Open Access Article
Open Access ArticleInsight into the capacity fading of layered lithium-rich oxides and its suppression via a film-forming electrolyte additive†
Jianhui Li,
Lidan Xing *,
Zaisheng Wang,
Wenqiang Tu,
Xuerui Yang,
Yilong Lin,
Yuqing Liao,
Mengqing Xu and
Weishan Li
*,
Zaisheng Wang,
Wenqiang Tu,
Xuerui Yang,
Yilong Lin,
Yuqing Liao,
Mengqing Xu and
Weishan Li *
*
Engineering Research Center of MTEES (Ministry of Education), Research Center of BMET (Guangdong Province), Key Lab. of ETESPG (GHEI), Innovative Platform for ITBMD (Guangzhou Municipality), School of Chemistry and Environment, South China Normal University, Guangzhou 510006, China. E-mail: xingld@scnu.edu.cn; liwsh@scnu.edu.cn
First published on 18th July 2018
Abstract
The capacity fading of layered lithium-rich oxide (Li1.2Mn0.54Ni0.13Co0.13O2, LLO) cathodes greatly hinders their practical application in next generation lithium ion batteries. It has been demonstrated in this work that the slow capacity fading of a LLO/Li cell within 120 cycles is mainly caused by electrolyte oxidation and LLO phase transformation with Ni dissolution. After 120 cycles, the dissolution of Mn becomes worse than that of Ni, leading to structural destruction of the generated spinel phase structure of LLO and fast capacity fading. Tripropyl borate (TPB) is proposed as a film-forming electrolyte additive, which shows a great capability to enhance the cycling stability of LLO/Li, with a capacity retention improvement from 21% to 78% after 250 cycles at 0.5C. Electrochemical and physical characterization demonstrated that the TPB-derived SEI film shows great capability to suppress electrolyte oxidation and the structural destruction of the generated spinel phase of LLO.
1. Introduction
Lithium ion batteries (LIBs) have been applied as the most promising energy storage/conversion system because of their high operating voltage, high energy density and cost-effectiveness.1–4 Although LIBs have been successfully commercialized, the energy density of LIBs needs to be improved to satisfy the demand of large-scale devices, such as electric vehicles (EVs) and hybrid electric vehicles (HEVs).5–10 The energy density of conventional LIBs, with the cathodes, such as LiCoO2 (∼140 mA h g−1) and LiFePO4 (∼160 mA h g−1), operating at 3.3–3.9 V, is limited.11,12 To achieve higher energy density, layered lithium-rich oxide (LLO) cathodes with chemical compositions of xLi2MnO3·(1 − x)LiMO2 (M = Co, Ni or Mn, 0 < x < 1) are held in high esteem because of their high operating voltage (4.8 V) and high reversible specific capacity (200 mA h g−1).13 However, the application of LLOs has been greatly restricted due to their poor cycling stability, caused by the irreversible layered-to-spinel phase transformation upon cycling.14–16 Moreover, carbonate-based electrolytes start to undergo oxidation at potentials beyond 4.3 V, generating polymers, gaseous products and HF. The former decomposition products increase the interfacial resistance and battery internal pressure, while the latter accelerates the structural transformation of the LLO.17–22Great efforts to improve the structural and cycling stability of LLOs have been made, including surface coating (such as with Al2O3, ZrO2, AlF3, CaF2 and polyaniline),23–27 Na, K, La and Mg element doping28–31 and the application of film-forming electrolyte additives.32–36 The usage of a film-forming electrolyte additive, in comparison with the other methods, has the advantages of simple operation, high performance, and cost-effectiveness,37 and the additive works by decomposing before the carbonate-based electrolyte to generate a solid electrolyte interface (SEI) film on the high voltage electrode surface. This SEI film effectively passivates the highly charged electrode surface, and therefore suppresses the direct electronic contact of the carbonate-based electrolyte with the electrode. The investigated film-forming electrolyte additives mainly include nitriles (such as 1,3,6-hexanetricarbonitrile and glycol bis(propionitrile) ether),38,39 phosphites (such as trimethyl phosphite, triethyl phosphite, triphenyl phosphite and tris(2,2,2-trifluoroethyl) phosphite)40–43 and silicohydrides (such as (trimethylsilyl)methanesulfonate and tris(trimethylsilyl) borate),44,45 which improve the cycling stability of LLOs to various extents. Furthermore, our previous work demonstrated that boracic electrolyte additives, such as trimethyl borate (TMB) and triethyl borate (TEB), show great capability to enhance the cycling stability of LLOs.33,35 Although tripropyl borate (TPB), whose structure is similar to that of TMB and TEB, has been demonstrated to improve the capacity retention and rate capability of a MCMB/Li1.1[Mn1/3Ni1/3Co1/3]0.9O2 full cell as an anion receptor additive,46 no prior study has been performed to investigate its film-forming capability and influence on the cycling stability of high voltage LLOs.
