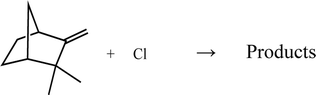 | (2) |
To the best of our knowledge, this work provides the first kinetic and product study for the reactions of Cl atoms with both monoterpenes. The aim of this study was to extend our earlier work on the reactivity of these monoterpenes toward OH radicals and O3 molecules.21–23 Kinetic data for these Cl reactions are needed to facilitate a better understanding of the oxidation mechanisms of these monoterpenes so that their role in tropospheric atmospheric chemistry can be better assessed. The measurements will also help to develop a more reliable structure–reactivity relationship for these biogenic compounds. Moreover, it has been identified reaction products due to the degradations of the studied monoterpenes with Cl atoms.
In addition to the kinetic experimental investigations, a correlation between the rate coefficients for the reactions of OH radicals with the monoterpenes studied in this work together with other monoterpenes and alkenes and those for the corresponding reactions with Cl atoms has been examined.
The atmospheric lifetimes of the BVOCs studied, with respect to reaction with Cl atoms, have been calculated with the rate coefficients obtained in this work and compared with the lifetimes of β-ocimene and camphene due to other homogeneous sinks in the troposphere. Products of the studied reactions has been identified using solid-phase microextraction (SPME) technique employing on-fiber carbonyl compound derivatization with detection system gas chromatograph-mass spectrometer (GC-MS).
2. Experimental
All the experiments were performed in a 1080 dm3 quartz–glass reaction chamber at a total pressure of 760 torr (760 torr = 101.325 kPa) and 298 ± 3 K in nitrogen. A detailed description of the reactor can be found elsewhere24 and only a brief description is provided here. A pumping system consisting of a turbo-molecular pump backed by a double-stage rotary fore pump was used to evacuate the reactor to 10−3 torr. Three magnetically coupled Teflon mixing fans are mounted inside the chamber to ensure homogeneous mixing of the reactants. The photolysis system consists of 32 low-pressure mercury lamps (Philips TUV 40 W, λmax = 254 nm), spaced evenly around the reaction vessel. The lamps are wired in parallel and can be switched individually, allowing a variation of the light intensity, and thus the photolysis frequency/radical production rate, within the chamber. The chamber is equipped with a White type multiple-reflection mirror system with a base length of (5.91 ± 0.01) m for sensitive in situ long path absorption monitoring of reactants and products in the IR spectral range 4000–700 cm−1. The White system was operated at 82 traverses, giving a total optical path length of (484.7 ± 0.8) m. The IR spectra were recorded with a spectral resolution of 1 cm−1 using a Nicolet Nexus FT-IR spectrometer, equipped with a liquid nitrogen cooled mercury-cadmium-telluride (MCT) detector. For the reaction of β-ocimene + Cl, 60 interferograms were co-added and for the reaction of camphene + Cl, 80 interferograms were co-added, for both reactions 20 spectra were recorded per experiment.Chlorine atoms were generated by the photolysis of oxalyl chloride with the germicidal lamps
| ClC(O)C(O)Cl + hν → 2Cl + 2CO | (3) |
The initial concentrations of reactants in ppmV (1 ppmV = 2.46 × 1013 molecule per cm3 at 298 K) were: β-ocimene, (2.1–2.7); camphene, (1.7–2.2); oxalyl chloride, (1.4–2.0); isobutene, (1.8–2.0) and propene, (3.7–4).
The reactants were monitored at the following infrared absorption frequencies (in cm−1): β-ocimene at 2700–3150; camphene at 882; isobutene at 890 and propene at 912.
Room temperature products identification experiments were carry out using SPME (grey fiber, PDMS/CAR/DVB, SUPELCO)/GC-MS (Varian CP3800) technique. Mixture of β-ocimene/ClC(O)C(O)Cl/air and camphene/ClC(O)C(O)Cl/air were flushed into the Teflon bag, respectively. The photolysis time was 10 s for each experiment, it was a experimentally considerable time in which the reactants have not been completely consumed with appreciable formation of the reaction products. In order to identify carbonyl products a 2 mL aqueous solution of o-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA) of 25 mg mL−1 was used as derivatizing agent. The PFBHA reacts with carbonyl compounds forming a stable oxime. The PFBHA was loaded on the SPME fiber during 1 min by head-space extraction. The fiber-PFBHA was exposed during 2 min for the β-ocimene–Cl reaction and 3 min for camphene–Cl reaction inside the chamber to produce the oxime on the fiber to be transferred to the GC-MS injector. The desorption time was 3 min at 200 °C. It was employed a capillary column HP-5MS (30 m × 0.250 mm, film 0.25 μm Agilent Part N 19091S-433). The temperature program was 80 °C for 3 min, 100 °C for 5 min, 200 °C for 5 min to 250 °C at a rate of 15 °C min−1 for the all experiments. The exposition times used were appropriate to detect these compounds, since larger exposition saturate the detector system used. In the mechanistic study, the time used between two consecutive withdrawals was 30 minutes.
