 Open Access Article
Open Access ArticleAdsorptive environmental applications of MXene nanomaterials: a review
Yujuan Zhang
 *a,
Lin Wang
*a,
Lin Wang
 b,
Ningning Zhanga and
Zhangjian Zhoua
b,
Ningning Zhanga and
Zhangjian Zhoua
aSchool of Materials Science and Engineering, University of Science and Technology Beijing, 100083, Beijing, China. E-mail: zhangyujuan@ustb.edu.cn
bLaboratory of Nuclear Energy Chemistry and Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, 100049, Beijing, China
First published on 30th May 2018
Abstract
Since titanium carbide Ti3C2 nanosheets were first produced in 2011, an increasing number of members of this new family of two-dimensional transition metal carbides/nitride (MXene) materials have been successfully synthesized. Due to their large specific surface area, hydrophilic nature and abundant highly active surface sites, MXenes have been demonstrated to adsorb a variety of environmental pollutants, including heavy metal ions, organic dyes, radionuclides, and gas molecules, and thus can be used for the removal of pollutants and even sensing. In this review, we summarize the recent research progress on MXene materials in the adsorptive remediation of environmental pollutants and highlight the main challenges in the future to understand the full potential of MXene materials in environmental systems.
1 Introduction
With the development of industrialization, environmental pollution has become increasingly serious worldwide, which poses serious effects on human health and the ecosystem. The most commonly encountered pollutants include heavy metal ions, organics, bio-toxins, and toxic gases. Thus, various physical, chemical and biological techniques have been developed to remedy pollutants, such as membrane filtration, precipitation, adsorption, solvent extraction, and ion exchange.1–4 Among them, adsorption is considered to be promising due to its simple, cost-effective and economical characteristics.1,5 Additionally, adsorption also avoids secondary pollution due to the production of harmful substances during remediation. For effective adsorption, adsorbents usually have a large specific surface area and proper functionalities for the adsorbates. To date, numerous porous materials have been developed as adsorbents for environmental pollutants, such as activated carbon, kaolinite, zeolites, chitosan, and metal–organic frameworks.6–11Two dimensional (2D) materials have garnered great attention owing to their unique physical and chemical properties, which differ from their corresponding bulk counterparts, since graphene was first produced by mechanical exfoliation into single-layers in 2004.12 Since the emergence of graphene, other 2D materials such as transition metal dichalcogenides (TMDs), hexagonal boron nitrides, metal oxides and hydroxides have been discovered and demonstrated in diverse applications.13–15 As is known, low-dimensional materials with the advantage of large surface areas have been considered as ideal adsorbents for various pollutants, e.g., ordered mesoporous silica, carbon-based nanomaterials (e.g., carbon nanotubes and graphene), and phosphorenes.16–20
Recently, a new family of 2D transition metal carbide/nitride materials known as MXene has been attracting tremendous interest from both theoretical and experimental physicists and chemists.21–26 Due to their unique structures, MXenes usually exhibit excellent properties such as high chemical stability, high electrical conductivity and environment-friendly characteristics. To date, MXenes have been reported as promising materials for application in semiconductors, hydrogen storage, supercapacitors, and lithium-ion batteries.27–34 Especially, their hydrophilic nature and abundant highly active functional sites on their surface render MXenes effective adsorbents for many molecular or ionic species, which consequently can be used for environmental pollutant purification or even sensing. Although several review papers on MXenes have been published,22,31–34 they generally cover all the application aspects of MXenes, and a special review on the progress of the application of MXenes in the environmental remediation area has not been reported to date. Herein, based on this fact, we focus and summarize in detail the recent research progress on MXene materials in the application of adsorptive remediation of environmental pollutants, including heavy metal ions, organic dyes, radionuclides and gaseous contaminants, and highlight the main challenges in the future to understand the full potential of MXene materials in environmental systems.
2 Structures and surface terminations of MXenes
The structure of a bare MXene can be described as n+1 layers of transition metal elements M covering n layers of X (where, X is C or N element.) in an (MX)nM arrangement. As shown in Fig. 1a, to date, at least three different formulae of MXenes, M2X, M3X2 and M4X3,22 have been confirmed. From the scanning electron microscope (SEM) images in Fig. 1b, the layered structures of MXenes are obvious. In addition, MXenes with two different transition metals have also been synthesized, which exist in two forms, i.e., solid solution phase (two transition metals randomly occupy the M-sites) and ordered phase (two transition metals are arranged layer by layer).31 For example, (Cr2V)C2 has an ordered structure, while (Ti and V)3C2 exist in solid solution.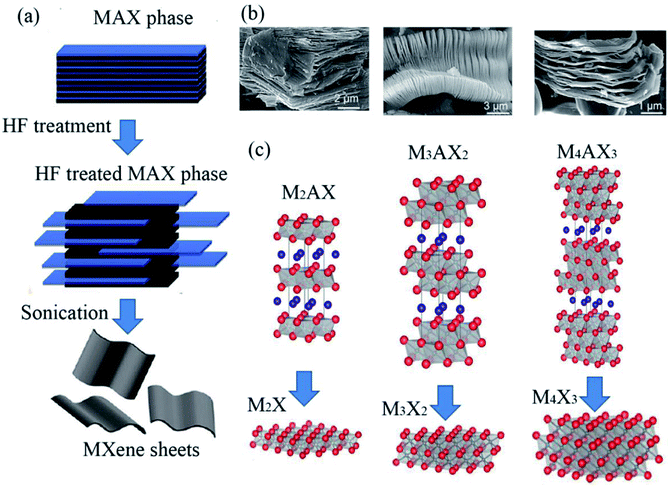 | ||
| Fig. 1 (a) Early reported three different structures of MXenes (non-terminated): M2X, M3X2 and M4X3. Reprinted from ref. 22 and 23 with permission. (b) SEM images of Ti2AlC, Ti3AlC2 and Ta4AlC3 after HF treatment (from right to left). (c) Schematic illustration of the synthesis of MXenes from MAX phases. Copyright 2013 John Wiley and Sons and Copyright 2012 American Chemical Society. | ||
Due to the important role of the specific surface area of MXene materials, the quality of MXene materials is a critical factor in environmental applications. In recent years, many preparation techniques have been developed to obtain high-quality flake nanomaterials. MXenes were first synthesized via a simple chemical etching method in 2011, where the “A” laminar component is exfoliated from the “MAX” matrix phase in hydrofluoric acid (HF), ammonium bifluoride (NH4HF2) or hydrochloric acid (HCl) combined with lithium fluoride (LiF), followed by sonication at room temperature,22,26 as shown in Fig. 1c. The term “MAX” represents the chemical composition of the parent compounds Mn+1AXn (n = 1, 2 and 3), where A stands for a group IIIA or IVA element (A = Al, Ga, In, Si, Ge, Sn, Pb, P, As, S and Cd). Recently, MXenes have also been synthesized from the laminated phase, which are categorized as derivatives of MAX phases (d-MAX). For example, Zr3C2 and Hf3C2 MXenes can be synthesized via the selective etching of Al–C and Si-alloyed Al–C sublayers from nanolaminated Zr3Al3C5 and Hf3[Al(Si)]4C6, respectively.35,36 Fig. 2 shows a schematic illustration of the synthesis of Hf3C2 MXene from the nanolaminated d-MAX phase Hf3[Al(Si)]4C6. During the etching process, the introduction of Si can significantly accelerate the etching of the layered ternary carbide Hf3Al4C6 through the weakened interfacial adhesion between the Hf–C and Al(Si)–C sublayers, as shown in Fig. 2c and d.