In this work, the film-forming mechanism of TPB and its influence on the long-term cycling performance of a LLO were investigated systematically. Electrochemical and physical characterization demonstrated that the structural destruction of the LLO within and after 120 cycles is mainly caused by the dissolution of Ni and Mn ions, respectively. Addition of a small amount of TPB additive effectively suppresses the electrolyte oxidation and structural destruction of the LLO via generating a protective compact SEI film on the LLO surface, resulting in a greatly improved cycling stability of the high voltage LLO. Importantly, the capacity retention of the LLO achieved by the application of TPB is higher than most of the reported literature values, as shown in Table S1 (see ESI†).
2. Experimental
2.1 Electrode and electrolyte preparation
In this work, LLO (Li1.2Mn0.54Ni0.13Co0.13O2) powders were synthesized by a sol–gel method, as in our previous works.35,47 For the preparation of the LLO cathode and assembly of Li/LLO refer to our previous work.35The standard (STD) (Tinci, China) electrolyte consists of 1.0 M LiPF6 in a mixed solvent of ethylene carbonate (EC)/ethyl methyl carbonate (EMC)/diethyl carbonate (DEC) (3/5/2 by weight). Various amounts of TPB (1, 2, 3, and 4 wt%) (Aladdin, China) were added into the STD electrolyte to obtain an additive-containing electrolyte. For the preparation of all the electrolytes, refer to our previous work.35
2.2 Electrochemical measurement and physical characterization
The charging/discharging tests and electrochemical impedance measurement (EIS) of the Li/LLO coin cells were performed as in our previous work.35 The V-type cells were charged at 0.1C with a cut-off voltage of 4.8 V and then maintained at 4.8 V for 3 days.To understand the capacity fading mechanism of the LLO electrodes upon short- and long-term cycling, nuclear magnetic resonance (NMR, Agilent VNMRS-600 spectrometer, USA), X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, USA), inductively coupled plasma atomic emission spectrometry (ICP AES, IRIS Intrepid II XSP, USA), scanning electron microscopy (SEM, JSM-6510, Japan) and transmission electron microscopy (TEM, JEM-2100, Japan) were performed. 19F NMR spectra were acquired to clarify the role of TPB in suppressing electrolyte oxidation and decomposition after storage at 4.8 V (V-type cell) with acetonitrile-d3. 19F NMR resonances were referenced to LiPF6 at −74 and −76 ppm.48 DMC solvent was used to wash off the residual electrolyte on the cycled electrodes before subsequent physical characterizations.
3. Results and discussion
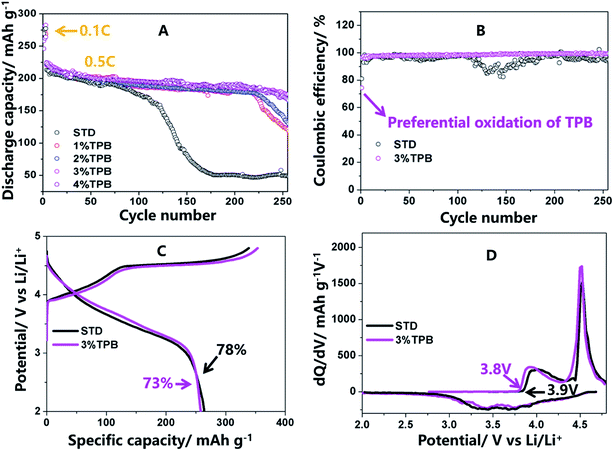
Fig. 1 presents the cycling stability, coulombic efficiency, initial charge/discharge profiles and corresponding dQ/dV curves of the LLO/Li cells in electrolytes with and without TPB. It can be seen from Fig. 1A that the discharge capacity of LLO cycled in STD electrolyte declines slowly within 120 cycles at 0.5C, which is mainly caused by the continuous decomposition of the STD electrolyte at high voltage and the layered-to-spinel phase transformation of the LLO.32,33,36,49 However, after 120 cycles, the capacity of LLO with the STD electrolyte drops dramatically, resulting in a capacity retention of 21% after 250 cycles, which could be ascribed to the further structural damage of the generated spinel phase.32,33,36 Interestingly, the addition of the TPB additive greatly suppressed the capacity fading of the LLO/Li cell. Specifically, the capacity retention of LLO/Li cells with 1%, 2%, 3% and 4% TPB-containing electrolytes was 55%, 59%, 78% and 76% after 250 cycles. The inferior cycling stability of the LLO/Li cells with 1% and 2% TPB-containing electrolytes can be explained by the inadequate amount of TPB additive. The capacity retentions of the LLO/Li cells with 3% and 4% TPB additive are similar and obviously higher than that of the 1% and 2% cells, indicating that 3% TPB additive is sufficient for LLO/Li cells. Next, the mechanism via which the 3% TPB additive improved the cycling stability of the LLO/Li cell was systematically investigated in this work.As shown in Fig. 1B and C, the initial coulombic efficiency of the LLO/Li cell cycling in 3% TPB-containing electrolyte is 5% lower than that cycling in the STD electrolyte, suggesting the co-decomposition reaction of the TPB additive.32,33,35,36,50 Importantly, in comparison with the STD electrolyte, the subsequent coulombic efficiency of the LLO/Li cell with 3% TPB-containing electrolyte becomes higher and steadier, confirming the great capability of the TPB additive to improve the interfacial stability of high voltage electrodes/electrolytes. The initial charging/discharging profiles (Fig. 1C) and the corresponding dQ/dV curves (Fig. 1D) of the LLO/Li cells consist of two stages: the stage below 4.5 V is ascribed to the oxidation of Ni2+ and Co3+, and the other long voltage plateau at around 4.5 V corresponds to the activation of Li2MnO3.41 The slightly lower onset oxidation potential of LLO/Li with the TPB additive can be observed from Fig. 1D, suggesting the preferential oxidation of the TPB additive.