3. Materials
The following chemicals, with purities as stated by the supplier, were used without further purification: nitrogen (Air Liquide, 99.999%), β-ocimene (Sigma-Aldrich, ≥90%), camphene (Aldrich, 95%), isobutene (Messer Griesheim, ≥99%), propene (Messer Griesheim, 99.5%) and oxalyl chloride (Sigma-Aldrich, ≥99%).4. Results
Rate coefficients for the reactions of Cl atoms with β-ocimene and camphene were determined by comparing their rate of decay with that of the corresponding decay of the reference compounds:| X + BVOC → Products, kBVOC | (4) |
| X + Reference → Products, kreference | (5) |
Provided that the reference compound and the reactant are lost only by reactions (4) and (5), then it can be shown that:
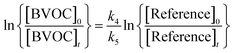 | (6) |
The relative rate technique relies on the assumption that both the monoterpenes and reference organics are removed solely by reaction with Cl atoms. To verify this assumption, various tests were performed to assess the loss of the reactants via reaction with oxalyl chloride, photolysis and wall deposition. These processes that could interfere with the kinetic determinations were found to be negligible for both the monoterpenes and the reference compounds. Mixtures of the monoterpenes and reference compounds with oxalyl chloride were stable in the dark when left in the chamber for the typical time span of the kinetic experiments (10–20 minutes). Moreover, in the absence of oxalyl chloride, photolysis of the mixtures (monoterpenes and reference compounds in air) did not show any decrease in the reactant concentrations over the time span of the experiments.
Fig. 1 and 2 show the kinetic data obtained from the experiments plotted according to eqn (6) for the reactions of Cl with the individual compounds studied measured relative to different reference compounds. Each plot represents a minimum of 2–3 experiments for each reference compound. Good linear relationships were obtained in all cases. The linearity of the plots with near-zero intercepts, combined with the fact that similar results were obtained for different initial concentrations of the unsaturated compound and reference organics, supports that complications due to secondary reactions in the experimental systems were negligible.
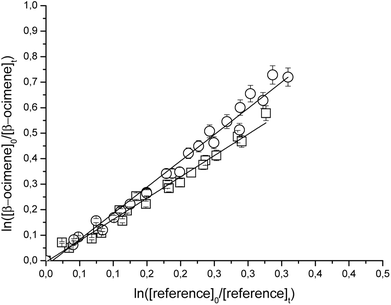 | ||
| Fig. 1 Relative rate data for the reaction of Cl with β-ocimene using isobutene (□) and propene (○) as reference compounds at 298 K and atmospheric pressure of air. | ||
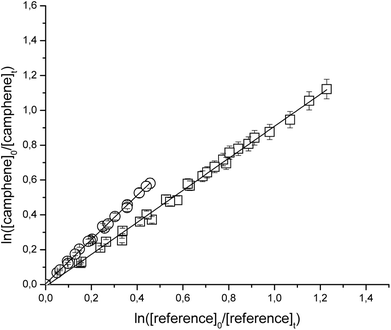 | ||
| Fig. 2 Relative rate data for the reaction of Cl with camphene using isobutene (□) and propene (○) as reference compounds at 298 K and atmospheric pressure of air. | ||
The kBVOC/kreference ratios determined from the slopes of the straight-line plots in Fig. 1 and 2 are listed in Table 1 together with the absolute values of the rate coefficients, kBVOC, calculated from the kBVOC/kreference ratios.
| Reaction | Reference | kBVOC/kreference | kBVOC × 1010 (cm3 per molecule per s) |
|---|---|---|---|
| β-Ocimene + Cl | Isobutene | 1.63 ± 0.05 | 5.5 ± 0.6 |
| Isobutene | 1.69 ± 0.05 | 5.7 ± 0.6 | |
| Isobutene | 1.63 ± 0.04 | 5.5 ± 0.6 | |
| Propene | 2.13 ± 0.09 | 5.6 ± 0.7 | |
| Propene | 1.96 ± 0.07 | 5.2 ± 0.6 | |
| Propene | 2.15 ± 0.07 | 5.7 ± 0.6 | |
| Average | 5.5 ± 0.7 | ||
| Camphene + Cl | Isobutene | 0.93 ± 0.02 | 3.2 ± 0.3 |
| Isobutene | 0.93 ± 0.01 | 3.2 ± 0.3 | |
| Isobutene | 0.94 ± 0.02 | 3.2 ± 0.3 | |
| Propene | 1.26 ± 0.04 | 3.3 ± 0.4 | |
| Propene | 1.27 ± 0.01 | 3.4 ± 0.3 | |
| Propene | 1.27 ± 0.02 | 3.4 ± 0.3 | |
| Average | 3.3 ± 0.4 |
In order to place on an absolute basis the rate coefficients for the reactions of Cl with the monoterpenes, the following values for the reactions of Cl with the reference compounds at 298 K were used: (3.40 ± 0.28) × 10−10 cm3 per molecule per s for Cl + isobutene25 and (2.64 ± 0.21) × 10−10 cm3 per molecule per s for Cl + propene.25 The errors for the ratios kBVOC/kreference are only the 2σ statistical errors, and the errors in the kBVOC was calculated using the propagation of relative error calculation method.
For both compounds, there is a good agreement between the values of kBVOC determined using two different reference compounds.