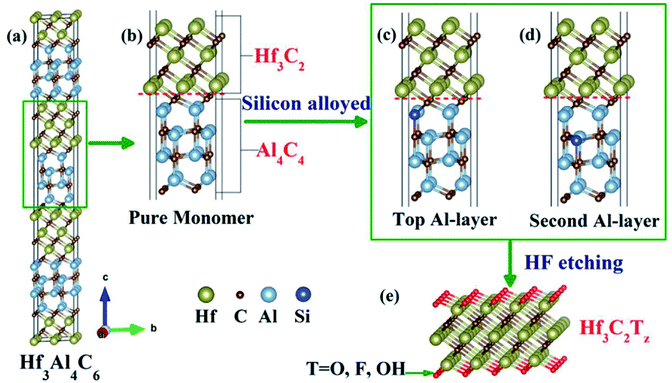 | ||
| Fig. 2 Schematic illustration of the synthesis of Hf3C2Tz MXene from nanolaminated phase Hf3[Al(Si)]4C6. (a) Structure of Hf3Al4C6, (b) structure of pure Hf3Al4C6 monomer, (c) and (d) structure of two Hf3Al4C6 monomers with different silicon alloying, and (e) side view of Hf3C2 MXene. Reprinted with permission of ref. 36 Copyright 2017 American Chemical Society. | ||
Surface functionalities are strongly relevant to the physical and chemical properties of MXene materials, which further affect their environmental applications. In various etching synthetic procedures, chemical functional groups such as oxygen (–O), hydroxyl (–OH) and fluorine (–F) often form on the surfaces of MXenes. Therefore, MXenes are generally written as Mn+1XnTx, where T refers to the surface terminated functional groups. For example, Ti3C2 MXene can have at least three formulae: Ti3C2(OH)2, Ti3C2O2 and Ti3C2F2. In practice, Ti3C2 MXene usually has a combination of these functional groups, the specific quantities of which are highly dependent on the synthetic methods.37,38 The random distribution of terminations on the surface of MXene has been confirmed by neutron scattering and NMR spectroscopy.38,39 In general, a hydrophilic surface is beneficial for the adsorption of polar or ionic species. It is shown that the –F group is not desirable when MXenes are used for adsorbents in most circumstances, which will be discussed in the following section. Fortunately, the –O and –OH groups are found to be more stable, and –F terminations will be replaced by OH groups upon rinsing and/or storage in water. Thus, most of the terminations of MXenes obtained using the chemical etching method are –OH and –O groups.40 This is probably why MXenes always exhibit very high adsorption capacities (in the unit of grams of adsorbate per gram of adsorbent) even compared to carbon-based nanomaterials, such as graphene, though the atomic weight of transition metal elements is usually large.
Recently, bare Mo2C MXene without functional groups has been successfully synthesized using the chemical vapor deposition method.41 The excellent physical properties of bare Mo2C MXene have also been systematically explored using density functional theory (DFT).42 The unterminated metallic atoms are considered to be highly active and easy to react with other substances. However, studies about the adsorption behaviors of MXenes without functional groups are scarce, and still need to be further studied.
3 Progress of MXenes in adsorption remediation of pollutants
3.1 Heavy metal ion adsorption
Heavy metal ions including Pb(II), Cr(IV), Hg(II), Cd(II) and Cu(II) are the most important pollutants in water and soil because of their high toxicity to humans and other living organisms. Adsorption is considered to be the most effective approach to remove heavy metal ions because other methods such as biological processes or chemical reactions cannot degrade them. Due to the abundant active sites on their surface, MXenes can adsorb metal ions through electrostatic and chemical interactions. To date, numerous studies have been carried out on heavy metal ion sequestration using MXenes.Among the various toxic heavy metal ions in water, Pb(II) is one of the most commonly encountered pollutants and its content in drinking water is strictly limited. In 2014, the Ti3C2(OH/ONa)xF2−x MXene material43 prepared via chemical exfoliation followed by alkalization intercalation was first reported to exhibit strong Pb(II) ion adsorption with a large uptake capacity (140 mg g−1) and high selectivity even when other competing cations such as Ca(II) and Mg(II) coexist at high levels. The intercalation of cations or small organic molecules can increase the distance of the interlayers of MXene and enhance the interaction between the surface functional groups and MXene layers, and therefore helps the adsorption process. Especially, after purification with the applied management of 4500 kg water per kg alk-MXene, the effluent Pb(II) content can reach as low as 2 μg L−1, which is below the drinking water standard set by the World Health Organization (10 μg L−1). The adsorption mechanism was elucidated to be Pb(II) ions being trapped within hydroxyl potential traps, forming strong bonds between Pb and oxygen atoms (hydroxyls losing H atoms), as shown in Fig. 3. First-principles calculations revealed that different hydroxyl sites and different functional groups have a great influence on the adsorption behaviors of Ti3C2(OH)xF2−x MXene for Pb(II) ions:44 the hydroxyl group vertical to the titanium atom shows a stronger trend of removing ions than other adsorption structures, and the terminated F occupation of alk-MXene decreases the efficiency of the adsorption while the intercalation of Li, Na, and K atoms accelerates it. DFT studies of the adsorption behaviors of Pb on different MXenes in the general form of M2X(OH)2 (M = Sc, Ti, V, Cr, Zr, Nb, Mo, Hf, Ta, and X = C or N)45 indicate that nitrides are basically more favorable for Pb ion adsorption than their carbide counterparts due to the different valence electron numbers between C and N atoms. In addition, Sc2C(OH)2 and Zr2C(OH)2 MXenes cannot be used for Pb removal. The weak ability of these two MXenes for Pb removal is mainly attributed to the energy difference between the sides of the adsorption reaction equation, i.e., the adsorption structures have positive formation energies.