Fig. 2 presents the impedance spectra of LLO/Li cells after 3, 120 and 250 cycles, which consist of a depressed semicircle at high frequency and a sloped line at low frequency. The semicircle reflects the electrode/electrolyte interfacial impedance, while the sloped line represents the diffusion of lithium ions in the electrode.51 After 3 cycles, the difference between the interfacial resistances of the LLO/Li cells with and without the additive is negligible. However, the interfacial resistance of the LLO/Li cell in STD electrolyte keeps growing during cycling. It can be noted that the increase of the interfacial resistance of the LLO/Li with STD electrolyte is slow and fast before and after 120 cycles, which is consistent with the change of capacity fading of LLO/Li with STD electrolyte shown in Fig. 1A. The slow increase of resistance before 120 cycles can be ascribed to the rise of electrode polarization caused by electrolyte decomposition, while the one after 120 cycles is due to the breakdown of the electronic conduction network (shedding of the LLO particulates).36 In contrast, the interfacial resistance of the LLO/Li cell with TPB stays almost constant in the subsequent cycles, indicating that the TPB additive not only suppresses the electrolyte oxidation and decomposition, but also hinders the structural destruction of the LLO.
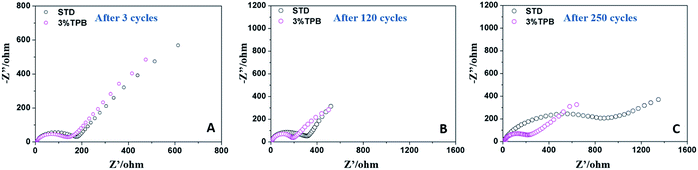 | ||
| Fig. 2 Electrochemical impedance spectra of the LLO after cycling in STD and 3% TPB-containing electrolytes after 3 cycles (A), 120 cycles (B) and 250 cycles (C). | ||
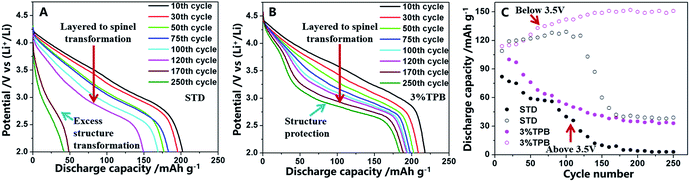
Selected discharge curves and the intersected discharge capacity above and below 3.5 V are presented in Fig. 3. As shown in Fig. 3A and B, in contrast to the cell with the STD electrolyte, whose voltage platform almost disappeared at the end of the 250th cycle, the discharge platform of the LLO/Li cell with 3% TPB-containing electrolyte drops to around 3.0 V and is maintained well in the subsequent cycles. The voltage decay to 3.0 V has been proved to be ascribed to the phase transformation from a layered to a spinel-like structure of LLO.36,52 Therefore, the change of the discharge voltage platform reveals that 3% TPB greatly improves the stability of the generated spinel phase structure of LLO, resulting in high capacity retention as shown in Fig. 1A. According to the reported literature,49,52 the discharge capacity of a LLO/Li cell includes capacity above and below 3.5 V, which is ascribed to the lithium deintercalation from the layered and spinel structure of the LLO, respectively. As shown in Fig. 3C, the discharge capacity of the LLO above 3.5 V decreases monotonously during cycling, while the capacity below 3.5 V shows the reverse effect, confirming the phase transformation of the LLO during cycling.49 Surprisingly, the capacity of the LLO/Li with the STD electrolyte, both above and below 3.5 V, decreases dramatically after 120 cycles, which clearly indicates the structural destruction of the LLO, including the remaining layered and generated spinel structure. This observation is in good agreement with the change of interfacial reaction resistance. However, after 170 cycles, the capacity of the LLO/Li with the TPB additive above and below 3.5 V stabilizes at around 32 and 150 mA h g−1, respectively, further confirming the great capability of the TPB additive to enhance the structural stability of the LLO, especially the generated spinel structure. The coexistence of two phase structures of LLO with the TPB additive reveals that the layered-to-spinel phase transformation reaction could be inhibited by stabilizing the generated spinel phase.
