Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Albatredines A and B, a pair of epimers with unusual natural heterocyclic skeletons from edible mushroom Albatrellus confluens†
Shuaibing Zhanga,
Ying Huangb,
Shijun Hec,
Heping Chena,
Zhenghui Lia,
Bin Wua,
Jianping Zuo*c,
Tao Feng *a and
Jikai Liu*a
*a and
Jikai Liu*a
aSchool of Pharmaceutical Sciences, South-Central University for Nationalities, Wuhan 430074, China. E-mail: tfeng@mail.scuec.edu.cn; jkliu@mail.kib.ac.cn
bLeibniz Research Group – Biobricks of Microbial Natural Product Syntheses, Leibniz Institute for Natural Product Research and Infection Biology (HKI), Adolf–Reichwein–Str. 23, 07745 Jena, Germany
cState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: jpzuo@simm.ac.cn
First published on 2nd July 2018
Abstract
A chemical study of the common species Albatrellus confluens present in Yunnan province, southwest China led to the identification of a pair of epimers named albatredines A (1) and B (2). They feature a natural unprecedented 1,2,4-oxadiazolidin-5-one skeleton. The acyl substitution pattern and complete configurational assignments were deduced from the comparison between experimental and theoretical 13C NMR and ECD data, respectively. Bioassay results showed that compound 1 exhibited a weak immunosuppressive activity against the concanavalin A-induced T lymphocyte cell proliferation (IC50 2.99 μM).
Introduction
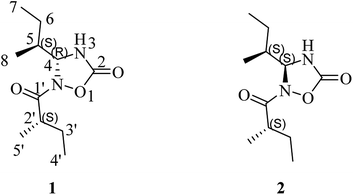
Nitrogen-containing heterocyclic scaffolds are basic and prevalent structural units of many drugs, such as Sovaldi, Abilify, and Nexium.1 Nitrogen-containing compounds originating from mushrooms are a large group of diverse secondary metabolites2,3 (characterized by various chiral structures with different bioactivities), such as enantiomers and epimers. These metabolites are not equally effective in blocking the effect of some receptors and may have a large difference in their ability to cure some diseases, the reason for which is still not known. For instance, in the formation of the peptide for the origin of life, L-amino acids were chosen instead of D-amino acids and D-sugars were selected instead of L-sugars, which was an amazing natural choice.4 However, non-crystallized natural products with multiple chiral centers, particularly the lower molecular weight compounds with more heteroatoms, dwarf the determination of their planar structures and absolute configurations to a large extent.The edible mushroom Albatrellus confluens, which belongs to the family Albatrellaceae, is widely distributed in China. Although the phytochemical investigations on A. confluens have been extensively reported,5–12 an interesting discovery has been made in the lower-polarity fraction of the extractions from the fruiting bodies of A. confluens collected in southwest China, Yunnan, which led to the isolation and structural elucidation of a pair of undescribed nitrogen-containing heterocyclic compounds albatredines A (1) and B (2) (Fig. 1). Structurally, albatredines A and B are quite unprecedented in nature, possessing five-membered 1,2,4-oxadiazolidin-5-one heterocyclic skeletons. Although the efforts to cultivate single crystals of these compounds have failed, the absolute configurations of these compounds were unambiguously determined by ECD calculations as well as 13C NMR calculations.13 Bioassay results indicated that compound 1 exhibited inhibitory activity against concanavalin A-induced T lymphocyte cell proliferation, an important target in the treatment of immunosuppression.