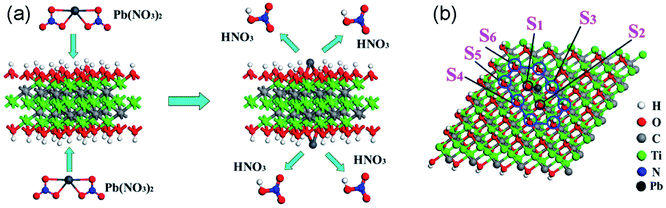 | ||
| Fig. 3 (a) Schematic illustration of the formation of the Ti3C2(O2H2−2mPbm) structure from Ti3C2(OH)2 to Ti3C2(O2H2−2mPbm); (b) top-view of the Ti3C2(O2H2−2mPbm) structure when the Pb coverage is 1/9 ML. S1–S6 represent the different atom sites of the hydroxyl. Reprinted from ref. 44 with permission. Copyright 2015 American Chemical Society. | ||
After Pb(II), Cr(VI) is listed as another important heavy metal pollutant in water, and its adsorption behavior on MXenes has also been investigated. Cr(VI) exists in the anionic form, i.e., Cr2O72−. Ying et al. reported that Ti3C2Tx MXene shows a high Cr(VI) adsorption capacity of 250 mg g−1 under optimized synthetic conditions (10% HF delamination, room temperature, pH = 5), and the residual concentration of Cr(VI) in the treated water is less than 5 ppm.46 This MXene not only removes Cr(VI) by reducing Cr(VI) to the less toxic Cr(III), but also adsorbs the reduced Cr(III) simultaneously. The initial adsorption of Cr(VI) on Ti3C2Tx is due to the electrostatic attraction between the positively charged surface of MXene and negatively charged Cr2O72− (at low pH, the hydroxyl groups on the surface of Ti3C2Tx MXene will be protonated). After adsorption, electrons are transferred from Ti3C2Tx to Cr2O72− with the assistance of H+, producing TiO2 and Cr(III) ions. The produced Cr(III) ions then interact with the [Ti–O] bond on the MXene and form the Ti–O–Cr(III) structure. Based on the reduction mechanism, it is confirmed that other oxidizing agents such as K3[Fe(CN)6], KMnO4, and NaAuCl4 can also be reduced to low oxidation states and removed by Ti3C2Tx MXene. By the in situ phase transformation of Ti3C2(OH)0.8F1.2 MXene under FeCl3 conditions, an urchin-like rutile TiO2–C nanocomposite with an abundance of (110) facets was fabricated, which displays a much higher Cr(VI) adsorption capacity of 225 mg g−1 than that of primitive Ti3C2(OH)0.8F1.2 MXene (∼62 mg g−1).47 Theoretical calculation revealed that the adsorption energies of all three types of Cr(VI) ions (including CrO42−, HCrO4−, and Cr2O72− ions) on rutile TiO2 are greater than that of water molecules, indicating that Cr(VI) ions can be adsorbed on rutile TiO2 surfaces in aqueous solution. After adsorption, the bridging oxo groups in Cr(VI) ions can further inhibit the adsorption of H2O molecules on the rutile TiO2 surfaces.
Besides Pb and Cr ions, first-principles calculations show that alkaline intercalated Ti3C2 MXene can also effectively remove a series of other heavy metal ions including Cu, Zn, Pd, and Cd, with formation energies ranging from −1.0 to −3.3 eV.44 The general chemical reaction of heavy metals Y (in nitrate form) uptake on alk-MXene can be written as Ti3C2(OH)2 + mY(NO3)2 → Ti3C2(O2H2−2mYm)2 + 2mHNO3. The calculated formation energies of Ti3C2(O2H2−2mYm)2 range from −1.0 to −3.3 eV for the Cu, Zn, Pd, and Cd elements. Ti3C2Tx MXene also acts as an effective adsorbent for Ba(II)48 and Cu(II) ions49 (two typical pollutants in water), and also applied in experiment. The formation of both Ba–O and Ba–F bonds is found to contribute to the Ba(II) ion adsorption process. The maximum adsorption capacity is found experimentally to be 9.3 mg g−1 for an initial barium concentration of 55 ppm, which is higher than that of other competitors such as activated carbon and carbon nanotubes. Furthermore, the removal efficiency can reach up to 100% under optimized conditions. Since other studies43 reported that –F groups hinder the adsorption of metal ions, more accurate investigations may be needed to clarify the effects of –F groups on the adsorption process. Regarding Cu(II) ions, the adsorption mechanism of ion exchange between the positively charged Cu ions and negatively charged terminal groups (–O and –OH) on the Ti3C2Tx surface was experimentally confirmed. After the ion exchange, a further oxidation-reduction reaction occurs, resulting in the formation of rutile TiO2 nanoparticles. The maximum Cu ion adsorption capacity of delaminated Ti3C2Tx was experimentally determined to be 78.45 mg g−1.
In addition to these ionic pollutants in water, MXenes have also shown strong adsorption capacity for free non-ionic metal atoms, and thus can be used as carriers for transition metal catalysts. Guo et al. studied the non-ionic Pb atom adsorption behaviors of different MXenes (including Ti3C2, V2C1 and Ti2C1) with different functional groups (including bare, H, OH, and F), and found that all the MXenes can effectively adsorb Pb atoms with a binding energy larger than 1 eV, except the F terminated ones.50 The theoretical Pb adsorption capacity was calculated to be as high as 2560 mg g−1 for bare Ti2C MXene and 1280 mg g−1 for H-terminated Ti2C MXene. They also found the bare and OH terminated MXenes of the three types show a high adsorption capability for Cu atoms. Yang et al. also predicted that Ti2C(OH)2 and Ti3C2(OH)2 MXene can effectively adsorb free non-ionic Au atoms with a high adsorption energy larger than 3 eV, and the O and F groups significantly reduce the adsorption ability.51 Since free non-ionic Pb, Cu and Au atoms are hard to obtain under common conditions, these studies may require future experimental validation.