Averaging the values of the rate coefficients and taking errors which encompass the extremes of both determinations for each reaction result in the following final values for the reaction rate coefficients at 298 K:
| k1 = (5.5 ± 0.7) × 10−10 cm3 per molecule per s |
| k2 = (3.3 ± 0.4) × 10−10 cm3 per molecule per s |
The errors quoted are twice the standard deviation arising from the least-squares fit of the straight lines, to which a contribution has been added to cover uncertainties in the reference rate coefficients.
Regarding to products identification, for the reaction of β-ocimene with Cl atoms the only identified product was acetone observed as oxime due to the derivatization with PFBHA. Chromatogram and mass spectra are presented on the ESI S1a and S1b,† respectively. For the reaction of camphene with Cl atoms, the positively identified products were formaldehyde and acetone, both were observed as formaldoxime and acetoxime, the chromatogram and mass spectra are presented in the ESI S2a, b, S3a and b,† respectively.
5. Discussion
Kinetics
To the best of our knowledge, no kinetic data on the reaction of Cl atoms with β-ocimene and camphene have been reported. The present study, thus, is the first measurement of the rate coefficients of the reactions (1) and (2) and therefore no direct comparison with the literature can be made for the same reactions. However, the rate coefficients for the reaction of β-ocimene with Cl atoms ((5.5 ± 0.7) × 10−10 cm3 per molecule per s) can be compared with the rate coefficients of the reactions of myrcene + Cl atoms ((6.6 ± 1.5) × 10−10 cm3 per molecule per s),26 taking into consideration the experimental errors, these rate coefficients present a reasonable agreement between them. On the other hand, comparing the reactivity toward Cl atoms of camphene and other bicyclic and exocyclic monoterpene, β-pinene, it can be observed that the value of the rate coefficient for the reactions of camphene + Cl atoms ((3.3 ± 0.4) × 10−10 cm3 per molecule per s) is 1.6 times lower than the rate coefficient for the reaction of β-pinene + Cl atoms ((5.3 ± 1.5) × 10−10 cm3 per molecule per s).27 However, the structure itself of these monoterpenes has a minor influence when they react with Cl atoms, which is expected due to the reactivity of VOCs with Cl atoms is collision-controlled.The rate coefficients of VOCs due to the reactions with Cl atoms and with OH radicals tend to present a linear correlation, in which this linearity indicates that the reaction mechanisms have similar pathway. In Fig. 3, it is shown the correlation between the rate coefficients due to reactions of OH radicals and Cl atoms with acyclic alkenes and monoterpenes (these rate coefficients are presented in Table 2), where the expression obtained is the following (rate coefficient in cm3 per molecule per s, r2 = 0.72):
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH= (3.2 ± 0.6) log kOH= (3.2 ± 0.6) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kCl + (20 ± 6) kCl + (20 ± 6) |
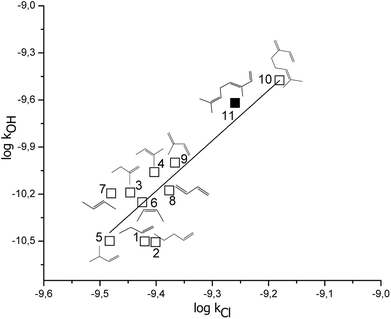 | ||
Fig. 3 Free energy correlation log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH vs. log kOH vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl for acyclic unsaturated terpenes and alkenes, bold square correspond to β-ocimene. Cl for acyclic unsaturated terpenes and alkenes, bold square correspond to β-ocimene. | ||
| No. | Compounds | kOH × 1011 (cm3 per molecule per s) | kCl × 1010 (cm3 per molecule per s) |
|---|---|---|---|
| a Ref. 36.b Ref. 26.c This work.d Ref. 25.e Ref. 28.f Ref. 27. | |||
| 1 | 1-Butene | 3.15a | 3.80d |
| 2 | 1-Pentene | 3.12a | 3.97d |
| 3 | 2-Methyl-1-butene | 6.46a | 3.58d |
| 4 | 2-Methyl-2-butene | 8.72a | 3.95d |
| 5 | 3-Methyl-1-butene | 3.17a | 3.29d |
| 6 | (Z)-2-Butene | 5.60a | 3.76d |
| 7 | (E)-2-Butene | 6.37a | 3.31d |
| 8 | 1,3-Butadiene | 6.66a | 4.20a |
| 9 | Isoprene | 10a | 4.30e |
| 10 | Myrcene | 33.5b | 6.60f |
| 11 | β-Ocimene | 24c | 5.5c |
A reasonable correlation has been obtained, therefore, is an indication of that the presented acyclic alkenes and monoterpenes has a similar mechanism when they react with OH radicals and Cl atoms. Additionally, in this correlation it can be observed that those compounds with a single double bond are group together, and that the reactivity increases when the compound presents two conjugated double bonds and it is even higher for the tri-unsaturated monoterpenes.