In our previous work, we have theoretically and experimentally demonstrated that the oxidation reaction of the carbonate-based electrolyte generates HF, which would accelerate the dissolution of transition metal ions from the cathode.36,53–55 Therefore, the ability of TPB to improve the cycling stability of LLO/Li cells may also rely on its suppression of electrolyte oxidation and the generation of HF. Fig. 4 presents the 19F NMR spectra of the STD and 3% TPB-containing electrolytes in a V-type cell after maintaining a constant voltage of 4.8 V for 3 days. The peaks at −74 and −76 ppm in the 19F NMR spectra are the characteristic peaks of LiPF6, which both appear in the STD and 3% TPB-containing electrolytes. The appearance of LiPO2F2 (−83 and −86 ppm), OPF3 (−87 and −90 ppm) and HF (190 ppm)56 confirms the oxidation of the electrolyte after storage at high voltage. It can be easily seen from Fig. 4 that the peaks of LiPO2F2, OPF3 and HF in the 3% TPB-containing electrolyte after storage are obviously weaker than those of the STD electrolyte, confirming that the preferential oxidation of TPB suppresses the oxidation of the STD electrolyte and the subsequent generation of HF, which may result in less transition metal dissolution. Indeed, as shown in Fig. 5, the detected content of Mn, Ni and Co on the lithium plate cycled with TPB is obviously lower in comparison to that of the STD electrolyte. It is important to mention that after 120 cycles, for the lithium plate with the STD electrolyte, the detected content of Ni is higher than that of Mn, while after 250 cycles, the content of Mn becomes obviously higher than that of Ni. In combination with the cycling stability of the LLO/Li cell with STD electrolyte shown in Fig. 1A, it can be concluded that the structural destruction of the LLO cell during short and long-term cycling is mainly caused by the dissolution of Ni and Mn, respectively.
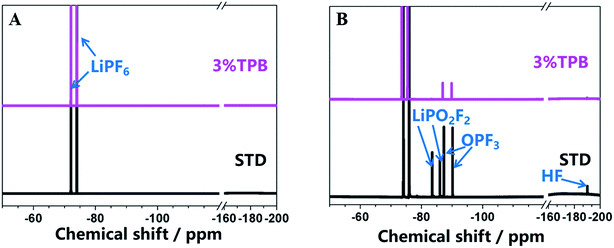 | ||
| Fig. 4 19F NMR spectra of the STD and 3% TPB-containing electrolyte before (A) and after (B) the V-cell test. | ||
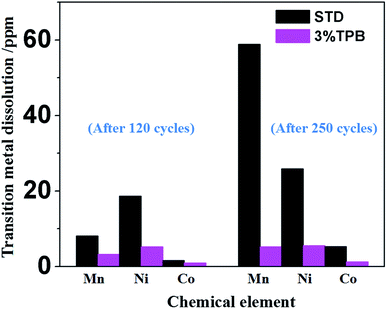 | ||
| Fig. 5 Contents of the deposited transition metals on the lithium electrode after 120 cycles and 250 cycles in STD and 3% TPB-containing electrolytes. | ||
The interfacial composition of the cycled LLO electrodes was analyzed by XPS, with the obtained results presented in Fig. 6. The C 1s, O 1s, F 1s and P 2p XPS spectra of fresh LLO and the LLO after 250 cycles in STD and 3% TPB-containing electrolytes are given in Fig. 6A. In the C1s spectra, the peaks at 290.3 and 285.7 eV correspond to the PVDF binder, while the peak at 284.8 eV is ascribed to acetylene black. The peak intensity of PVDF and acetylene black after cycling with the STD electrolyte is weaker than that after cycling with the 3% TPB-containing electrolyte, indicating that the deposition of the electrolyte decomposition products in the former case is thicker than that in the latter. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289 eV) and C–O (286 eV) peaks that correspond to electrolyte decomposition products such as ROCO2Li, ROLi and Li2CO3 (ref. 57) after cycling in the STD electrolyte are stronger than those for the 3% TPB-containing electrolyte, further confirming the capability of 3% TPB to suppress electrolyte oxidation. In the O 1s spectra, the peak at 529.9 eV is ascribed to the metallic oxide (Me-O), while the C
O (289 eV) and C–O (286 eV) peaks that correspond to electrolyte decomposition products such as ROCO2Li, ROLi and Li2CO3 (ref. 57) after cycling in the STD electrolyte are stronger than those for the 3% TPB-containing electrolyte, further confirming the capability of 3% TPB to suppress electrolyte oxidation. In the O 1s spectra, the peak at 529.9 eV is ascribed to the metallic oxide (Me-O), while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531.7 eV) and C–O (533.4 eV) peaks are assigned to the polycarbonates generated from electrolyte decomposition.58–63 The stronger Me-O and weaker C
O (531.7 eV) and C–O (533.4 eV) peaks are assigned to the polycarbonates generated from electrolyte decomposition.58–63 The stronger Me-O and weaker C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O peaks in the O 1s spectrum of the 3% TPB electrolyte system also confirms the thinner layer of decomposition products after cycling. Similarly, in the F 1s and P 2p spectra, the LiF and LixPOyFz peaks, which reflect the co-decomposition of LiPF6, are weaker in STD electrolyte than in 3% TPB-containing electrolyte.
