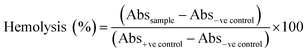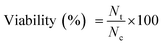Open Access Article
Open Access ArticleDevelopment of banana (Musa balbisiana) pseudo stem fiber as a surgical bio-tool to avert post-operative wound infections†
Himadri Kalita‡
*a,
Ankita Hazarika‡*a,
Raghuram Kandimalla‡b,
Sanjeeb Kalita‡b and
Rajlakshmi Devi *a
*a
aLife Sciences Division, Institute of Advanced Study in Science and Technology, Guwahati, Assam, India. E-mail: kalitahimadri123@gmail.com; ankitahazarikabiotech@gmail.com; Tel: +91-9706107073 Tel: +91-9706053605
bDrug Discovery Laboratory, Institute of Advanced Study in Science and Technology, Guwahati, Assam 781035, India. E-mail: biochemistry.iasst@gmail.com; Tel: +91-9706033567
First published on 31st October 2018
Abstract
The search to develop an ideal suture material encourages us to explore novel suture biomaterials with superior characteristics to the current commercially available products. Surgical sutures play a crucial role in the development of post-operative wound infection by acting as a substrate for biofilm formation which leads to dehisced wounds. In this context, the present invention meets this need by fabricating banana (Musa balbisiana) fibre into an advanced antimicrobials releasing suture biomaterial (BSc) for the prevention of post-operative wound infection. Suture material developed from banana pseudo stem fiber was impregnated with chloramphenicol, clotrimazole and growth factors with the aid of a hydro-gel system. The fabricated suture material was found to be biocompatible towards human erythrocytes and L929 mouse fibroblast cells. BSc exhibited promising physico-chemical characteristics which were comparable to the commercially available Bombyx mori silk fibroin (BMSF) suture. BSc displayed a biphasic release pattern with sustained release of chloramphenicol for up to 140 h. Apart from being environment friendly and having a facile fabrication method, this advanced suture biomaterial showed broad spectrum in vitro antimicrobial activity against bacterial and fungal pathogens. BSc successfully impeded biofilm formation on its surface, as is evident from the confocal microscopy analysis. This contributes to superior wound healing efficacy in terms of reduced microbial burden and a subsequent decrease in the inflammatory cytokine levels. Histopathological observations further supported the pronounced healing efficacy of BSc sutured wounds. The findings of this study establish the banana pseudo stem fiber as a novel advanced suture biomaterial to prevent post-operative wound infections.
1. Introduction
Suture material of natural or synthetic origin can be used for tissue ligation during surgery. Mechanical stability, tissue compatibility, thermal stability, and superior tensile strength are the prerequisites for successful tissue adherence at the healing site.1 These properties depend on the chemical composition of the suture and the surface impregnated additives.1,2 An ideal suture material possessing all of these essential characteristics has not been realized to date. Knot security, tissue reactivity, and tensile strength are the key disadvantages associated with the commercially available suture materials.3,4 Mitigation of the shortcomings of commercially available sutures encouraged us to develop a novel suture biomaterial with superior characteristics. Plant originated fibers are biocompatible and can be developed as a suture material with lower costs, that are eco-friendly and have a facile fabrication process.5 Bananas (Musa balbisiana, family: Musaceae) are mainly available in India, South Asia, Southeast Asia, and Southern China. India is the largest producer of bananas, accounting for 16 million tons per year. Large-scale production of fiber from bananas could tremendously contribute towards the uptake of a banana fiber based industrial venture.6 After harvesting the fruits, the discarded waste of the pseudo stem is processed for banana fiber preparation, which serves as a prospective raw material for textiles, handicrafts or the banana paper manufacturing industry.6 Banana fibers are light weight, have soft fibers,6 and are mainly composed of cellulose, lignin, hemicelluloses, and pectin, and have microfibrillar angles which give superior tensile properties.7An ideal suture material should have an antimicrobial property, which can speed up the healing process, and maintain a sustained drug release to the wound area. Post-operative wound infections remains to be a predominant factor contributing to delayed wound healing processes which leads to prolonged hospital stays.8,9 Surgical sutures are critical in the settlement of microbial colonies and subsequent establishing of infections. Microbial pathogens exploit the suture surface as a substratum for biofilm formation and spread towards deeper tissues through capillary action.1 To prevent suture mediated surgical site infections (SSI), antimicrobial coated sutures with sustained release properties are a valuable alternative strategy. Antibiotics can be applied to sutures in two different ways: passive coating, based on cationic biopolymers that prevent the attachment of bacteria to the suture biomaterial, and active coatings that release drugs into the tissue to inhibit bacterial growth.10,11 Although active strategies are more effective; passive strategies are believed to be more biocompatible than the former. Efficient antimicrobial activity with a profound biofilm obstruction ability can be achieved by coating the biomaterial with a broad spectrum antimicrobial agent like chloramphenicol and clotrimazole, which are active against different wound specific microbes such as Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans.12 Along with antimicrobial agents, impregnation of growth factors in biomedical devices has also been reported to prevent the formation of biofilms.1 However, there has been less significant progress in the development of suture material with sustained drug release for longer duration and efficient wound healing.13 The healing process can be further accelerated by using growth factors and tissue regenerative phyto-constituents on the suture surface.1,14 However, there is not a single suture material that is commercially available with advanced antimicrobial, inherent healing, and controlled drug release properties. The natural hydrogel system, which possesses inherent wound healing characteristics was used in this study to impregnate the suture material with antimicrobial agents.