In Fig. 4, it is presented the correlation of the rate coefficients for the reactions of cyclic alkenes and terpenes with OH radicals and Cl atoms (these rate coefficients are shown in Table 3), which to our knowledge is the first correlation of this type involving cyclic compounds. The following expression has been obtained (rate coefficient in cm3 per molecule per s, r2 = 0.82):
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH = (1.4 ± 0.2) log kOH = (1.4 ± 0.2) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kCl + (3 ± 2) kCl + (3 ± 2) |
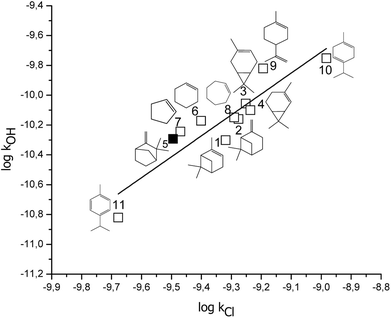 | ||
Fig. 4 Free energy correlation log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH vs. log kOH vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl for cyclic unsaturated terpenes and alkenes, bold square correspond to camphene. Cl for cyclic unsaturated terpenes and alkenes, bold square correspond to camphene. | ||
| No. | Compounds | kOH × 1011 (cm3 per molecule per s) | kCl × 1010 (cm3 per molecule per s) |
|---|---|---|---|
| a Ref. 37.b Ref. 38.c Ref. 39.d This work.e Ref. 36.f Ref. 40.g Ref. 27.h Ref. 41. | |||
| 1 | α-Pinene | 5.00a | 4.80g |
| 2 | β-Pinene | 6.92a | 5.30g |
| 3 | 3-Carene | 8.8b | 5.60g |
| 4 | 2-Carene | 7.95c | 5.80g |
| 5 | Camphene | 5.1d | 3.3d |
| 6 | Cyclohexene | 6.74e | 3.97b |
| 7 | Cyclopentene | 5.71e | 3.39b |
| 8 | Cycloheptene | 7.09e | 5.12b |
| 9 | Limonene | 15.9a | 6.40g |
| 10 | γ-Terpinene | 17.7f | 10.4h |
| 11 | p-Cymene | 1.51c | 2.10g |
This correlation presents good linearity and also indicates similarity on the reaction mechanisms between the reactions of cyclic compounds with Cl atoms and OH radicals, respectively. Both correlations can be used to estimate the value of a rate coefficient when only one of them is available as experimental data.
Product identification
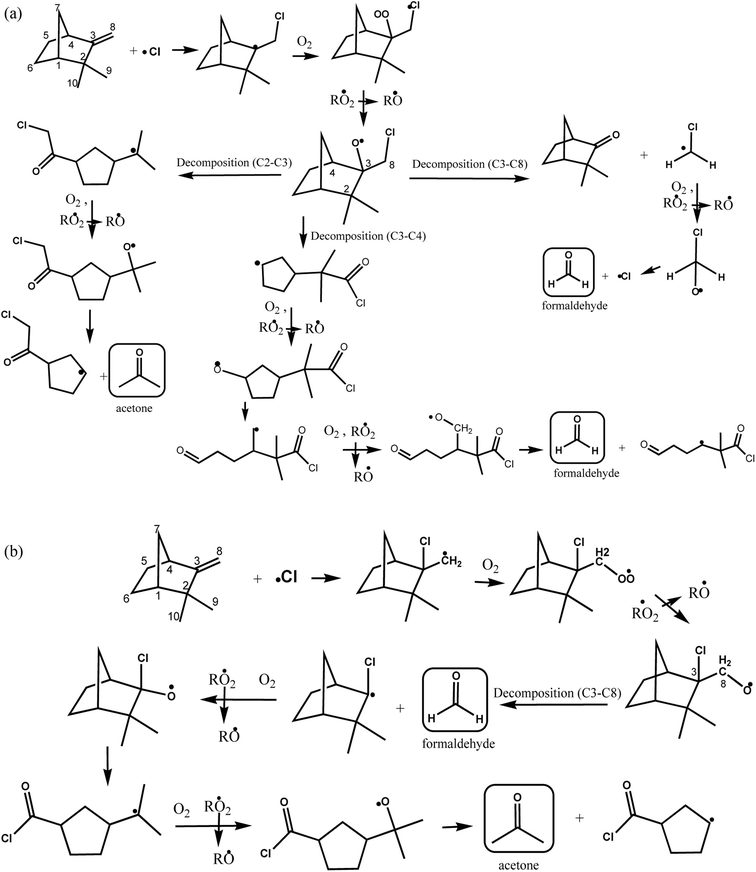
According to the mechanism presented in Fig. 5, the primary formation of acetone can be generated through the addition of Cl atom to the C6 and C7 in the double bond of the group (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in the structure of β-ocimene. However, secondary formation of acetone is also possible since β-ocimene degradation leads to the formation of several products which can also react with Cl atoms (only acetone primary formation is presented in Fig. 5).
CH– in the structure of β-ocimene. However, secondary formation of acetone is also possible since β-ocimene degradation leads to the formation of several products which can also react with Cl atoms (only acetone primary formation is presented in Fig. 5).