O and C–O peaks in the O 1s spectrum of the 3% TPB electrolyte system also confirms the thinner layer of decomposition products after cycling. Similarly, in the F 1s and P 2p spectra, the LiF and LixPOyFz peaks, which reflect the co-decomposition of LiPF6, are weaker in STD electrolyte than in 3% TPB-containing electrolyte.
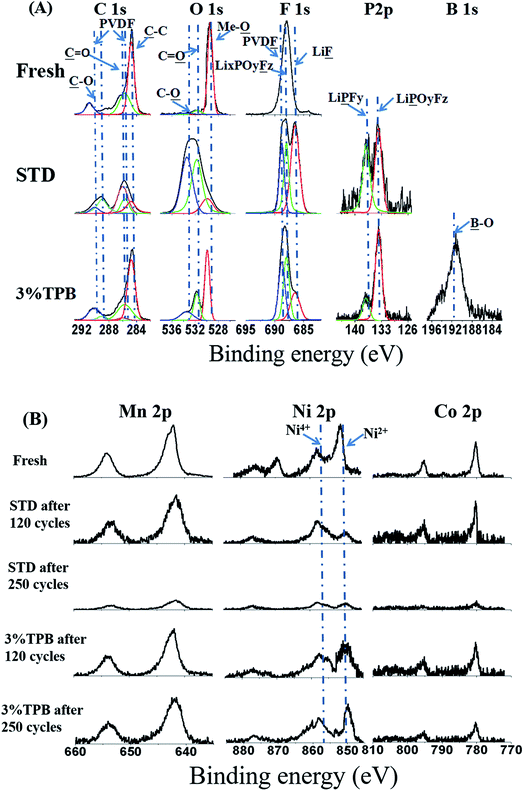 | ||
| Fig. 6 The C 1s, O 1s, F 1s and P 2p (A) and Mn 2p, Ni 2p and Co 2p (B) XPS spectra of fresh LLO electrodes and the LLO electrodes cycled in STD and 3% TPB-containing electrolytes. | ||
Fig. 6B presents the Mn 2p, Ni 2p and Co 2p XPS spectra of a fresh LLO electrode and the LLO electrodes after 120 and 250 cycles. In the Ni 2p spectra, there are peaks of Ni4+ and Ni2+ at 856 eV and 850 eV, respectively.64 It is obvious that the peak of Ni2+ for the LLO with the STD electrolyte becomes weaker than that of Ni4+ after 120 cycles, and both peaks almost disappear after 250 cycles. Gu et al. proposed that Ni ions would preferentially segregate on the surface facets during cycling,65 and this segregated Ni may be easily dissolved, leading to fast voltage fading of the LLO and the transformation of the structure from layer to spinel.66 This in good agreement with our observation of more severe Ni dissolution after 120 cycles in STD electrolyte (see Fig. 5). In contrast, the change of Mn 2p and Co 2p of the LLO in STD electrolyte after 120 cycles is negligible. After 250 cycles, the peaks of Mn 2p, Ni 2p and Co 2p almost disappear, which can be ascribed to the thick covering of electrolyte decomposition products and the dissolution of metal ions. However, the peak intensities in the Mn 2p, Ni 2p and Co 2p spectra of the LLO after cycling in 3% TPB-containing electrolyte remain similar to those of the fresh one, confirming the great capability of TPB to suppress the structural damage of the LLO and electrolyte decomposition.
Fig. 7 presents the SEM and TEM images of the fresh and cycled LLO in STD and 3% TPB-containing electrolyte. A smooth and clean surface (Fig. 7A) and obvious layered structure (Fig. 7B) can be observed on the fresh LLO particles.41 The detailed lattice fringe presents the typical crystal (001) plane of Li2MnO3 and (003) plane of LiMn1/3Ni1/3Co1/3O2.35 However, after cycling in STD electrolyte, as shown in Fig. 7C and D, the LLO surface is apparently covered by a thick and irregular polymer film resulting from the continuous decomposition of the STD electrolyte at high voltage.67 Moreover, the LLO particles lose their crystal feature, indicating their severe structural damage after 250 cycles. In contrast, the surface morphology of the LLO cycled in 3% TPB-containing electrolyte compares well with the fresh one, see Fig. 7A and E, except that there is a uniform thin SEI film covering the LLO surface after 250 cycles with the TPB additive (see Fig. 7F).