Besides adsorptive removal, MXene materials can also be applied for sensing trace amounts of heavy metal ions. An alk-Ti3C2 modified glassy carbon electrode is reported to exhibit a highly sensitive electrochemical response to Cd(II), Pb(II), Cu(II) and Hg(II) ions using square-wave anodic stripping voltammetry.52 Under optimized conditions, the electrode displays a detection limit of 0.098 μM, 0.041 μM, 0.032 μM and 0.130 μM for Cd(II), Pb(II), Cu(II) and Hg(II), respectively, which is superior to most of the state-of-the-art sensors. Moreover, the peak potentials of each heavy metal are well defined and sufficiently separated during the simultaneous voltammetric determination of four target heavy metal ions, which is very suitable for the simultaneous and selective detection of co-existing metal ions.
Overall, MXenes show strong adsorption capabilities for metal ions and non-ionic atoms, which are even stronger than that of carbon nanotubes and graphene oxide,17–19 even if the atomic weight of the carbon element is usually much lower than transition metal elements in MXenes (for example, the typical Pb(II) uptake capacity of oxidized carbon nanotubes is below 100 mg g−1). These unique adsorption properties are generally attributed to the natural surface-functionalization of MXenes during the synthetic process using acidic fluoride-containing solutions.
3.2 Organic dye adsorption
Organic dyes are also an important type of pollutant in water. Dyes usually have a stable molecular structure and are difficult to biodegrade. The presence of dyes in water can directly impact the ecosystem and even the health of human beings through the contamination of drinking water supplies. Dyes usually exist in the form of cations or anions in water, and thus can be adsorbed by specific adsorbents. Several groups have investigated the possibility of removing organic dye pollutants using MXene materials, especially combined with the UV photocatalytic technique to further degrade them.The charged state of a dye is a critical factor to its adsorption on MXenes. Studies of the adsorptive properties of the cationic dye methylene blue (MB) and anionic dye acid blue 80 (AB80) on Ti3C2Tx MXene53 reveal that MB can be strongly and irreversibly bonded to Ti3C2Tx (with the adsorption capacity of ∼39 mg g−1), while AB80 can hardly adsorb on MXene. The preferential adsorption of the cationic dye over anionic dye is attributed to the electrostatic interactions between the positively charged dye cations and the negatively charged surfaces of MXenes in aqueous solution. UV light is also found to significantly enhance the degradation rate of MB and AB80 dye in solutions containing both the dye and Ti3C2Tx MXene. Similar to Pb ion adsorption,43 expanding the interlayer spacing of MXene is also an efficient approach to improve its dye adsorption performance. Zheng et al. reported that the interlayer spacing of Ti3C2Tx MXene can be increased by up to 29% via LiOH treatment and 28% via NaOH treatment.54 The adsorption capacity of LiOH–Ti3C2Tx and NaOH–Ti3C2Tx for MB reaches 121 mg g−1 and 189 mg g−1, respectively, in comparison with that of the pristine Ti3C2Tx MXene without alkaline treatment (100 mg g−1).
Semiconductor/MXene nanocomposites have also been studied to enhance photocatalytic activity. Gao et al. synthesized TiO2/Ti3C2 nanocomposites via a hydrothermal process,55 which showed improved photocatalytic activity for methyl orange degradation under ultraviolet light irradiation compared to simplex TiO2 or Ti3C2. Their studies suggested that the TiO2/Ti3C2 composite exhibits more effective electron–hole separation than pure TiO2 or Ti3C2 under UV irradiation. To further improve the photocatalytic activity of the TiO2/Ti3C2 complex, Peng et al. synthesized a hybrid comprised of (001) facets of TiO2 nanosheets and layered Ti3C2 via the facile in situ hydrothermal partial oxidation of Ti3C2.56 The catalytic activity is highly dependent on the hydrothermal time (Fig. 4a). The unique hybrid significantly enhances the photocatalytic degradation of methyl orange dye. The result was attributed to the fact that the highly active (001) facets of TiO2 allow the high-efficiency photogeneration of electron–hole pairs, which are substantially promoted by the hole trapping effect by the interfacial Schottky junction with 2D Ti3C2 acting as a reservoir of holes, as illustrated in Fig. 4b. This design not only allows for high-efficiency separation of electron–hole pairs, but also overcomes the problem of their rapid recombination. Zhou et al. synthesized a CeO2/Ti3C2 nanocomposite via a one-step hydrothermal method based on the electrostatic attraction between Ce3+ ions and Ti3C2 nanosheets.57 The composite shows excellent photocatalytic activity toward the degradation of Rhodamine B compared with pure Ti3C2 and CeO2, which is attributed to the enhanced utilization of solar energy.
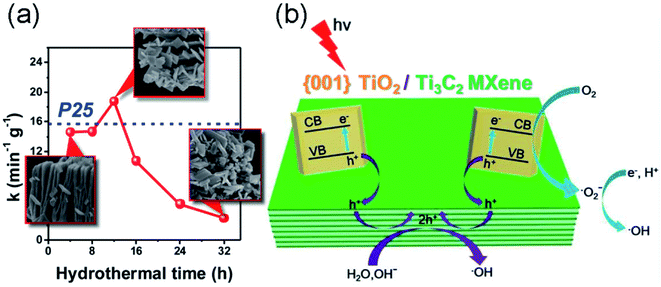 | ||
| Fig. 4 (a) Photocatalytic degradation rate constant (k) of (001)TiO2/Ti3C2 prepared for different hydrothermal times. (b) Schematic illustration of the charge-transfer process over (001)TiO2/Ti3C2. Reprinted from ref. 56 with permission. Copyright 2016 American Chemical Society. | ||
From the above literature, two basic conclusions can be derived, the adsorption mechanism of dyes on MXenes is electrostatic attraction and semiconductor/MXene nanocomposites are an effective approach for the photocatalytic degradation of dyes compared to their simplex component counterparts.