Results and discussion
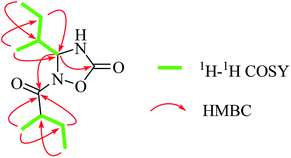
(+)-HRESIMS analysis of 1 revealed a major ion peak at m/z 229.1544, which was consistent with the molecular formula C11H21N2O3 [M + H]+. First inspection of the 1H NMR spectrum (Table 1) evidenced two 2-methylbutyl units with methyl signals at δH 0.92 (t, 3H, J = 7.5 Hz, H-7), 0.98 (d, 3H, J = 6.8 Hz, H-8), 0.95 (t, J = 7.5 Hz, 3H, H-4′) and 1.08 (d, J = 6.9 Hz, 3H, H-5′), which was also supported by the 1H–1H COSY correlations of H-4/H-5/H-6/H-7 and H-5/H-8 together with H-5′/H-2′/H-3′/H-4′ as well as the HMBC correlations of H-8/C-4 and C-6; H-7/C-5, H-6/C-8 and C-4; H-4′/C-2′, H-3′/C-5′, and C-1′; and H-5′/C-1′. The critical HMBC correlations from the proton at δH 5.55 (d, 1H, J = 4.4 Hz, H-4) to C-2 and C-1′ suggested that the two carbonyl moieties (δC 157.1; 182.5) were located at the meta position of C-4 (Fig. 2). Because no HMBC was observed from the H–N signal, we compared the experimental and theoretical 13C NMR chemical shifts of all possible substitution patterns. Choosing the location of the 2-methylbutyryl on one primary amine in five-membered heterocycles afforded two possible substitutions, and forming a four-membered heterocycle afforded one possible substitution. Among the three possible substitution patterns, the most probable locations of the acyls are shown in Fig. 1 with 99.92% confidence (Table S20†).14–17 Accordingly, the planar structure of 1 was determined to be a new natural N-containing derivative bearing a 1,2,4-oxadiazolidin-5-one group.| No. | δCa | δHa | δCb | δHb |
|---|---|---|---|---|
| a Measured in methanol-d4.b Measured in DMSO-d6. | ||||
| 2 | 157.12 | 154.64 | ||
| 4 | 76.58 | 5.55(d, 4.4) | 74.67 | 5.46(dd, 4.7,1.9) |
| 5 | 41.77 | 1.70(m) | 39.8 | 1.59(m) |
| 6 | 24.95 | 1.53(m), 1.16(m) | 23.36 | 1.45(m), 1.08(m) |
| 7 | 11.71 | 0.92(t, 7.4) | 11.22 | 0.91(t, 7.3) |
| 8 | 13.33 | 0.98(d, 6.8) | 12.89 | 0.87(d, 6.8) |
| 1′ | 182.45 | 180.22 | ||
| 2′ | 39.64 | 2.87(m) | 37.63 | 2.78(m) |
| 3′ | 28.02 | 1.66(m), 1.53(m) | 26.39 | 1.59(m), 1.48(m) |
| 4′ | 11.69 | 0.95(t, 7.4) | 11.16 | 0.85(t, 7.4) |
| 5′ | 15.79 | 1.08(d, 6.9) | 15.26 | 1.01(d, 6.9) |
| –NH | 9.16(s) | |||
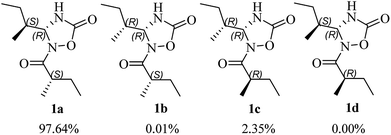
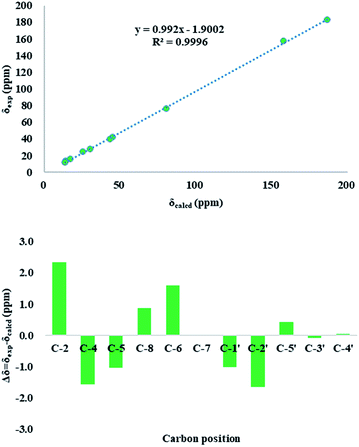
The use of the DP4+ probability was also required to determine the relative configuration of 1. On the basis of the relative configuration, four stereoisomers, (4R,5S,2′S)-1 (1a), (4R,5R,2′S)-1 (1b), (4R,5R,2′R)-1 (1c), (4R,5S,2′R)-1 (1d) were observed. As shown in Fig. 3, the relative configuration was established as 4R*,5S*,2′S* with 97.64% confidence (Table S13†). The results also revealed that the correlation coefficient (R2) between calculated and experimental data from the linear regression analysis was 0.9996, and the root-mean-square deviation (RMSD) was 1.21 ppm, which further provided solid evidence for the structural rationality of 1 (Fig. 4).
Electronic circular dichroism (ECD) calculations were used to determine the absolute configuration of 1 by the time dependent density functional theory (TDDFT) method at the CAM-B3LYP/6-311+G (d,p) level in gas phase. Two stereoisomers, (4R,5S,2′S)-1 (1a) and (4S,5R,2′R)-1 (1f), exist on the basis of the relative configuration. Comparison of theoretically calculated and experimental ECD curves (Fig. 5) showed that the calculated curves of 1a were similar to the experimental data, indicating the absolute configuration of 1 as 4R,5S,2′S.