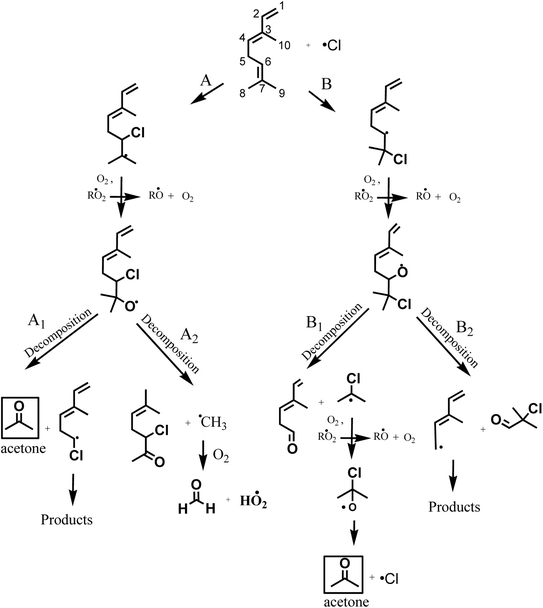 | ||
| Fig. 5 Proposed reaction mechanism for β-ocimene initiated by addition of Cl atom to C6 and C7 on the double bond. | ||
If the addition of Cl atoms is produced in the C6 of the double bond (Fig. 5, channel A), the alcoxy radical formed could decompose forming acetone and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH·Cl radical (channel A1) or decompose forming CH3C(CH3)
CHCH2CH·Cl radical (channel A1) or decompose forming CH3C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH(Cl)C(O)CH3 and ·CH3 radical that could lead to the formaldehyde formation.
CHCH2CH(Cl)C(O)CH3 and ·CH3 radical that could lead to the formaldehyde formation.
When the addition of Cl atoms is produced in C7 the alkoxy radical formed could generate through a decomposition CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2C(O)H and ·C(CH3)2Cl radical (channel B1) that after the subsequent reactions with O2 in the absence of NOx could produce ·OC(CH3)2Cl radical that through an elimination of Cl atom could lead to the formation of acetone. If the alcoxy radical formed decompose by channel B2 of Fig. 5, the products formed will be ClC(CH3)2C(O)H and CH2
CHCH2C(O)H and ·C(CH3)2Cl radical (channel B1) that after the subsequent reactions with O2 in the absence of NOx could produce ·OC(CH3)2Cl radical that through an elimination of Cl atom could lead to the formation of acetone. If the alcoxy radical formed decompose by channel B2 of Fig. 5, the products formed will be ClC(CH3)2C(O)H and CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(CH3)
CHC(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2˙ radical.
CHCH2˙ radical.
According to the products identified in the experimental conditions performed in this work, we have demonstrated that the addition of Cl atoms is produced on C6–C7 of the double bond of β-ocimene leading to the formation of acetone through channels A1 and B1 of Fig. 5.
On the other hand, for the reaction of camphene with Cl atoms, the proposed reaction mechanisms is presented in Fig. 6(a and b) by the addition of Cl atom to the C8 and C3 of this double bond, respectively. Acetone can be formed through decomposition of C2–C3 bond from the formed alkoxide when Cl atom is added to C8 (Fig. 6a), and through decomposition of C3–C8 bond from the alkoxide, which was formed due to the addition of Cl atom to C3 of the double bond (Fig. 6b), while, formaldehyde can be generated through the decomposition of C3–C8 and C3–C4 bonds from the alkoxides (Fig. 6a), and it can also be formed due to the alkoxide decomposition C3–C8 formed from the addition of Cl atom to C3 (Fig. 6b). The degradation of these two studied monoterpenes were expected to generate acetone since they contain on their structures the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)2 (β-ocimene) and
C(CH3)2 (β-ocimene) and ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C(CH3)2 (camphene) entities, product that was also formed due to the reaction of these terpenes with OH radicals (Gaona-Colmán et al., 2016a and 2017)21,23. Ketones and aldehydes are functional groups also reported as products during the reaction of acyclic and cyclic alkenes with Cl atoms.28,29
C(CH3)2 (camphene) entities, product that was also formed due to the reaction of these terpenes with OH radicals (Gaona-Colmán et al., 2016a and 2017)21,23. Ketones and aldehydes are functional groups also reported as products during the reaction of acyclic and cyclic alkenes with Cl atoms.28,29
Atmospheric implications
In Table 4 is presented the estimated tropospheric lifetimes of β-ocimene and camphene respect to their degradations toward the main atmospheric oxidants. These lifetimes have been estimated using the rate coefficients and the typical tropospheric concentrations of the oxidants: [OH] = 2 × 106 molecules per cm3,30 [O3] = 7 × 1011 molecules per cm3,31 [NO3] = 5 × 108 molecules per cm3 (ref. 32) and [Cl] = 1 × 104 molecules per cm3.33 Additionally, it is presented the global lifetimes taking into account the tropospheric losses of the studied compounds according to the following expression:| Compound | kOH × 1011a | τOH (hours) | kO3 × 1018a | τO3 (hours) | kNO3 × 1013a | τNO3 (hours) | kCl × 1010a | τCl (hours) | τglobal (hours) |
|---|---|---|---|---|---|---|---|---|---|
| a k in (cm3 per molecule per s).b Ref. 42.c Ref. 21.d Ref. 26.e Ref. 43.f Ref. 22.g Ref. 44.h This work.i Ref. 23.j Ref. 45.k Ref. 46. | |||||||||
| β-Ocimene | 25b | 0.6 | 385d | 1 | 240g | 0.017 | 5.5h | 51 | 0.02 |
| 23.6c | 0.6 | 556e | 0.7 | ||||||
| 374f | 1 | ||||||||
| Camphene | 5.1i | 3 | 0.51i | 778 | 6.54j | 0.85 | 3.3h | 84 | 0.7 |