3.3 Radionuclide pollutant adsorption
With the rapid development of the nuclear industry and the peaceful utilization of nuclear energy, nuclear waste pollution has become a challenging environmental concern. As is known, the contamination of some long-lived actinides can be a significant hazard even at trace amount due to their long-term radiological and chemical toxicities. The unique characteristics of MXenes such as high ability to resist strong radiation and good chemical compatibility with molten salt harsh materials give them the potential to clean up radionuclides for nuclear waste treatment.Using multi-layered vanadium carbide V2CTx nanosheets as a case study, MXenes have been shown to be competitive inorganic adsorbents for actinide capture from aqueous solution.58,59 Uranium, which normally exists as UO22+ in most processing and environmental conditions, is one of the most important radionuclides due to its central role in the nuclear energy industry and its relative enrichment in nature. It is confirmed that V2CTx MXene can adsorb uranium U(VI) with a high uptake capacity of 174 mg g−1, along with fast sorption kinetics and desirable selectivity.58 Density functional theory calculations in combination with X-ray absorption fine structure characterization suggest that uranyl ions prefer to coordinate with hydroxyl groups bonded to the V sites of MXene via the formation of bidentate inner-sphere complexes, as shown in Fig. 5. According to the uranium speciation diagram, uranium ions can exist with different ligands such as aqueous, carbonate and hydroxylated species. To specify the adsorption behaviors of uranium on MXene, the adsorption properties of hydroxylated V2C nanosheets for uranyl ions with different ligands in the general form [UO2(L1)x(L2)y(L3)z]n (where, L1, L2 and L3 stand for H2O, OH and CO3, respectively)59 are further clarified. All uranyl species can bond strongly with V2C(OH)2 nanosheets with high adsorption energies greater than 3 eV. Among the studied uranyl species, aquouranyl [UO2(H2O)5]2+ bonds the strongest to the hydroxylated V2C nanosheet. It is also found that the terminated –F groups on the V2C nanosheets can weaken the adsorption capability for uranyl ions.
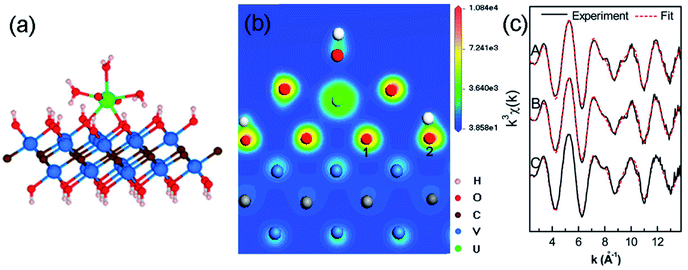 | ||
| Fig. 5 Adsorption configuration of a U ion on V2CTx nanosheets as analyzed by DFT and X-ray adsorption spectroscopic approaches. (a) Bidentate inner-sphere adsorption configuration of a uranium ion on V2CTx nanosheets, (b) charge density distribution of the adsorption structure by DFT simulations, (c) raw U LIII-edge k3-weighted extended X-ray absorption fine structure (EXAFS) spectra and the best theoretical fits of U adsorbed on V2CTx nanosheets under different conditions. Reprinted from ref. 58 with permission. Copyright 2015 and 2016 American Chemical Society. | ||
Titanium carbide Ti3C2Tx, as the typical material of MXene, with the merits of good development and inexpensive preparation, is the ideal adsorbent for radionuclide elimination with respect to technology and the economy. DFT studies of the adsorption behaviors of U(VI) ions on Ti3C2(OH)2 MXene60 have shown that U(VI) ions can form strong bonds with MXene in aqueous solution regardless of the presence of anionic ligands such as OH−, Cl− and NO3−. Chemical interactions and hydrogen bonds are considered as the main adsorption interactions. Based on the stable adsorption configuration, the theoretical adsorption capacity can approach 595.3 mg g−1 for the [UO2(H2O)5]2+ species.
In experiment, a major challenge for radionuclide removal using Ti3C2Tx as the adsorbent material is the contradiction between the small interlayer space of Ti3C2Tx MXene and the large hydrated radionuclide ionic radius, which limits the radionuclide adsorption capacity. Delamination of multilayered Ti3C2Tx into nanoflakes is an ideal approach to significantly improve the adsorption capacity. Furthermore, hydration is found to be an effective method to enhance the adsorption properties of U(VI) ions on MXenes.61 Through this approach, the presence of hydrophilic groups and van der Waals weak interactions can increase the interlayered distance of MXenes, and therefore enhance their adsorption capacity for U ions.
Briefly, MXene materials have been demonstrated to be effective adsorbents for uranium ions both experimentally and theoretically. Chemical interactions and hydrogen bonds are the main adsorption mechanism. Except for uranium, the adsorption of other radionuclide ions (e.g., Np and Pu ions) on MXenes are still unknown and need further investigation.
3.4 Gaseous contaminant adsorption
Gaseous contaminants mainly include toxic inorganic gases (NOx, SOx, H2S, NH3, and CO), and volatile organic compounds (VOCs), most of which can cause serious diseases to humans and other living organisms. Thus, both effectively sensing and removing them are of high importance. A number of studies have been carried out on gaseous contaminant adsorption using MXenes.Regarding inorganic gases, first-principles calculations of the adsorption behaviors of NH3, H2, CH4, CO, CO2, N2, NO2 and O2 on monolayer Ti2CO2 reveal that only NH3 can be chemisorbed on Ti2CO2 (adsorption energy of −0.37 eV), while the others are physisorbed on Ti2CO2 with low adsorption energies, as shown in Fig. 6a62. The electrical conductivity of Ti2CO2 changes significantly after the adsorption of NH3, indicating that Ti2CO2 can be a potential NH3 sensor with high sensitivity (Fig. 6b). Additionally, the interaction between NH3 and Ti2CO2 can be further enhanced by applying strain on the nanosheet. The adsorption behavior of NH3 on a series of O-terminated semiconducting MXenes with the general form M2CO2 (M = Sc, Ti, Zr, and Hf) were investigated using first-principles simulations.63 All the studied MXenes can chemisorb NH3 with notable charge transfer. The adsorbed NH3 can be further released from the MXenes by injecting electrons into them. These results indicate that M2CO2 MXenes are very suitable as NH3 sensors. The density functional theory investigations by Morales-Garcia et al. showed that bare M2C (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) MXenes without surface functional groups can effectively adsorb CO2 even at low CO2 partial pressures and high temperatures, and thus can act as very promising candidates for carbon dioxide capture, storage, and activation.64
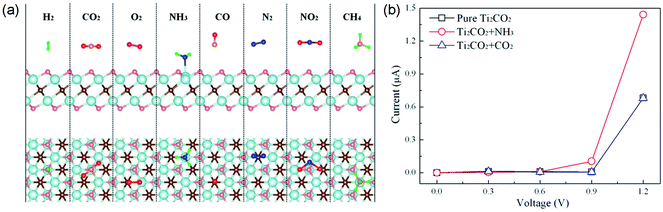 | ||
| Fig. 6 (a) Schematic illustration of the adsorption of NH3, H2, CH4, CO, CO2, N2, NO2 and O2 molecules on monolayer Ti2CO2. (b) Current–voltage relationship before and after the adsorption of NH3 or CO2 molecule on monolayer Ti2CO2. Reprinted from ref. 62 with permission. Copyright 2015 American Chemical Society. | ||
Besides the adsorption of inorganic gaseous contaminants, MXene materials can also be utilized to fabricate gas sensors for VOCs. Kim et al. reported that Ti3C2Tx MXene exhibits a very low detection limit of 50–100 ppb for VOC gases (including acetone, ethanol, and propanal) and ammonia at room temperature.65 The high metallic conductivity and fully functionalized surface of Ti3C2Tx MXene lead to an ultrahigh signal-to-noise ratio, which is superior to conventional semiconductor channel materials and other well-known 2D materials (e.g., MoS2). The terminal hydroxyl (–OH) groups on the surface of Ti3C2Tx are mainly responsible for the adsorptive detection of the target species.