The present study aims to develop a biocompatible, cost effective suture biomaterial from the waste material of banana pseudo stems by imparting antimicrobial functionalities onto it to achieve accelerated healing of post-operative wounds. The broad spectrum antimicrobial property was achieved by impregnating the suture with antimicrobial drugs (chloramphenicol and clotrimazole) and growth factors (nerve and epithelial growth factors) with the aid of an aloe-vera (AV) and gum acacia (GA) based hydrogel system.
2. Materials and method
2.1. Materials
Banana (Musa balbisiana) fiber was obtained from Ramie research station (22°3′N, 84°24′E), Sorbhog, Assam, India. All of the therapeutic molecules and chemicals used in this study were procured from Sigma Aldrich, USA and Merck, Germany. ELISA kits (IL-1β & TNF-α) were procured from R&D systems, USA. The LIVE/DEAD BacLight bacterial viability kit was purchased from Life technologies, USA.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) at 95 °C for 120 min. After degumming the fiber was washed thoroughly in distilled water to remove the traces of NaOH and dried at 37 °C at 65% relative humidity. Furthermore, the degummed fiber was braided using a manual braiding technique, which was used for the preparation of banana suture (BS). The braided suture was further sterilized using the moist heat sterilization method in an auto clave (121 °C, 100 kPa pressure) for 20 min and kept in a desiccator prior to use.
7) at 95 °C for 120 min. After degumming the fiber was washed thoroughly in distilled water to remove the traces of NaOH and dried at 37 °C at 65% relative humidity. Furthermore, the degummed fiber was braided using a manual braiding technique, which was used for the preparation of banana suture (BS). The braided suture was further sterilized using the moist heat sterilization method in an auto clave (121 °C, 100 kPa pressure) for 20 min and kept in a desiccator prior to use.The coating material was prepared by dissolving 4.4% of the antimicrobial agents (chloramphenicol and clotrimazole at 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), nerve growth factor (250 ng ml−1) and epithelial growth factor (1 ng ml−1) (Sigma, USA) in a mixture of 0.1% hydrogel base. The hydrogel consisted of AV and GA. AV is a semi liquid gel, whereas GA is a natural adhesive agent, together both the materials help the successful impregnation of drugs onto the surface of the suture material. The braided, sterilized banana suture was dip-coated in the coating material and dried at 37 °C for 24 h to form the advanced antimicrobials releasing suture biomaterial (BSc). The percentage of drug impregnation is calculated from eqn (1).
1), nerve growth factor (250 ng ml−1) and epithelial growth factor (1 ng ml−1) (Sigma, USA) in a mixture of 0.1% hydrogel base. The hydrogel consisted of AV and GA. AV is a semi liquid gel, whereas GA is a natural adhesive agent, together both the materials help the successful impregnation of drugs onto the surface of the suture material. The braided, sterilized banana suture was dip-coated in the coating material and dried at 37 °C for 24 h to form the advanced antimicrobials releasing suture biomaterial (BSc). The percentage of drug impregnation is calculated from eqn (1).
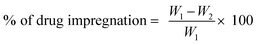 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dissolved in the hydrogel; W2 is the total weight of the remaining drugs in the hydrogel after coating on the suture (BS). W2 is calculated from the concentration values obtained from the calibration curve created using UV spectrophotometric analysis of the samples.
1) dissolved in the hydrogel; W2 is the total weight of the remaining drugs in the hydrogel after coating on the suture (BS). W2 is calculated from the concentration values obtained from the calibration curve created using UV spectrophotometric analysis of the samples.