| 5.33j | 3 | 0.90j | 441 | ||||||
| 0.45k | 882 | ||||||||
Since photolytic loss of β-ocimene and camphene is not relevant in the troposphere, because they do not absorb in the actinic region, and wet and dry deposition are expected to be of minor importance due to the low solubility in water and they volatility, respectively. The global tropospheric lifetimes was calculated considering the reaction with the oxidants, as it is presented in Table 4, these global lifetimes are very short for both compounds. The calculated tropospheric lifetimes due to reaction with each oxidants listed in Table 4 indicate that the reactions with OH radicals, NO3 radicals and O3 molecules are most important atmospheric degradation pathways for β-ocimene, while the reaction with Cl atoms could be important in regions where they are more available, i.e. coastal region or highly industrialized areas. On the other hand, the main tropospheric oxidation for camphene will occur due to reactions with OH and NO3 radicals, and the degradation due to reactions with Cl atoms could also be important in the previously mentioned region. However, camphene loss due to reaction with O3 is the less relevant tropospheric reactions. According to the identified products obtained in this work, the degradations of β-ocimene and camphene due to reaction with Cl atoms lead to the formation of acetone, one of the most abundant oxygenated compounds in the atmosphere. Acetone roles in the troposphere are the contribution on the HOx cycle and the formation of peroxyacetyl nitrate, a well-known NOx reservoir.34 Additionally, formaldehyde was also an identified product from the reaction of camphene with Cl atoms, thus, this reaction is another photochemical source of formaldehyde in the outdoor air. This aldehyde also contributes to the HOx cycle and is considered one of the main indoor pollutants. It is important to take into account the indoor/outdoor ratio in order to assess the risk for human health due to the exposure of formaldehyde35 and this requires the identification all the primary and secondary sources of this aldehyde in the troposphere.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge the EU project EUROCHAMP, CONICET (Argentina), ANPCyT-FONCYT (Argentina), SECyT-UNC (Córdoba, Argentina) for financial support of this research.References
- J. Kesselmeier and M. Staudt, Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology, J. Atmos. Chem., 1999, 33, 23–88 CrossRef.
- R. Atkinson and J. Arey, Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review, Atmos. Environ., 2003, 37, 197–219 CrossRef.
- C. W. Spicer, E. G. Chapman, B. J. Finlayson-Pitts, R. A. Plastridge, J. M. Hubbe, J. D. Fast and C. M. Berkowitz, Unexpectedly high concentrations of molecular chlorine in coastal air, Nature, 1998, 394, 353–356 CrossRef.
- R. Vogt, P. J. Crutzen and R. A. Sander, A mechanism for halogen release from sea-salt aerosol in the remote marine boundary layer, Nature, 1996, 383, 327–330 CrossRef.
- B. J. Finlayson-Pitts, Chlorine atoms as a potential tropospheric oxidant in the marine boundary layer, Res. Chem. Intermed., 1993, 19, 235 CrossRef.
- G. Sarwar and P. W. Bhave, Modeling the effect of chlorine emissions on ozone levels over the Eastern United States, J. Appl. Meteorol. Clim., 2007, 46, 1009–1019 CrossRef.
- T. P. Riedel, T. H. Bertram, T. A. Crisp, E. J. Williams, B. M. Lerner, A. Vlasenko, S. M. Li, J. Gilman, J. de Gouw, D. M. Bon, N. L. Wagner, S. S. Browm and J. A. Thornton, Nitryl chloride and molecular chlorine in the coastal marine boundary layer, Environ. Sci. Technol., 2012, 46, 10463–10470 CrossRef PubMed.
- G. L. Philips, M. J. Tang, J. Thieser, B. Brickwedde, G. Schuster, B. Bohn, J. Lelieveld and J. N. Crowley, Significant concentrations of nitryl chloride observed in rural continental Europe associated with the influence of sea salt chloride and anthropogenic emissions, Geophys. Res. Lett., 2012, 39, L10811 Search PubMed.
- J. A. Thornton, J. P. Kercher, T. P. Riedel, N. L. Wagner, J. Cozic, J. S. Holloway, W. P. Dubé, G. M. Wolfe, P. K. Quinn, A. M. Middlebrook, B. Alexander and S. S. Brown, A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry, Nature, 2010, 464, 271–274 CrossRef PubMed.
- T. P. Riedel, N. L. Wagner, W. P. Dubé, A. M. Middlebrook, C. J. Young, F. Öztürz, R. Bahreini, T. C. VandenBoer, D. E. Wolfe, E. J. Williams, J. M. Roberts, S. S. Brown and J. A. Thornton, Chlorine activation within urban or power plant plumes: Vertically resolved ClNO2 and Cl2 measurements from a tall tower in a polluted continental setting, J. Geophys. Res.: Atmos., 2013, 118, 8702–8715 Search PubMed.