3.5 Adsorption of other pollutants
In addition to the abovementioned pollutants, the adsorption of some other pollutants have also been demonstrated using MXene materials due to their active surface structures.Zhang et al. synthesized a novel sandwiched MXene-iron oxide (MXI) 2D material by selectively exfoliating an Al layer followed by magnetic ferric oxide intercalation for phosphate sequestration in water.66 Compared with commercial adsorbents, the MXI nanocomposite exhibits superior treatment capacities (2100 kg and 2400 kg kg−1 sorbent in simulated and real phosphate wastewater tests, respectively), with fast kinetics. The grown ultrafine nano-Fe2O3 particles intercalate into the interior layers of MXene, releasing the overlapped layers and further forming Ti–OH terminated layered surface within the MXene and Fe–OH exchanged sites of ferric oxide, and contribute to the unique phosphate sequestration behaviors. Additionally, after phosphate adsorption, the material can be recycled using binary alkaline brine solutions. Wu et al. fabricated an electrochemical tyrosinase biosensor based on the Ti3C2Tx MXene material, which demonstrates good repeatability and reproducibility, long-term stability and high recovery for the detection of phenol in water.67 The adsorption mechanism involves the oxidation–reduction of phenol by the tyrosinase biosensor.
For clarity, the specific uptake capacities of MXenes and their derivatives for several pollutants are listed in Table 1.
| MXene or derivative | Pollutant | Uptake capacity | Reference |
|---|---|---|---|
| Ti3C2(OH/ONa)xF2−x | Pb(II) | 140 mg g−1 | 43 |
| Ti3C2Tx | Cr(VI) | 250 mg g−1 | 46 |
| Ti3C2(OH)0.8F1.2 | Cr(VI) | 62 mg g−1 | 47 |
| TiO2–C nanocomposite | Cr(VI) | 225 mg g−1 | 47 |
| Ti3C2Tx | Ba(II) | 9.3 mg g−1 (for initial barium concentration of 55 ppm) | 48 |
| Ti3C2Tx | Cu(II) | 78.45 mg g−1 | 49 |
| Ti3C2Tx | Methylene blue | 39 mg g−1 | 53 |
| Ti3C2Tx | Methylene blue | 100 mg g−1 | 54 |
| LiOH–Ti3C2Tx | Methylene blue | 121 mg g−1 | 54 |
| NaOH–Ti3C2Tx | Methylene blue | 189 mg g−1 | 54 |
| V2CTx | U(VI) | 174 mg g−1 | 58 |
| V2C(OH)2 | U(VI) | 536 mg g−1 (theoretical) | 58 |
| Ti2C | Pb | 2560 mg g−1 (theoretical) | 45 |
| Ti2CH2 | Pb | 1280 mg g−1 (theoretical) | 45 |
| Ti3C2(OH)2 | U(VI) | 595.3 mg g−1 (theoretical for [UO2(H2O)5]2+) | 60 |
| M2C (M = Ti, Zr, Hf, V, CO2, Nb, Ta, Cr, Mo, and W) | 103–363 mg g−1 (theoretical) | 64 |
Besides adsorption, MXene materials can also be fabricated into a membrane form and used as a sieve for the filtration of pollutant species in wastewater. Ren et al. fabricated Ti3C2Tx membranes using a vacuum filtration method and conducted permeation tests for a series of cations.68 Their results revealed that the separation properties of the membranes are highly dependent on the size and charge of the cations, where, the permeation of cations decreases with an increase in size and charge, and cations with hydration radii larger than the interlayer spacing are nonpermeable (e.g. methylene blue ions). The Ti3C2Tx membranes demonstrated a better performance than graphene oxide in the separation of higher charged cations under the same test conditions. The hydrophilic nature of Ti3C2Tx accompanied by the presence of H2O between the layers is attributed to the ultrafast water flux of 37.4 L (bar h m2)−1. Ding et al. fabricated Ti3C2Tx membranes with expanded microchannels to further increase the water flux.69 During the fabrication process, colloidal Fe(OH)3 was employed as the intercalation medium to increase the interlayer spacing. The resulting Ti3C2Tx membranes exhibited an excellent water permeation performance (higher than 1000 L (bar h m2)−1) with a favorable rejection rate (over 90%) for Evans blue molecules. Berdiyorov et al. investigated the mechanism of the charge-selective permeation property of Ti3C2(OH)2 MXene using first-principles density functional theory.70 They found that this phenomenon originates from the charged nature of the MXene layers, where, ions with different charge states have different energy barriers for the intercalation between the MXene layers due to electrostatic interactions. The calculated energy barriers are 0.14 eV, 0.26 eV, and 0.34 eV for Na+, Mg2+, and Al3+, respectively.
4 Summary and outlook
In summary, 2D transition metal carbides/nitrides, i.e. MXenes, have demonstrated very promising results in the adsorptive remediation of polar and ionic pollutants, including heavy metal ions, organic dyes, radionuclides and gaseous pollutants. Their overall performances seem better than that of conventional pollutant adsorbents and even other low-dimensional materials, such as carbon nanotubes and graphene. Nevertheless, there are still many open questions that need to be addressed before these materials can be used practically.Although theoretical simulations have predicted a large number of MXene materials, the number of the experimentally synthesized MXenes is limited. For example, calculations have shown that Hf2CO2 MXene possesses excellent thermal and electrical properties to be a promising semiconductor,55 but it is yet to be synthesized experimentally. Thus, the synthesis of the large number of MXenes becomes an important research direction. We expect there are still many MXenes that are more suitable as adsorbents, and worth investigating. Comparative studies between the properties of the newly obtained and readily available MXenes should be conducted.