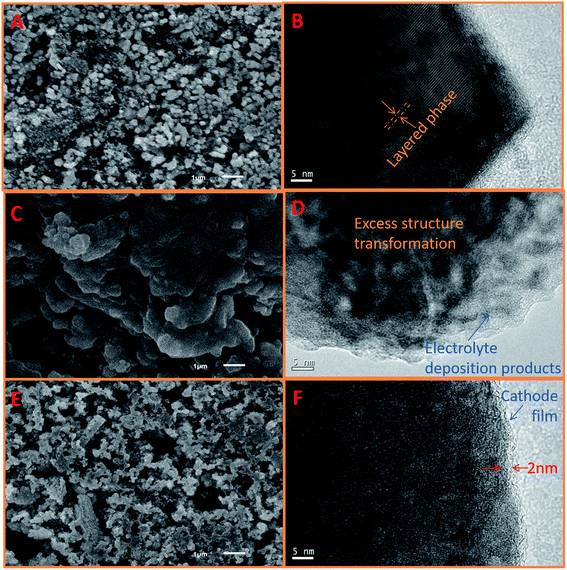 | ||
| Fig. 7 SEM and TEM images of fresh LLO (A and B) and LLO in STD (C and D) and 3% TPB-containing (E and F) electrolytes after 250 cycles at 0.5C rate. | ||
4. Conclusion
It has been demonstrated in this work that the capacity fading of LLO/Li cells during short-term cycling is mainly caused by electrolyte decomposition and layered-to-spinel phase transformation, while that during long-term cycling is triggered by structural destruction of the generated spinel phase. The ICP results reveal that Ni dissolution is more severe than Mn and Co dissolution within 120 cycles; however, Mn dissolution becomes even worse in the subsequent cycles. Tripropyl borate (TPB) as an electrolyte film-forming additive shows higher oxidation activity than STD electrolyte, and generates a condensed protective SEI film on the LLO surface during the initial charging process, resulting in a significantly improved cycling stability of high voltage LLO/Li cells. This TPB-derived SEI film suppresses the continuous oxidation of the electrolyte, and therefore hinders the generation of harmful decomposition products, especially HF, which plays an important role in restraining the transition metal dissolution and structural destruction of the LLO during cycling.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21573080), Guangdong Program for Support of Top-notch Young Professionals (2015TQ01N870) and Distinguished Young Scholars (2017B030306013), and the Science and Technology Planning Project of Guangdong Province (Grant No. 2017B090901020).References
- H. B. Rong, M. Q. Xu, L. D. Xing and W. S. Li, J. Power Sources, 2014, 261, 148 CrossRef.
- M. M. Thackeray, C. Wolverton and E. D. Isaacs, Energy Environ. Sci., 2012, 5, 7854 RSC.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef PubMed.
- J. Tollefson, Nature, 2008, 456, 436 CrossRef PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 CrossRef.
- L. W. Su, Y. Jing and Z. Zhou, Nanoscale, 2011, 3, 3967 RSC.
- J. Zhao, W. H. Li, H. Z. Xu, L. S. Sun, C. Q. Li and F. Q. Liu, RSC Adv., 2016, 6, 70972 RSC.
- S. Byun, J. Park, W. A. Appiah, M. H. Ryou and Y. M. Lee, RSC Adv., 2017, 7, 10915 RSC.
- S. Y. Zuo, Z. G. Wu, S. K. Li, D. Yan, Y. H. Liu, F. Y. Wang, R. F. Zhuo, B. S. Geng, J. Wang and P. X. Yan, RSC Adv., 2017, 7, 18054 RSC.
- K. Kang, Y. S. Meng, J. Breger, C. P. Grey and G. Ceder, Science, 2006, 311, 977 CrossRef PubMed.
- A. R. Armstrong, M. Holzapfel, P. Novak, C. S. Hohnson, S. H. Kang, M. M. Thackeray and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 8694 CrossRef PubMed.
- Z. H. Lu, D. D. MacNeil and J. R. Dahn, Electrochem. Solid-State Lett., 2001, 4, A191 CrossRef.
- A. D. Robertson and P. G. Bruce, Chem. Mater., 2003, 15, 1984 CrossRef.
- Y. W. Denis, K. Yanagida, Y. Kato and H. Nakamura, J. Electrochem. Soc., 2009, 156, A417 CrossRef.
- S. H. Yu, T. Yoon, J. Mun, S. Park, Y. S. Kang, J. H. Park, S. M. Oh and Y. E. Sung, J. Mater. Chem. A, 2013, 1, 2833 RSC.