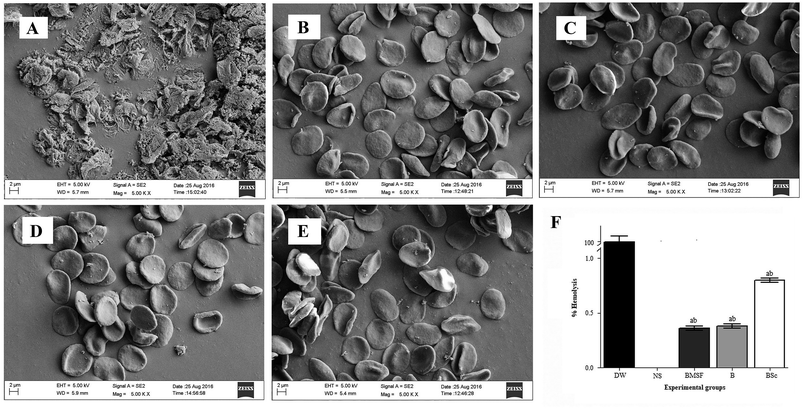
2.2.3.1. Hemocompatibility. A hemolysis experiment was performed according to a standard protocol.15,16 Blood was collected from a healthy human volunteer in sodium citrate siliconized tubes. Informed consent was obtained from the human volunteer before conducting the experiment and the experiment was performed 3 h after collection of the blood. BMSF, BS, and BSc sutures were separately incubated with 10 ml of blood (blood and PBS in 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) for 1 h at 37 °C. After 1 h the tubes were centrifuged and the optical density of the supernatant was measured at 545 nm using a UV-spectrophotometer (Shimadzu, UV 1800, Japan). The experiment was performed in triplicate and the percentage of hemolysis was calculated as follows:
9) for 1 h at 37 °C. After 1 h the tubes were centrifuged and the optical density of the supernatant was measured at 545 nm using a UV-spectrophotometer (Shimadzu, UV 1800, Japan). The experiment was performed in triplicate and the percentage of hemolysis was calculated as follows:2.2.3.2. Effect on morphology of human erythrocyte. Suture materials were incubated with mammalian erythrocyte and its morphology was observed under FESEM.17 after the incubation period (from the above experiment) the pellet of erythrocytes was collected and fixed in 3% glutaraldehyde for 4 h. The fixed cells were washed with 0.2 mM phosphate buffer saline (PBS) and incubated in PBS for 6 h. The incubated cells were subjected to an acetone dehydration process (30, 50, 70, 90, 95 and 100%) and finally treated with tetra methyl saline for 15 min to achieve complete dryness, the powdered erythrocytes were then observed under FESEM.
2.2.3.3. Cytocompatibility. For the cytotoxicity measurement a MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed against a L929 mouse fibroblast cell line (NCCS, Pune, India).15 L929 cells were seeded on 96 well plates (1 × 104 cell per well) in Dulbecco's Modified Eagle's Medium (DMEM) and allowed to attach for 24 h. The cultured cells were then incubated with BMSF, BS, and BSc in DMEM for 24, 48, and 72 h at 37 °C in a humidified incubator. This was followed by removal of the medium and adding MTT dye before being incubated again for 4 h. Finally, DMSO was added to each well and the absorbance was measured at 570 nm using a micro-plate reader. Cells without suture were considered as the positive control. Cell viability was expressed as the percentage of the control according to the following equation:
In which, Nt is the absorbance of the cells treated with sample and Nc is the absorbance of the un-treated cells.
In the agar diffusion method, bacterial and fungal cultures were grown on nutrient agar and sabouraud chloramphenicol agar plates respectively.15 BMSF, BS, and BSc sutures were placed on the agar plates and incubated at 37 °C for 24 h (bacteria) and 28 °C for 72 h (fungus). After completion of the incubation period, the zone of inhibition was measured for each suture sample. The experiment was repeated three times and the results are represented as the mean ± SD.
The direct contact test method was also performed to evaluate the antibacterial ability of BMSF, BS, and BSc.19,20 The test materials (2 cm) were co-inoculated in a nutrient broth medium containing S. aureus and E. coli at a concentration of 107 CFU ml−1 and incubated at 37 °C for 21 days. After the incubation period, the bacterial solution (30 μl) was collected and inoculated on a nutrient agar plate and incubated at 37 °C for 24 h. Furthermore, the culture plates were photographed to evaluate the difference in the microbial colony forming unit (CFU).
In order to examine the live and dead bacterial cell population, after the stipulated period of incubation (7 days) the culture medium was removed and suture samples were washed with PBS and a LIVE/DEAD® assay using Backlight™ (Invitrogen) stain was then performed. During the staining process sutures were kept in the dark for 30 min and then subjected to confocal microscopy analysis.21
2.2.7.1. Experimental animal. The Institutional Animal Ethics Committee (IAEC) of IASST (Institute of Advanced Study in Science and Technology) approved all of the experimental protocols (IASST/IAEC/2015-16/751). All of the experiments were conducted in accordance with CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines. Male Wistar albino rats (10 weeks old, 150–170 g, n = 18) were obtained from the animal house facility of IASST and randomly divided into three experimental groups with six animals each. Animals were housed in individual cages at an ambient temperature of 27 ± 3 °C and a relative humidity of 50 ± 5% with a 12 h light–dark cycle. Rats were given ad libitum access to food and water.23,24
2.2.7.2. Animal grouping. BMSF: animals sutured with BMSF and infected with S. aureus (2 × 108 cells per ml) 24 h after surgery.
BS: animals sutured with BS and infected with S. aureus (2 × 108 cells per ml) 24 h after surgery.
BSc: animals sutured with BSc and infected with S. aureus (2 × 108 cells per ml) 24 h after surgery.