Apparently, the functional groups on the surfaces and delamination conditions of MXenes are strongly related to their adsorption capabilities (in fact, also to most other chemical properties). Large discrepancies are often found between the theoretical and experimental uptake capacities, which are generally attributed to non-ideal surface conditions (e.g. ref. 58). Therefore, the development of new synthetic procedures and post treatments to achieve uniform functional groups (such as hydroxyl, fluorine or oxygen) and full delamination are highly necessary. Non-terminated MXenes, the surfaces of which are comprised of metal atoms, are considered highly reactive,45 and thus need to be further studied for adsorption applications, especially experimentally.
Since it is reported that Ti3C2Tx MXene can be oxidized to titania over a long period in water,53 the stability of MXene materials needs to be critically considered when used as adsorbents, especially in water. Therefore, the stability of various MXenes in water together with their adsorption capacities are highly worth investigating. Highly stable and strongly adsorptive MXenes are desirable in practical applications.
Toxicity is a significant issue in all applications. It is very recently reported that delaminated Ti3C2 MXene has effects on the viability of both normal and cancerous cells.70 Although this is not an exciting result, deeper investigations on the toxicity of MXenes are needed.
To evaluate the reliability and lifespan of recycled MXenes used as adsorbent materials, more careful investigations are still required. Especially, clarification of the exact mechanism of the decline in performance after repeated use is of high importance for practical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11505006, 11675192) and Beijing Natural Science Foundation (Grant No. 2182042), and Zhengzhou Ruitai Refractory Technology Co. Ltd, China.References
- G. F. Vandegrift, D. T. Reed and I. R. Tasker, Environmental Remediation: Removing Organic and Metal Ion Pollutants, ACS Publications, 1992 Search PubMed.
- B. Gu, Y. Ku and P. M. Jardine, Environ. Sci. Technol., 2004, 38, 3184–3188 CrossRef PubMed.
- Q. Zhang, N. Wang, L. Zhao, T. Xu and Y. Cheng, ACS Appl. Mater. Interfaces, 2013, 5, 1907–1912 Search PubMed.
- F. Fu, L. Xie, B. Tang, Q. Wang and S. Jiang, Chem. Eng. J., 2012, 189, 283–287 CrossRef.
- S. Kerisit and C. Liu, Environ. Sci. Technol., 2014, 48, 3899–3907 CrossRef PubMed.
- K. Y. Foo and B. H. Hameed, J. Hazard. Mater., 2010, 175, 1–11 CrossRef PubMed.
- S. S. Gupta and K. G. Bhattacharyya, Phys. Chem. Chem. Phys., 2012, 14, 6698–6723 RSC.
- S. Wang and Y. Peng, Chem. Eng. J., 2010, 156, 11–24 CrossRef.
- W. W. Ngah, L. C. Teong and M. Hanafiah, Carbohydr. Polym., 2011, 83, 1446–1456 CrossRef.
- A. A. Adeyemo, I. O. Adeoye and O. S. Bello, Toxicol. Environ. Chem., 2012, 94, 1846–1863 CrossRef.
- L. Yuan, M. Tian, J. Lan, X. Cao, X. Wang, Z. Chai, J. Gibson and W. Shi, Chem. Commun., 2018, 54, 370–373 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef PubMed.
- J. N. Coleman, M. Lotya, A. O Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De and R. J. Smith, Science, 2011, 331, 568–571 CrossRef PubMed.
- R. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082–5104 CrossRef PubMed.
- L. Yuan, Z. Bai, R. Zhao, Y. Liu, Z. Li, S. Chu, L. Zheng, J. Zhang, Y. Zhao and Z. Chai, ACS Appl. Mater. Interfaces, 2014, 6, 4786–4796 Search PubMed.
- K. V. Wong and B. Bachelier, J. Energy Resour. Technol., 2014, 136, 021601 CrossRef.
- F. Perreault, A. F. De Faria and M. Elimelech, Chem. Soc. Rev., 2015, 44, 5861–5896 RSC.
- S. Wang, H. Sun, H. Ang and M. O. Tadé, Chem. Eng. J., 2013, 226, 336–347 CrossRef.
- O. Chen, Y. Lin, W. Cao and C. Chang, Mater. Lett., 2017, 190, 280–282 CrossRef.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef PubMed.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef PubMed.
- M. Khazaei, M. Arai, T. Sasaki, C. Y. Chung, N. S. Venkataramanan, M. Estili, Y. Sakka and Y. Kawazoe, Adv. Funct. Mater., 2013, 23, 2185–2192 CrossRef.
- B. Anasori, Y. Xie, M. Beidaghi, J. Lu, B. C. Hosler, L. Hultman, P. R. Kent, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2015, 9, 9507–9516 CrossRef PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 Search PubMed.
- Y. Lee, S. B. Cho and Y. Chung, ACS Appl. Mater. Interfaces, 2014, 6, 14724–14728 Search PubMed.
- J. Guo, Y. Sun, B. Liu, Q. Zhang and Q. Peng, J. Alloys Compd., 2017, 712, 752–759 CrossRef.
- Q. Hu, D. Sun, Q. Wu, H. Wang, L. Wang, B. Liu, A. Zhou and J. He, J. Phys. Chem. A, 2013, 117, 14253–14260 CrossRef PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef.
- J. Lei, X. Zhang and Z. Zhou, Front. Phys., 2015, 10, 276–286 CrossRef.
- M. Ashton, R. G. Hennig and S. B. Sinnott, Appl. Phys. Lett., 2016, 108, 023901 CrossRef.
- V. M. H. Ng, H. Huang, K. Zhou, P. S. Lee, W. Que, J. Z. Xu and L. B. Kong, J. Mater. Chem. A, 2017, 5, 3039–3068 Search PubMed.
- J. Zhou, X. Zha, F. Y. Chen, Q. Ye, P. Eklund, S. Du and Q. Huang, Angew. Chem., Int. Ed., 2016, 128, 5092–5097 CrossRef.
- J. Zhou, X. Zha, X. Zhou, F. Chen, G. Gao, S. Wang, C. Shen, T. Chen, C. Zhi and P. Eklund, ACS Nano, 2017, 11, 3841–3850 CrossRef PubMed.
- X. Wang, C. Garnero, G. Rochard, D. Magne, S. Morisset, S. Hurand, P. Chartier, J. Rousseau, T. Cabioc'H and C. Coutanceau, J. Mater. Chem. A, 2017, 5, 22012–22023 Search PubMed.
- M. A. Hope, A. C. Forse, K. J. Griffith, M. R. Lukatskaya, M. Ghidiu, Y. Gogotsi and C. P. Grey, Phys. Chem. Chem. Phys., 2016, 18, 5099–5102 RSC.