- Y. S. Kang, T. Yoon, S. S. Lee, J. Mun, M. S. Park, J. H. Park, S. G. Doo, L. Y. Song and S. M. Oh, Electrochem. Commun., 2013, 27, 26 CrossRef.
- F. Rosciano, J. F. Colin, F. L. Mantia, N. Tran and P. Novak, Electrochem. Solid-State Lett., 2009, 12, A140 CrossRef.
- B. Xu, C. R. Fell, M. Chi and Y. S. Meng, Energy Environ. Sci., 2011, 4, 2223 RSC.
- H. J. Yu, H. J. Kim, Y. R. Wang, P. He, D. Asakura, Y. Nakamura and H. S. Zhou, Phys. Chem. Chem. Phys., 2012, 14, 6584 RSC.
- A. Boulineau, L. Simonin, J. F. Colin, C. Bourbon and S. Patoux, Nano Lett., 2013, 13, 3857 CrossRef PubMed.
- J. M. Zheng, M. Gu, J. Xiao, P. J. Zuo, C. M. Wang and J. G. Zhang, Nano Lett., 2013, 13, 3824 CrossRef PubMed.
- G. S. Zou, X. K. Yang, X. Y. Wang, L. Ge, H. B. Shu, Y. S. Bai, C. Wu, H. P. Guo, L. Hu and X. Yi, et al., J. Solid State Electrochem., 2014, 18, 1789 CrossRef.
- Z. Y. Wang, E. Z. Liu, L. C. Guo, C. S. Shi, C. N. He, J. J. Li and N. Q. Zhao, Surf. Coat. Technol., 2013, 235, 570 CrossRef.
- G. R. Li, X. Feng, Y. Ding, S. H. Ye and X. P. Gao, Electrochim. Acta, 2012, 78, 308 CrossRef.
- X. Y. Liu, J. L. Liu, T. Huang and A. S. Yu, Electrochim. Acta, 2013, 109, 52 CrossRef.
- D. H. Cho, H. Yashiro, Y. K. Sun and S. T. Myung, J. Electrochem. Soc., 2014, 161, A142 CrossRef.
- R. P. Qing, J. L. Shi, D. D. Xiao, X. D. Zhang, Y. X. Yin, Y. B. Zhai, L. Gu and Y. G. Guo, Adv. Energy Mater., 2015, 5, 1501914 Search PubMed.
- Z. Zheng, X. D. Guo, Y. J. Zhong, W. B. Hua, C. H. Shen, S. L. Chou and X. S. Yang, Electrochim. Acta, 2016, 188, 336 CrossRef.
- R. Yu, G. Wang, M. Liu, X. Zhang, X. Wang, H. Shu, X. Yang and W. Huang, J. Power Sources, 2016, 335, 65 CrossRef.
- R. Yu, X. Wang, Y. Fu, L. Wang, S. Cai, M. Liu, B. Lu, G. Wang, D. Wang, Q. Ren and X. Yang, J. Mater. Chem. A, 2016, 4, 4941 RSC.
- J. Li, L. Xing, R. Zhang, M. Chen, Z. Wang, M. Xu and W. Li, J. Power Sources, 2015, 285, 360 CrossRef.
- J. Li, L. Xing, L. P. Zhang, L. Yu, W. Z. Fan, M. Q. Xu and W. S. Li, J. Power Sources, 2016, 324, 17 CrossRef.
- J. G. Han, S. J. Lee, J. Lee, J. S. Kim, K. T. Lee and N. S. Choi, ACS Appl. Mater. Interfaces, 2015, 7, 8319 CrossRef PubMed.
- J. Li, L. Zhang, L. Yu, W. Fan, Z. Wang, X. Yang, Y. Lin, L. Xing, M. Xu and W. Li, J. Phys. Chem. C, 2017, 120, 26899 CrossRef.
- J. Li, L. Xing, J. Chen, H. Zhou, M. Xu and W. Li, J. Electrochem. Soc., 2016, 163, A2258 CrossRef.
- A. M. Haregewoin, A. S. Wotango and B. J. Hwang, Energy Environ. Sci., 2016, 9, 1955 RSC.
- L. Wang, Y. Ma, Q. Lin, Z. Zhou, X. Cheng, P. Zuo, C. Du, Y. Gao and G. Yin, J. Power Sources, 2016, 361, 227 CrossRef.
- P. Hong, M. Xu, X. Zheng, Y. Zhu, Y. Liao, L. Xing, Q. Huang, H. Wan, Y. Yang and W. Li, J. Power Sources, 2016, 329, 216 CrossRef.
- Z. D. Li, Y. C. Zhang, H. F. Xiang, X. H. Ma, Q. F. Yuan, Q. S. Wang and C. H. Chen, J. Power Sources, 2013, 240, 471 CrossRef.