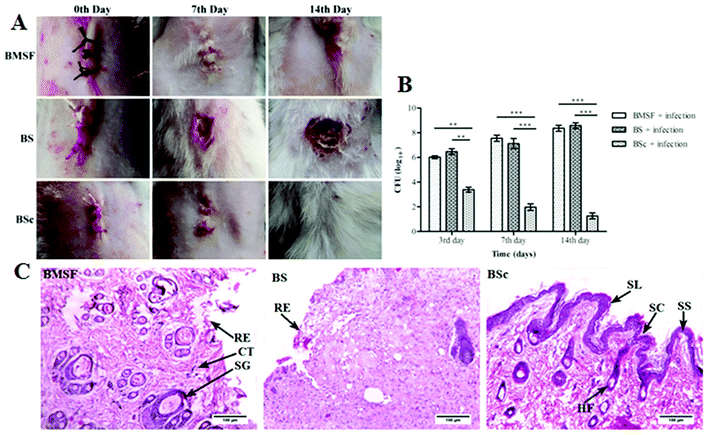
2.2.7.3. Surgical protocol. Animals were anesthetized with a ketamine (80 mg kg−1) and xylazine (10 mg kg−1) cocktail. Hair on the dorsal side of the animals was removed and the skin was cleaned with povidone iodine solution. A 25 mm thickness wound was incised on the dorsal side using a surgical scalpel. The wounded area was closed with BMSF, BS, or BSc and covered with a cotton gauze. After 24 h the wounds were infected by inoculation with 2 × 108 cells per ml of S. aureus. On the third, seventh and 14th post-operative day tissue samples were excised, pulverized, and homogenized in sterile PBS and plated on a nutrient agar medium to tally the CFU count of S. aureus grown after incubation for 24 h at 37 °C.25 Blood was collected using the retro orbital route from all of the animals on the third, seventh, and 14th day and serum was separated to measure the inflammatory markers, tumor necrosis factor (TNF-α) and interleukin (IL)-1β by using ELISA kits from R&D systems as per the instructions given by the manufacturer (Invitrogen). On the 14th post-surgical day the animals were sacrificed and tissue samples from the surgical site were collected and preserved in 10% buffered formalin. Fixed tissues were further subjected to the ethanol dehydration process (30, 70, 90, and 100% v/v) and after processing tissue paraffin blocks were prepared. Paraffin embedded tissues were sectioned at 5 μm thickness using a microtome and stained with hematoxylin–eosin and examined under a bright field microscope (LEICA EC3, Germany).26,27
3. Results and discussion
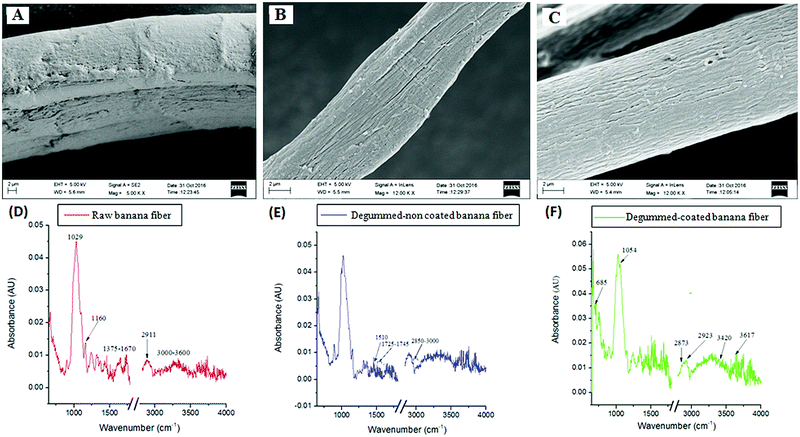
3.1. Physico-chemical characterization of the sutures
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, C–H alkynes and alkane respectively), 1054 cm−1 (C–N stretch, aliphatic amine), 2160 cm−1 (C
C, C–H alkynes and alkane respectively), 1054 cm−1 (C–N stretch, aliphatic amine), 2160 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching), 2111 cm−1 (C
C stretching), 2111 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching), 2860 cm−1 (C–H stretching, alkane), 2923 cm−1 (C–H stretch, alkane), 3429 cm−1 (C–N stretch, amine), and 2921 cm−1 (C–H stretch). Phytochemicals such as aliphatic amines, alkynes, alkanes, carbonyl are mainly present in the innermost part of the plant leaves.34 The presence of alkynes, alkanes and amines comes from the natural base material AV-GA and thus confirmed the presence of the coating material on the surface of the BSc.