- H. Wang, M. Naguib, K. Page, D. J. Wesolowski and Y. Gogotsi, Chem. Mater., 2015, 28, 349–359 CrossRef.
- Y. Xie, M. Naguib, V. N. Mochalin, M. W. Barsoum, Y. Gogotsi, X. Yu, K. Nam, X. Yang, A. I. Kolesnikov and P. R. Kent, J. Am. Chem. Soc., 2014, 136, 6385–6394 CrossRef PubMed.
- C. Xu, L. Wang, Z. Liu, L. Chen, J. Guo, N. Kang, X. Ma, H. Cheng and W. Ren, Nat. Mater., 2015, 14, 1135 CrossRef PubMed.
- X. Zha, J. Yin, Y. Zhou, Q. Huang, K. Luo, J. Lang, J. S. Francisco, J. He and S. Du, J. Phys. Chem. C, 2016, 120, 15082–15088 Search PubMed.
- Q. Peng, J. Guo, Q. Zhang, J. Xiang, B. Liu, A. Zhou, R. Liu and Y. Tian, J. Am. Chem. Soc., 2014, 136, 4113–4116 CrossRef PubMed.
- J. Guo, Q. Peng, H. Fu, G. Zou and Q. Zhang, J. Phys. Chem. C, 2015, 119, 20923–20930 Search PubMed.
- J. Guo, H. Fu, G. Zou, Q. Zhang, Z. Zhang and Q. Peng, J. Alloys Compd., 2016, 684, 504–509 CrossRef.
- Y. Ying, Y. Liu, X. Wang, Y. Mao, W. Cao, P. Hu and X. Peng, ACS Appl. Mater. Interfaces, 2015, 7, 1795–1803 Search PubMed.
- G. Zou, J. Guo, Q. Peng, A. Zhou, Q. Zhang and B. Liu, J. Mater. Chem. A, 2016, 4, 489–499 Search PubMed.
- A. K. Fard, G. Mckay, R. Chamoun, T. Rhadfi, H. Preud'Homme and M. A. Atieh, Chem. Eng. J., 2017, 317, 331–342 CrossRef.
- A. Shahzad, K. Rasool, W. Miran, M. Nawaz, J. Jang, K. Mahmoud and D. S. Lee, ACS Sustainable Chem. Eng., 2017, 5, 11481–11488 CrossRef.
- X. Guo, X. Zhang, S. Zhao, Q. Huang and J. Xue, Phys. Chem. Chem. Phys., 2016, 18, 228–233 RSC.
- J. Yang, S. Zhang, J. Ji and S. Wei, Acta Phys.-Chim. Sin., 2015, 31, 369–376 Search PubMed.
- X. Zhu, B. Liu, H. Hou, Z. Huang, K. M. Zeinu, L. Huang, X. Yuan, D. Guo, J. Hu and J. Yang, Electrochim. Acta, 2017, 248, 46–57 CrossRef.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334–14338 Search PubMed.
- W. Zheng, P. Zhang, W. Tian, X. Qin, Y. Zhang and Z. M. Sun, Mater. Chem. Phys., 2018, 206, 270–276 CrossRef.
- Y. Gao, L. Wang, A. Zhou, Z. Li, J. Chen, H. Bala, Q. Hu and X. Cao, Mater. Lett., 2015, 150, 62–64 CrossRef.
- C. Peng, X. Yang, Y. Li, H. Yu, H. Wang and F. Peng, ACS Appl. Mater. Interfaces, 2016, 8, 6051–6060 Search PubMed.
- W. Zhou, J. Zhu, F. Wang, M. Cao and T. Zhao, Mater. Lett., 2017, 206, 237–240 CrossRef.
- L. Wang, L. Yuan, K. Chen, Y. Zhang, Q. Deng, S. Du, Q. Huang, L. Zheng, J. Zhang and Z. Chai, ACS Appl. Mater. Interfaces, 2016, 8, 16396–16403 Search PubMed.
- Y. Zhang, Z. Zhou, J. Lan, C. Ge, Z. Chai, P. Zhang and W. Shi, Appl. Surf. Sci., 2017, 426, 572–578 CrossRef.
- Y. Zhang, J. Lan, L. Wang, Q. Wu, C. Wang, T. Bo, Z. Chai and W. Shi, J. Hazard. Mater., 2016, 308, 402–410 CrossRef PubMed.
- L. Wang, W. Tao, L. Yuan, Z. Liu, Q. Huang, Z. Chai, J. K. Gibson and W. Shi, Chem. Commun., 2017, 53, 12084–12087 RSC.
- X. Yu, Y. Li, J. Cheng, Z. Liu, Q. Li, W. Li, X. Yang and B. Xiao, ACS Appl. Mater. Interfaces, 2015, 7, 13707–13713 Search PubMed.
- B. Xiao, Y. Li, X. Yu and J. Cheng, Sens. Actuators, B, 2016, 235, 103–109 CrossRef.
- A. Morales-Garcia, A. F. Fernández, F. Viñes and F. Illas, J. Mater. Chem. A, 2018, 6, 3381–3385 Search PubMed.
- S. J. Kim, H. J. Koh, C. E. Ren, O. Kwon, K. Maleski, S. Y. Cho, B. Anasori, C. K. Kim, Y. K. Choi and J. Kim, ACS Nano, 2018, 12, 986–993 CrossRef PubMed.
- Q. Zhang, J. Teng, G. Zou, Q. Peng, Q. Du, T. Jiao and J. Xiang, Nanoscale, 2016, 8, 7085–7093 RSC.
- L. Wu, X. Lu, Dhanjai, Z. S. Wu, Y. Dong, X. Wang, S. Zheng and J. Chen, Biosens. Bioelectron., 2018, 107, 69 CrossRef PubMed.
- C. E. Ren, K. B. Hatzell, M. Alhabeb, Z. Ling, K. A. Mahmoud and Y. Gogotsi, J. Phys. Chem. Lett., 2015, 6, 4026–4031 CrossRef PubMed.
- L. Ding, Y. Wei, Y. Wang, H. Chen, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 1825–1829 CrossRef PubMed.
- G. R. Berdiyorov, M. E. Madjet and K. A. Mahmoud, Appl. Phys. Lett., 2016, 108, 113110 CrossRef.
- A. M. Jastrzębska, A. Szuplewska, T. Wojciechowski, M. Chudy, W. Ziemkowska, L. Chlubny, A. Rozmysłowska and A. Olszyna, J. Hazard. Mater., 2017, 339, 1–8 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |