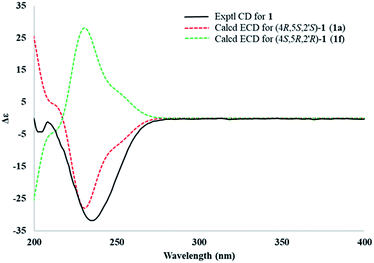 | ||
| Fig. 5 Comparison between the experimental (line) and theoretical (dashed) ECD spectra of the two diastereoisomers of 1. | ||
Compound 2 was isolated as a white powder. Its molecular formula was determined as C11H21N2O3 by HRESIMS, which was the same as that of 1. The NMR data (Tables 1 and 2), UV and IR spectra of 2 and 1 had remarkable resemblances. In addition, the experimental ECD spectra of 1 and 2 also possessed mirror symmetry. All the abovementioned results illustrated that 1 and 2 were a pair of epimers. Correspondingly, calculated ECD (Fig. S27†) and DP4+ probabilities (Table S13†) were also used to tackle the absolute configurations, which were confirmed as 4S,5S,2′S.
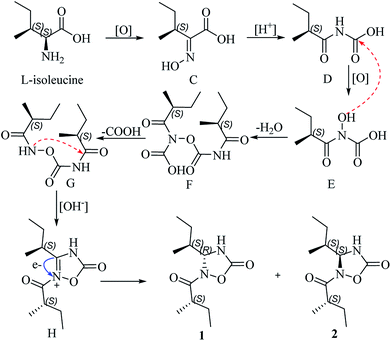
Albatredines A and B represent nor-peptides with unprecedented natural heterocyclic skeletons, which aroused our interest in their plausible biogenesis. Biosynthetically, this pair of epimers might possibly be traced back to L-isoleucine since they possessed this common side chain. The probable biogenetic pathways for 1 and 2 are proposed (Scheme 1). L-isoleucine was oxygenated at the amino site to give the key (S,E)-2-(hydroxyimino)-3-methylpentanoic acid (C), which could induce a rearrangement reaction under acidic condition to afford D. Furthermore, the generation of D was also followed by an oxidation at the amino site to generate E, which proceeded with D to form the intermediate F involving the elimination of water. Correspondingly, a decarboxylative reaction of F took place to produce G, which could trigger an intramolecular SN2 nucleophilic addition in basic condition to yield the intermediate H. The intermediate H further underwent an electron transfer to give a pair of epimers at C-4.
Immunosuppressants are an important class of clinical drugs and an essential prerequisite for successful organ transplantation and treatment of various autoimmune diseases, such as psoriasis, pemphigus, myasthenia gravis, multiple sclerosis, systemic lupus and rheumatoid arthritis.18 Thus, we have evaluated the immunosuppressive activity of 1 and 2 by a previously reported method.19 In the experiment, cyclosporin A (CsA) was used as the positive control. Compound 1 exhibited weak activity against concanavalin A-induced T lymphocyte cell proliferation with an IC50 value of 2.99 μM (Table 3). However, compound 2 did not show any activity, which could explain that there is a difference between the epimers to some extent.
| No. | CC50 (μM) | ConA-induced T-cell proliferation | LPS-induced B-cell proliferation | ||
|---|---|---|---|---|---|
| IC50 (μM) | SIa | IC50 (μM) | SIa | ||
| a SI was determined as the ratio of the concentration of the compound that reduced the cell viability to 50% (CC50) to the concentration of the compound needed to inhibit the proliferation by 50% relative to the control value (IC50). “-” stands for inactive. | |||||
| 1 | 21.05 | 2.99 | 7.04 | 11.46 | 1.84 |
| 2 | >160 | >200 | - | >200 | - |
| CsA | 4.63 | 0.02 | 189.43 | 0.77 | 5.99 |
Conclusions
In summary, chemical investigations on the famous edible mushroom A. confluens were conducted, which resulted in the isolation of a pair of epimers, albatredines A (1) and B (2), bearing a previously undescribed natural heterocyclic scaffold. Their structures were unambiguously determined through extensive spectroscopic analysis in combination with computational approaches. Moreover, immunosuppressive bioassay results suggested that the discovery of new types of heterocyclic compounds such as these from natural sources provides new possibilities to explore new immunosuppressants by the inhibition of T cell proliferation.Experimental section
General experimental procedures
Optical rotations (OR) were recorded on a JASCO P-1020 digital polarimeter (Horiba, Kyoto, Japan). UV/Vis spectra were obtained using a Shimadzu UV2401PC spectrometer (Shimadzu, Kyoto, Japan). CD spectra were obtained on an Applied Photophysics Chirascan Circular Dichroism Spectrometer (Applied Photophysics Limited, Leatherhead, Surrey, UK). IR spectra were obtained using a Bruker Tensor 27 FT-IR spectrometer (Bruker Optics, Inc., Billerica, MA) with KBr pellets. HR ESI-MS spectra were recorded on an Agilent 6200 Q-TOF MS system (Agilent Technologies, Santa Clara, CA, USA). NMR spectra were obtained on a Bruker Avance III 600 MHz spectrometer (Bruker BioSpin GmbH, Karlsruhe, Germany). Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd., P. R. China) and Sephadex LH-20 (Amersham Biosciences, Sweden) were used for column chromatography (CC). Medium-pressure liquid chromatography (MPLC) was performed on a Büchi Sepacore System equipped with pump manager C-615, pump modules C-605 and a fraction collector C-660 (Büchi Labortechnik AG, Switzerland), and columns packed with Chromatorex C-18 (40–75 μm, Fuji Silysia Chemical Ltd., Japan). Preparative HPLC was performed on an Agilent 1260 liquid chromatography system equipped with two types of Zorbax SB-C18 columns (9.4 mm × 150 mm and 21.2 mm × 150 mm, particle size 5 μm). Chiral phase HPLC analysis/preparation was performed on an Agilent 1100 liquid chromatography system equipped with a Diacel CHIRAL-PAK AS-H column (particle size 5 μm, dimensions 4.6 mm × 250 mm).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–1
0–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford sixteen fractions (A–P). Fraction C (2.2 g) was subjected to MPLC with a gradient solvent system of MeOH/H2O (v/v 20
1) to afford sixteen fractions (A–P). Fraction C (2.2 g) was subjected to MPLC with a gradient solvent system of MeOH/H2O (v/v 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80–100
80–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 25 mL min−1) to obtain 20 subfractions (C1–C20). Subfraction C9 (150 mg) was further purified by Sephadex LH-20 (MeOH) to give 12 subfractions (C9-1 to C9-12). Generation of C9-5 was followed by prep-HPLC to yield compounds 1 (4.4 mg, MeCN–H2O: 28%, 20 min, 10 mL min−1) and 2 (4.9 mg, MeCN–H2O: 30%, 18 min, 10 mL min−1).
0, 25 mL min−1) to obtain 20 subfractions (C1–C20). Subfraction C9 (150 mg) was further purified by Sephadex LH-20 (MeOH) to give 12 subfractions (C9-1 to C9-12). Generation of C9-5 was followed by prep-HPLC to yield compounds 1 (4.4 mg, MeCN–H2O: 28%, 20 min, 10 mL min−1) and 2 (4.9 mg, MeCN–H2O: 30%, 18 min, 10 mL min−1).
Albatredine A (1). White powder; [α]20D − 179.6 (c 0.10 MeOH); UV (MeOH) λmax (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 208 nm (3.91) CD (MeOH) λ (Δε) 235 (−39.6); IR (KBr) νmax 3290, 2969, 1785, 1701, 1462, 1203, 937 cm−1; HRESI(+)MS m/z 229.1544 [M + H]+ (calcd. for C11H20N2O3H, 229.1547).
ε) 208 nm (3.91) CD (MeOH) λ (Δε) 235 (−39.6); IR (KBr) νmax 3290, 2969, 1785, 1701, 1462, 1203, 937 cm−1; HRESI(+)MS m/z 229.1544 [M + H]+ (calcd. for C11H20N2O3H, 229.1547).
Albatredine B (2). White powder; [α]20D + 56.3 (c 0.10 MeOH); UV (MeOH) λmax (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 207 nm (3.20); CD (MeOH) λ (Δε) 234 (+31.8); IR (KBr) νmax 3286, 2968, 1786, 1700, 1462, 1203, 937 cm−1; HRESI(+)MS m/z 229.1547 [M + H]+ (calcd. for C11H20N2O3H, 229.1547).
ε) 207 nm (3.20); CD (MeOH) λ (Δε) 234 (+31.8); IR (KBr) νmax 3286, 2968, 1786, 1700, 1462, 1203, 937 cm−1; HRESI(+)MS m/z 229.1547 [M + H]+ (calcd. for C11H20N2O3H, 229.1547).