C stretching), 2860 cm−1 (C–H stretching, alkane), 2923 cm−1 (C–H stretch, alkane), 3429 cm−1 (C–N stretch, amine), and 2921 cm−1 (C–H stretch). Phytochemicals such as aliphatic amines, alkynes, alkanes, carbonyl are mainly present in the innermost part of the plant leaves.34 The presence of alkynes, alkanes and amines comes from the natural base material AV-GA and thus confirmed the presence of the coating material on the surface of the BSc.| Sample | Max. load (kN) | Stress (MPa) | Stress at auto break (MPa) | Young's modulus (MPa) | Toughness (MPa) | Maximum percent strain | % strain at auto break |
|---|---|---|---|---|---|---|---|
| a All results were expressed in mean ± SD (N = 10). | |||||||
| BMSF | 0.07 ± 0.0068 | 0.50 ± 0.04 | 0.40 ± 0.08 | 5 ± 0.8 | 0.18 ± 0.01 | 38.22 ± 2 | 33.87 ± 4 |
| Suture made with raw fiber | 0.048 ± 0.001 | 0.42 ± 0.004 | 0.32 ± 0.01 | 3.99 ± 0.7 | 0.16 ± 0.001 | 35.23 ± 2 | 28.10 ± 3 |
| Suture made with degummed fiber | 0.05 ± 0.002 | 0.40 ± 0.01 | 0.34 ± 0.007 | 3.68 ± 0.9 | 0.168 ± 0.003 | 35.9 ± 3.1 | 29.56 ± 2 |
| Antimicrobials coated suture | 0.051 ± 0.004 | 0.44 ± 0.06 | 0.33 ± 0.003 | 4.12 ± 0.78 | 0.166 ± 0.004 | 35 ± 4 | 29 ± 3 |
3.2. In vitro biocompatibility evaluation
3.3. Anti-thrombogenic properties
An in vitro blood clotting test was performed to evaluate the antithrombogenic properties of the fabricated suture materials. After incubating the sutures with blood, the clotting experiment was conducted and the weight of the clot was measured at different time intervals (30, 45 and 60 min). Fig. S5 (ESI†) depicts the thrombus formation after incubation of blood with different sutures at different time points. In the control experiment, the thrombus percentage was found to be 2.4 ± 0.1% and 80.43 ± 4% at 30 and 60 min respectively. Incubation of blood with BS and BSc decreases the percentage of thrombus formation. Whereas the percentage of thrombus formed was found to be 2.1 ± 0.34% and 2 ± 0.6% at 30 min and 73 ± 2% and 75 ± 3% at 60 min for BS and BSc respectively. It can be concluded from the above findings that BS and BSc were found to be compatible with human erythrocytes.3.4. Antimicrobial properties
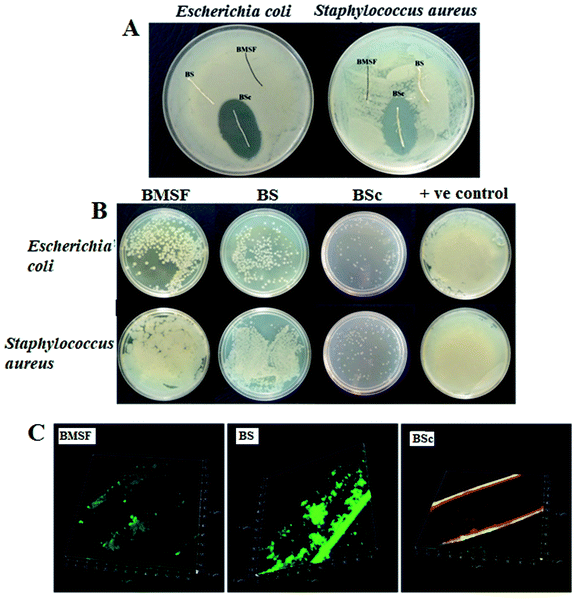
The antimicrobial activity of the BMSF, BS, and BSc sutures were evaluated using the agar diffusion test (Fig. 3A). BSc was found to exhibit a significant antimicrobial activity against the tested pathogenic bacteria E. coli, S. aureus, and opportunistic fungus C. albicans with a clear zone of inhibition (32 ± 2, 29 ± 3, and 6 ± 0.5 mm respectively), whereas the BS and BMSF sutures did not show any inhibition of bacterial growth on its surface. This clearly indicates the successful impregnation of the antimicrobial drugs on the surface of BSc resulting in the inhibited growth of the tested microorganisms. Chloramphenicol is a broad spectrum antibacterial agent which interrupts the bacterial protein synthesis machinery and is active against various bacterial strains like E. coli, and S. aureus. Whereas, clotrimazole acts against a variety of fungal pathogens by disrupting their cell wall permeability. Along with these antimicrobial agents, the natural base materials AV and GA also possess inherent antimicrobial activities. Direct contact of the Gram positive S. aureus and Gram negative E. coli with the sutures for 21 days further established the antimicrobial activity of the BMSF, BS, and BSc sutures. Fig. 3B represents the colonies of the re-cultivated bacteria from the BMSF, BS, and BSc treatment groups. The test results clearly showed a lower number of bacterial colonies on the agar plates for BSc, which significantly indicates the poor survival frequency of E. coli and S. aureus on the plates. The re-cultivated bacterial colonies of BS and BMSF are clearly visualized on the agar medium. The existence of these bacterial colonies implies that bacteria can survive on the BS and BMSF. The presence of the natural (AV and GA) base material and antimicrobial agents (chloramphenicol and clotrimazole) on the BSc prevented the growth of bacteria on the suture,39,40Florescence staining and confocal microscopy analysis was used to visualize and verify the capability of the coating material to fight against viable bacterial colonization and biofilm formation (Fig. 3C). After 21 days of incubation with S. aureus, a large number of viable bacteria (green) and an insignificant number of dead bacteria (red) were observed on the BMSF and BS suture surfaces. On the other hand, the number of viable bacteria was significantly lower on the BSc suture surface. The presence of the antimicrobial agents and the natural base material on BSc inhibits the growth of bacteria on the suture surface and prevented biofilm formation.