- C. B. Faxon and D. T. Allen, Chlorine chemistry in urban atmospheres: a review, Environ. Chem., 2013, 10, 221–233 CrossRef.
- C. B. Faxon, J. K. Bean and L. Hildebrandt Ruiz, Inland concentrations of Cl2 and ClNO2 in southeast Texas suggest chlorine chemistry significantly contributes to atmospheric reactivity, Atmosphere, 2015, 6, 1487–1506 CrossRef.
- J. Ouyang, G.-S. Yang, L.-L. Ma, M. Luo, L. Zheng, Q. Huo, Y.-D. Zhao, T.-D. Hu, Z.-F. Cai and D.-D. Xu, Chlorine levels and species in fine and size resolved atmospheric particles by X-ray absorption near-edge structure spectroscopy analysis in Beijing, China, Chemosphere, 2018, 196, 393–401 CrossRef PubMed.
- R. W. Talbot, M. O. Andreae, H. Berresheim, D. J. Jacob and K. M. Beecher, Sources and sinks of formic, acetic, and pyruvic acids over central Amazonia 2. Wet season, J. Geophys. Res., 1999, 95, 16799–16811 CrossRef.
- R. J. Griffin, C. I. David, R. C. Flagan and J. H. Seinfeld, Organic aerosol formation from the oxidation of biogenic hydrocarbons, J. Geophys. Res., 1999, 104, 3555–3567 CrossRef.
- J. D. Fuentes, M. Lerdau, R. Atkinson, D. Baldocchi, J. W. Bottenheim, P. Ciccioli, B. Lamb, C. Geron, L. Gu, A. Guenther, T. D. Sharkey and W. Stockwell, Biogenic hydrocarbons in the atmospheric boundary layer: a review, Bull. Am. Meteorol. Soc., 2000, 81, 1537–1576 CrossRef.
- M. Komenda and R. Koppmann, Monoterpene emissions from Scots pine (Pinus sylvestris): field studies of emission rate variabilities, J. Geophys. Res., 2002, 107(D13), 4161 CrossRef.
- S. M. Noe, P. Ciccioli, E. Brancaleoni, F. Loreto and Ü. Niinemets, Emissions of monoterpenes linalool and ocimene respond differently to environmental changes due to differences in physico-chemical characteristics, Atmos. Environ., 2006, 40, 4649–4662 CrossRef.
- M. Reichstein, M. Bahn, P. Ciais, D. Frank, M. D. Mahecha, S. I. Seneviratne, J. Zscheischler, C. Beer, N. Buchmann, D. C. Frank, D. Papale, A. Rammig, P. Smith, K. Thonicke, M. van der Velde, S. Vicca, A. Walz and M. Wattenbach, Climate extremes and the carbon cycle, Nature, 2013, 500(7462), 287–295 CrossRef PubMed.
- D. F. Zhao, A. Buchholz, R. Tillmann, E. Kleist, C. Wu, F. Rubach, A. Kiendler-Scharr, Y. Rudich, J. Wildt and T. Mentel, Environmental conditions regulate the impact of plants on cloud formation, Nat. Commun., 2017, 8, 14067 CrossRef PubMed.
- E. Gaona-Colmán, M. B. Blanco, I. Barnes and M. A. Teruel, Effect of NOx on product yields and Arrhenius parameters of gas-phase oxidation of β-ocimene initiated by OH radicals, RSC Adv., 2016a, 6, 92795–92803 Search PubMed.
- E. Gaona-Colmán, M. B. Blanco, I. Barnes and M. A. Teruel, Gas-phase ozonolysis of β-ocimene. Temperature dependent rate coefficients and product distribution, Atmos. Environ., 2016b, 147, 46–54 Search PubMed.
- E. Gaona-Colmán, M. B. Blanco, I. Barnes, P. Wiesen and M. A. Teruel, OH- and O3-initiated atmospheric degradation of camphene: Temperature dependent rate coefficients, product yields and mechanisms, RSC Adv., 2017, 7, 2733–2744 RSC.
- I. Barnes, K. H. Becker and N. Mihalopoulos, An FTIR product study of the photooxidation of dimethyl disulfide, J. Atmos. Chem., 1994, 18, 267–289 CrossRef.
- M. J. Ezell, W. Wang, A. A. Ezell, G. Soskin and B. J. Finlayson-Pitts, Kinetics of reactions of chlorine atoms with a series of alkenes at 1 atm and 298 K: structure and reactivity, Phys. Chem. Chem. Phys., 2002, 4, 5813–5820 RSC.
- D. Kim, P. S. Stevens and A. Hites, Rate constants for the gas-phase reactions of OH and O3 with β-ocimene, β-myrcene and α- and β-farnesene as a function of temperature, J. Phys. Chem. A, 2011, 115, 500–506 CrossRef PubMed.
- B. Finlayson-Pitts, C. J. Keoshian, B. Buehler and A. A. Ezell, Kinetics of reaction of chlorine atoms with some biogenic organics, Int. J. Chem. Kinet., 1999, 31, 491–499 CrossRef.
- J. J. Orlando, G. S. Tyndall, E. C. Apel, D. D. Riemer and S. E. Paulson, Rate coefficients and mechanisms of the reaction of Cl-atoms with a series of unsaturated hydrocarbons under atmospheric conditions, Int. J. Chem. Kinet., 2003, 35, 334–353 CrossRef.
- A. Sharma, K. K. Pushpa, S. Dhanya, P. D. Naik and P. N. Bajaj, Rate coefficients and products for gas-phase reactions of chlorine atoms with cyclic unsaturated hydrocarbons at 298 K, Int. J. Chem. Kinet., 2009, 42, 98–105 CrossRef.
- R. Hein, P. J. Crutzen and M. Heinmann, An inverse modeling approach to investigate the global atmospheric methane cycle, Global Biogeochem. Cycles, 1997, 11, 43–76 CrossRef.
- J. A. Logan, Tropospheric ozone: seasonal behavior, trends, and anthropogenic influence, J. Geophys. Res.: Atmos., 1985, 90, 10463–10482 CrossRef.
- Y. Shu and R. Atkinson, Atmospheric lifetimes and fates of a series of sesquiterpenes, J. Geophys. Res., 1995, 100, 7275–7282 CrossRef.
- O. W. Wingenter, M. K. Kubo, N. J. Blake, T. W. Smith, D. R. Blake and F. S. Rowland, Hydrocarbon and halocarbon measurements as photochemical and dynamical indicators of atmospheric hydroxyl, atomic chlorine, and vertical mixing obtained during Lagrangian flights, J. Geophys. Res., 1996, 101, 4331–4340 CrossRef.
- L. Hu, D. B. Millet, S. Y. Kim, K. C. Wells, T. J. Griffis, E. V. Fischer, D. Helmig, J. Hueber and A. J. Curtis, North American acetone sources determined from tall tower measurements and inverse modeling, Atmos. Chem. Phys., 2013, 13, 3379–3392 CrossRef.
- T. Salthammer, Formaldehyde in the Ambient Atmosphere: From an Indoor Pollutant to an Outdoor Pollutant?, Angew. Chem., Int. Ed., 2013, 52, 3320–3327 CrossRef PubMed.
- NIST Chemical Kinetics Database, Standard Reference Database 17, Version 7.0 (Web Version), http://kinetics.nist.gov/kinetics/index.jsp Search PubMed.
- K. J. Gill and R. A. Hites, Rate constants for the gas-phase reactions of the hydroxyl radical with isoprene, α- and β-pinene, and limonene as a function of temperature, J. Phys. Chem. A, 2002, 106, 2538–2544 CrossRef.
- J. G. Calvert, R. Atkinson, J. A. Kerr, S. Madronich, G. K. Moorgat, T. J. Wallington and G. Yarwood, The mechanisms of atmospheric oxidation of alkenes, Oxford University Press, Oxford, 2000 Search PubMed.
- S. B. Corchnoy and R. Atkinson, Kinetics of the gas-phase reactions of OH and NO3 radicals with 2-carene, 1,8-cineoIe, p-cymene, and terpinolene, Environ. Sci. Technol., 1990, 24(10), 1497–1502 CrossRef.
- Y. Shu and R. Atkinson, Rate constants for the gas-phase reactions of O3 with a series of terpenes and OH radical formation from the O3 reactions with sesquiterpenes at 296 ± 2 K, Int. J. Chem. Kinet., 1994, 26, 1193–1205 CrossRef.
- Q. K. Timerghazin and P. A. Ariya, Kinetics of the gas-phase reaction of atomic chlorine with selected monoterpenes, Phys. Chem. Chem. Phys., 2001, 3, 3981–3986 RSC.
- R. Atkinson, S. M. Aschmann and J. N. Pitts Jr, Rate constants for the gas-phase reactions of the OH radical with a series of monoterpenes at 294 K, Int. J. Chem. Kinet., 1986, 18, 287–299 CrossRef.
- R. Atkinson, D. Hasegawa and S. M. Aschmann, Rate constants for the gas-phase reactions of O3 with a series of monoterpenes and related compounds at 296 ± 2 K, Int. J. Chem. Kinet., 1990a, 22, 871–887 Search PubMed.
- R. Atkinson, S. M. Aschmann, A. M. Winer and J. N. Pitts Jr, Kinetics and atmospheric implications of the gas-phase reactions of NO3 radicals with a series of monoterpenes and related organics at 294 ± 2 K, Environ. Sci. Technol., 1985, 19, 159–163 CrossRef.
- R. Atkinson, S. M. Aschmann and J. Arey, Rate constants for the gas-phase reactions of OH and NO3 radicals and O3 with sabinene and camphene at 296 ± 2 K, Atmos. Environ., Part A, 1990b, 24(10), 2647–2654 Search PubMed.
- D. Johnson, A. R. Rickard, C. D. McGill and G. Marston, The influence of orbital asymmetry on the kinetics of the gas-phase reactions of ozone with unsaturated compounds, Phys. Chem. Chem. Phys., 2000, 2, 323–328 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04931a |
| This journal is © The Royal Society of Chemistry 2018 |