Immunosuppressive activities assay
Computational methods
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81561148013, 81373289), and the Key Projects of Technological Innovation of Hubei Province (No. 2016ACA138). Computational resources used in this work were supported in part by the HPC Center, Kunming Institute of Botany, CAS, China and the Supercomputing Environment of Chinese Academy of Science (ScGrid).Notes and references
- P. Martins, J. Jesus, S. Santos, L. R. Raposo, C. Roma-Rodrigues, P. V. Baptista and A. R. Fernandes, Molecules, 2015, 20, 16852–16891 CrossRef PubMed.
- J. Liu, Chem. Rev., 2005, 105, 2723–2744 CrossRef PubMed.
- M. Jiang, T. Feng and J. Liu, Nat. Prod. Rep., 2011, 28, 783–808 RSC.
- H. J. Zhu, Organic Stereochemistry: Experimental and Computational Methods, Wiley-VCH, 2015 Search PubMed.
- Z. Ding, Z. Dong and J. Liu, Helv. Chim. Acta, 2001, 84, 259–262 CrossRef.
- H. Guo, Z. Li, T. Feng and J. Liu, J. Asian Nat. Prod. Res., 2015, 17, 107–113 CrossRef PubMed.
- W. Yang, J. Liu, Q. Chen, Y. Liu, Z. Ding, Z. Shen and Z. Chen, Planta Med., 2003, 69, 715–719 CrossRef PubMed.
- M. Ye, X. Luo, L. Li, Y. Shi, M. Tan, X. Weng, W. Li, J. Liu and Y. Cao, Cancer Lett., 2007, 258, 199–207 CrossRef PubMed.
- Z. Zhou, R. Liu, M. Jiang, L. Zhang, Y. Niu, Y. Zhu, Z. Dong and J. Liu, Chem. Pharm. Bull., 2009, 57, 975–978 CrossRef PubMed.
- F. Wang, D. Luo and J. Liu, J. Antibiot., 2005, 58, 412–415 CrossRef PubMed.
- H. Kawagishi, A. Tanaka, K. Sugiyama, H. Mori, H. Sakamoto, Y. Ishiguro, K. Kobayashi and M. Uramato, Phytochemistry, 1996, 42, 547–548 CrossRef PubMed.
- X. Yang, C. Qin, F. Wang, Z. J. Dong and J. Liu, Chem. Biodiversity, 2008, 5, 484–489 CrossRef PubMed.
- Z. Zhao, H. Chen, B. Wu, L. Zhang, Z. Li, T. Feng and J. Liu, J. Org. Chem., 2017, 82, 7974–7979 CrossRef PubMed.
- P. O. Guillen, K. B. Jaramillo, G. Gentajouve, F. Sinniger, J. Rodriguez and O. P. Thomas, Org. Lett., 2017, 19, 1558–1561 CrossRef PubMed.
- M. M. Zanardi, A. G. Suárez and A. M. Sarotti, J. Org. Chem., 2017, 82, 1873–1879 CrossRef PubMed.
- N. Grimblat, M. M. Zanardi and A. M. Sarotti, J. Org. Chem., 2015, 80, 12526–12534 CrossRef PubMed.
- S. G. Smith and J. M. Goodman, J. Am. Chem. Soc., 2010, 132, 12946–12959 CrossRef PubMed.
- N. A. Dangroo, J. Singh, A. A. Dar, N. Gupta, P. K. Chinthakindi, A. Kaul, M. A. Khuroo and P. L. Sangwan, Eur. J. Med. Chem., 2016, 120, 160–169 CrossRef PubMed.
- Y. Fan, L. Gan, H. Liu, H. Li, C. Xu, J. Zuo, J. Ding and J. Yue, Org. Lett., 2017, 19, 4580–4583 CrossRef PubMed.
- H. Goto and E. Osawa, J. Am. Chem. Soc., 1989, 111, 8950–8951 CrossRef.
- H. Gotō and E. Ōsawa, J. Chem. Soc., Perkin Trans. 2, 1993, 187–198 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- T. Bruhn, A. Schaumlöffel, Y. Hemberger and G. S. Bringmann, SpecDis, version 1.60; University of Würzburg, Würzburg, Germany, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: One PDF document. See DOI: 10.1039/c8ra04226h |
| This journal is © The Royal Society of Chemistry 2018 |