3.5. In vitro drug release kinetics
BSc demonstrated a biphasic release pattern with a slow and sustained release of chloramphenicol for up to 144 h in all of the tested pH conditions (Fig. 4). It was observed that the drug loaded on BSc was at a concentration of 8 μg cm−1 of the suture. At pH 7.7, initially at 24 h 35 ± 3% drug was released and this gradually increased with the increase in the incubation period. The initial burst release of the loaded drug was due to the loose association of the drug (antimicrobial agent) with the surface of the base material loaded suture. Also, the PBS, which acts as a physiological saline when used as the drug release medium helps the steady release of the drugs to the target site. After incubation for 144 h, the slope of the curve reached a plateau at 87 ± 3%. Furthermore, at pH 6.3 and 6.8, the suture shows a similar trend in the drug release, in which a release of 70 ± 3% and 78 ± 4% were observed respectively. The natural base materials, AV and GA used together as the drug loading agent, facilitate the sustained drug release.41,42 AV assists the drug release mechanism through a biphasic mode; initially by breaking the drug embedded polymeric matrix, followed by diffusion.41 Also, gum acacia possesses the ability to hold the loaded drug in the wound area for a longer duration.42 To maintain the aseptic condition of the wound for a longer duration of time, sustained release is the most desirable property, which in turn is crucial for accelerated wound healing.22 The drug release process starts before the formation of the anatomical barrier and is completed after the wound healing process.43,44 Studies have reported that the alkaline environment accelerates the hydrolytic degradation which enhances the drug release process.45 The same result has been achieved in this study by release of the maximum percentage of drug (87 ± 3%) at alkaline pH 7.7.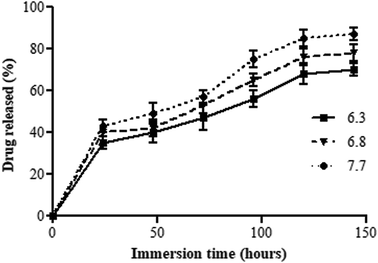 | ||
| Fig. 4 In vitro drug release percentage of BSc in PBS at three different pH values of 6.3, 6.6, and 7.7, up to 144 h. | ||
3.6. Wound healing efficacy analysis
4. Conclusion
In the present study, an attempt was made to develop a novel suture biomaterial from the pseudo stem of bananas which is an agricultural byproduct. Furthermore, the suture was functionalized with an AV-GA based hydrogel containing antimicrobial agents and growth factors. The surface modified suture possesses excellent tensile strength along with the desirable physico-chemical properties of an ideal suture. The fabricated suture was found to be biocompatible and also exhibited the sustained release of drugs for up to 144 h. The BSc suture exhibited a significant antimicrobial activity against infectious microbes such as S. aureus, E. coli, and C. albicans in both in vitro and in vivo conditions. Furthermore, the BSc sutured animals showed pronounced wound healing through the reduction of infection and related inflammatory markers at the wound site. Additionally, the findings of this study could potentially contribute towards the promotion of banana cultivators by adding value to the agricultural waste.Author contributions
All of the first authors made a significant contribution towards the conceptualization of the study and agreed with the content of the manuscript. Himadri Kalita and Ankita Hazarika conceived, designed and performed the experiments, analyzed the data and jointly wrote the manuscript. Raghuram Kandimalla and Sanjeeb Kalita performed the biocompatibility studies, antimicrobial assays, in vivo experiments, and contributed towards the data analysis, manuscript writing and corrections. The final approval of the manuscript was done by Rajlakshmi Devi.Conflicts of interest
A portion of this work reported in this article was used to file an Indian patent application (201631039603) dated 21rd November 2016.Acknowledgements
The authors acknowledge the Department of Science and Technology, Government of India, New Delhi, for financial support and the Institute of Advanced Study in Science and Technology (IASST), Guwahati, Assam, for providing the necessary facilities. We thank Ramie research station, Sarbhog, Assam, India and Mr Haren Medhi for providing the plant fiber. The authors also acknowledge Mr Subrata Goswami, a technical assistant at IASST for evaluating the mechanical properties; Mr Bikash Sarma and Achyut Konwar, PhD scholars at IASST for helping in conducting the FESEM and TGA analysis. All of the authors are grateful to Dr Anupam Banerjee, Manager Application Support, Leica Microsystem and Dr Bula Choudhury, Senior Scientist, Guwahati Biotech Park for their immense help with confocal microscopy.References
- J. Pedro, C. Serrano, L. García-fern, M. Barbeck, S. Ghanaati, R. Unger, J. Kirkpatrick, E. Arzt, L. Funk and P. Tur, Biomaterials, 2015, 52, 291–300 CrossRef PubMed.