- W. Tu, P. Xia, X. Zheng, C. Ye, M. Xu and W. Li, J. Power Sources, 2017, 341, 348 CrossRef.
- Z. Zhou, Y. Ma, L. Wang, P. Zuo, X. Cheng, C. Du, G. Yin and Y. Gao, Electrochim. Acta, 2016, 216, 44 CrossRef.
- J. Pires, A. Castets, L. Timperman, J. Santos-Peña, E. Dumont, S. Levasseur, C. Tessier, R. Dedryvère and M. Anouti, J. Power Sources, 2015, 296, 413 CrossRef.
- S. H. Lim, W. Cho, Y. Kim and T. Yim, J. Power Sources, 2016, 336, 465 CrossRef.
- A. Birrozzi, N. Laszczynski, M. Hekmatfar, J. Zamory, G. Giffin and S. Passerini, J. Power Sources, 2016, 325, 525 CrossRef.
- Z. H. Chen, J. Liu and K. Amine, Electrochim. Acta, 2008, 53, 3267 CrossRef.
- M. Chen, X. Xiang, D. Chen, Y. Liao, Q. Huang and W. Li, J. Power Sources, 2015, 279, 197 CrossRef.
- M. H. Liu, F. Dai, Z. Ma, M. Ruthkosky and L. Yang, J. Power Sources, 2014, 268, 37 CrossRef.
- B. H. Song, Z. W. Liu, M. O. Lai and L. Lu, Phys. Chem. Chem. Phys., 2012, 14, 12875 RSC.
- X. R. Yang, J. H. Li, L. D. Xing, Y. H. Liao, M. Q. Xu, Q. M. Huang and W. S. Li, Electrochim. Acta, 2017, 227, 24 CrossRef.
- J. Zhang, J. Wang, J. Yang and Y. N. Li, Electrochim. Acta, 2014, 117, 99 CrossRef.
- J. M. Zheng, M. Gu, A. Genc, J. Xiao, P. H. Xu, X. L. Chen, Z. H. Zhu, W. B. Zhao, L. Pullan and C. M. Wang, et al., Nano Lett., 2014, 14, 2628 CrossRef PubMed.
- Y. M. Zhu, X. Y. Luo, M. Q. Xu, L. P. Zhang, L. Yu, W. Z. Fan and W. S. Li, J. Power Sources, 2016, 317, 65 CrossRef.
- L. Xing, O. Borodin, G. Smith and W. Li, J. Phys. Chem. A, 2011, 115, 13896 CrossRef PubMed.
- Y. T. Wang, L. D. Xing, W. S. Li and D. Bedrov, J. Phys. Chem. Lett., 2013, 4, 3992 CrossRef.
- M. Xu, L. Hao, Y. Liu, W. Li, L. Xing and B. Li, J. Phys. Chem. C, 2011, 115, 6085 CrossRef.
- X. X. Zuo, C. J. Fan, J. S. Liu, X. Xiao, J. H. Wu and J. M. Nan, J. Power Sources, 2013, 229, 308 CrossRef.
- B. Li, Y. Q. Wang, H. B. Lin, J. S. Liu, L. D. Xing, M. Q. Xu and W. S. Li, Electrochim. Acta, 2014, 14, 263 CrossRef.
- P. Verma, P. Maire and P. Novák, Electrochim. Acta, 2010, 55, 6332 CrossRef.
- M. Xu, A. Xiao, W. Li and B. L. Lucht, J. Electrochem. Soc., 2010, 157, A115 CrossRef.
- D. Aurbach, I. Weissman, A. Schechter and H. Cohen, Langmuir, 1996, 12, 3991 CrossRef.
- W. Li, A. Xiao, B. L. Lucht, M. C. Smart and B. V. Ratnakumar, J. Electrochem. Soc., 2008, 155, A648 CrossRef.
- Y. Yang, T. Markmaitree and B. L. Lucht, J. Power Sources, 2011, 196, 2251 CrossRef.
- X. Wei, S. Zhang, Z. Du, P. Yang, J. Wang and Y. Ren, Electrochim. Acta, 2013, 107, 549 CrossRef.
- M. Gu, I. Belharouak, A. Genc, D. Wang, Z. Wang, K. Amine, F. Gao, G. Zhou, S. Thevuthasan and D. R. Baer, et al., Nano Lett., 2012, 12, 5186 CrossRef PubMed.
- J. Zheng, S. Myeong, W. Cho, P. Yan, J. Xiao, C. Wang, J. Cho and J. Zhang, Adv. Energy Mater., 2017, 7, 1601284 CrossRef.
- W. N. Huang, L. D. Xing, Y. T. Wang, M. Q. Xu, W. S. Li, F. C. Xie and S. G. Xia, J. Power Sources, 2014, 267, 560 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03852j |
| This journal is © The Royal Society of Chemistry 2018 |