- A. Paige, V. Giuseppe and A. Travaglia, J. Inorg. Biochem., 2016, 161, 1–8 CrossRef PubMed.
- C. Justinger, M. R. Moussavian, C. Schlueter, B. Kopp, O. Kollmar and M. K. Schilling, Surgery, 2009, 145, 330–334 CrossRef PubMed.
- K. P. Chellamani, D. Veerasubramanian and R. S. V. Balaji, J. Acad. Ind. Res., 2013, 1, 778–782 Search PubMed.
- R. Kandimalla, S. Kalita, B. Choudhury, D. Devi, D. Kalita, K. Kalita, S. Dash and J. Kotoky, Mater. Sci. Eng., C, 2016, 62, 816–822 CrossRef CAS PubMed.
- N. Venkateshwaran and A. Elayaperumal, J. Reinf. Plast. Compos., 2010, 29, 2387–2396 CrossRef CAS.
- P. Gañán, J. Cruz, S. Garbizu, A. Arbelaiz and I. Mondragon, J. Appl. Polym. Sci., 2004, 94, 1489–1495 CrossRef.
- S. Guo and L. A. Dipietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- X. Liu, T. Lin, J. Fang, G. Yao, H. Zhao, M. Dodson and X. Wang, J. Biomed. Mater. Res., Part A, 2010, 94, 499–508 Search PubMed.
- M. Kazemzadeh-Narbat, B. F. L. Lai, C. Ding, J. N. Kizhakkedathu, R. E. W. Hancock and R. Wang, Biomaterials, 2013, 34, 5969–5977 CrossRef CAS PubMed.
- S. Forbes, A. J. McBain, S. Felton-Smith, T. A. Jowitt, H. L. Birchenough and C. B. Dobson, Biomaterials, 2013, 34, 5453–5464 CrossRef CAS PubMed.
- S. Kalita, R. Kandimalla, B. Devi, B. Kalita, K. Kalita, M. Deka, A. Chandra Kataki, A. Sharma and J. Kotoky, RSC Adv., 2017, 7, 1749–1758 RSC.
- F. Kashiwabuchi, K. S. Parikh, R. Omiadze, S. Zhang, L. Luo, H. V Patel, Q. Xu, L. M. Ensign, H.-Q. Mao, J. Hanes and P. J. McDonnell, Transl. Vis. Sci. Technol., 2017, 6, 1 Search PubMed.
- L. Chun-hui, W. Chang-hai, X. Zhi-liang and W. Yi, Process Biochem., 2007, 42, 961–970 CrossRef.
- A. Konwar, S. Kalita, J. Kotoky and D. Chowdhury, ACS Appl. Mater. Interfaces, 2016, 8, 20625–20634 CrossRef CAS PubMed.
- S. Kalita, R. Kandimalla, A. C. Bhowal, J. Kotoky and S. Kundu, Sci. Rep., 2018, 8, 5778 CrossRef PubMed.
- H. Kalita, A. Hazarika, S. Kalita, R. Kandimalla and R. Devi, RSC Adv., 2017, 7, 32637–32646 RSC.
- A. Jyoti, D. Gogoi, R. Kandimalla, S. Kalita, Y. B. Chaudhari, M. R. Khan, J. Kotoky and J. Chutia, Mater. Sci. Eng. C, 2016, 60, 475–484 CrossRef PubMed.
- J. Li, G. Wang, H. Zhu, M. Zhang, X. Zheng, Z. Di, X. Liu and X. Wang, Sci. Rep., 2014, 4, 4359 CrossRef PubMed.
- A. Konwar, R. Kandimalla, S. Kalita and D. Chowdhury, ACS Sustainable Chem. Eng., 2018, 6, 5806–5817 CrossRef CAS.
- G. Limbert, R. Bryan, R. Cotton, P. Young, L. Hall-Stoodley, S. Kathju and P. Stoodley, Acta Biomater., 2013, 9, 6641–6652 CrossRef CAS PubMed.
- X. Chen, D. Hou, L. Wang, Q. Zhang, J. Zou and G. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 22394–22403 CrossRef CAS PubMed.
- A. Hazarika, H. Kalita, M. Chandra Kalita and R. Devi, Nutrition, 2017, 38, 95–101 CrossRef CAS PubMed.
- H. Kalita, D. C. Boruah, M. Deori, A. Hazarika, R. Sarma, S. Kumari, R. Kandimalla, J. Kotoky and R. Devi, Front. Pharmacol., 2016, 7, 1–19, DOI:10.3389/fphar.2016.00102.
- A. J. Choudhury, D. Gogoi, J. Chutia, R. Kandimalla, S. Kalita, Y. B. Choudhury, M. R. Khan, K. Kalita and J. Kotoky, Surgery, 2016, 159, 539–547 CrossRef PubMed.
- N. Bhardwaj, Y. P. Singh, D. Devi, R. Kandimalla, J. Kotoky and B. B. Mandal, J. Mater. Chem. B, 2016, 4, 3670–3684 RSC.
- A. Hazarika, H. Kalita, D. Chandra, M. Chandra and R. Devi, Nutrition, 2016, 32, 1081–1091 CrossRef CAS PubMed.
- J. T. Kim and A. N. Netravali, Composites, Part A, 2010, 41, 1245–1252 CrossRef.
- S. Kalia, K. Thakur, A. Celli, M. A. Kiechel and C. L. Schauer, J. Environ. Chem. Eng., 2013, 1, 97–112 CrossRef CAS.
- V. K. S. M. Moghaddasi Sharrif, Int. J. Biol. Med. Res., 2011, 2, 466–471 Search PubMed.
- F. Al-Rimawi and M. Kharoaf, Chromatogr. Res. Int., 2011, 2011, 1–6 CrossRef.
- M. Boopalan, M. J. Umapathy and P. Jenyfer, Silicon, 2012, 4, 145–149 CrossRef CAS.
- S. Delvasto, E. F. Toro, F. Perdomo and R. M. de Gutiérrez, Constr. Build. Mater., 2010, 24, 187–192 CrossRef.
- L. S. Kassama, Am. Int. J. Contemp. Res., 2015, 5, 30–39 Search PubMed.
- S. Kalia, B. S. Kaith and I. Kaur, Polym. Eng. Sci., 2009, 49, 1253–1272 CrossRef CAS.
- V. Mhuka, S. Dube and M. M. Nindi, Int. J. Biol. Macromol., 2013, 52, 305–311 CrossRef CAS PubMed.
- S. Henkelman, G. Rakhorst, J. Blanton and W. van Oeveren, Mater. Sci. Eng., C, 2009, 29, 1650–1654 CrossRef CAS.
- E. Carolina, D. João, D. Masi, A. Carlos, L. Campos, F. David, J. De Masi, M. Aurelio, S. Ratti, I. Shin, R. David and J. De Mais, Braz. J. Otorhinolaryngol., 2016, 82, 512–521 CrossRef PubMed.
- A. E. Krausz, B. L. Adler, V. Cabral, M. Navati, J. Doerner, R. A. Charafeddine, D. Chandra, H. Liang, L. Gunther, A. Clendaniel, S. Harper, J. M. Friedman, J. D. Nosanchuk and A. J. Friedman, Nanomedicine, 2015, 11, 195–206 CrossRef CAS PubMed.
- K. A. Juby, C. Dwivedi, M. Kumar, S. Kota, H. S. Misra and P. N. Bajaj, Carbohydr. Polym., 2012, 89, 906–913 CrossRef CAS PubMed.
- M. Tummalapalli, M. Berthet, B. Verrier, B. L. Deopura, M. S. Alam and B. Gupta, Int. J. Biol. Macromol., 2016, 82, 104–113 CrossRef CAS PubMed.
- B. A. Aderibigbe, K. Varaprasad, E. R. Sadiku, S. S. Ray, X. Y. Mbianda, M. C. Fotsing, S. J. Owonubi and S. C. Agwuncha, Int. J. Biol. Macromol., 2015, 73, 115–123 CrossRef CAS PubMed.
- T. Dai, M. Tanaka, Y.-Y. Huang and M. R. Hamblin, Expert Rev. Anti-Infect. Ther., 2011, 9, 857–879 CrossRef CAS PubMed.
- G. Gainza, S. Villullas, J. L. Pedraz, R. M. Hernandez and M. Igartua, Nanomedicine, 2015, 11, 1551–1573 CrossRef CAS PubMed.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS.
- G. E. Magoulas, O. N. Kostopoulou, T. Garnelis, C. M. Athanassopoulos, G. G. Kournoutou, M. Leotsinidis, G. P. Dinos, D. Papaioannou and D. L. Kalpaxis, Bioorg. Med. Chem., 2015, 23, 3163–3174 CrossRef CAS PubMed.
- S. O. Sequeira, C. A. T. Laia, A. J. L. Phillips, E. J. Cabrita and M. F. Macedo, J. Cult. Herit., 2017, 24, 45–52, DOI:10.1016/j.culher.2016.12.004.
- G. Schulze-Tanzil, O. Al-Sadi, E. Wiegand, W. Ertel, C. Busch, B. Kohl and T. Pufe, Scand. J. Med. Sci. Sports, 2011, 21, 337–351 CrossRef CAS PubMed.
- R. Kandimalla, S. Kalita, B. Choudhury, S. Dash, K. Kalita and J. Kotoky, Front. Pharmacol., 2016, 7, 1–8, DOI:10.3389/fphar.2016.00198.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04470h |
| ‡ First authors. |
| This journal is © The Royal Society of Chemistry 2018 |