Open Access Article
Open Access ArticleDesign, synthesis and biological evaluation of N-arylsulfonyl carbazoles as novel anticancer agents†
Xin You‡
a,
Daqian Zhu‡ab,
Wenhua Lua,
Yichen Suna,
Shuang Qiaoab,
Bingling Luoab,
Yongliang Duab,
Rongbiao Pib,
Yumin Hua,
Peng Huang*a and
Shijun Wen *ab
*ab
aSun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University, 651 Dongfeng East Road, Guangzhou 510060, China. E-mail: wenshj@sysucc.org.cn; huangpeng@sysucc.org.cn
bSchool of Pharmaceutical Sciences, Sun Yat-sen University, 132 Waihuan East Road, Guangzhou 510006, China
First published on 10th May 2018
Abstract
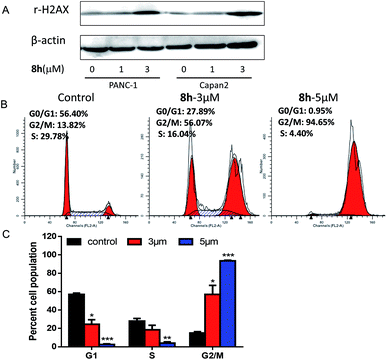
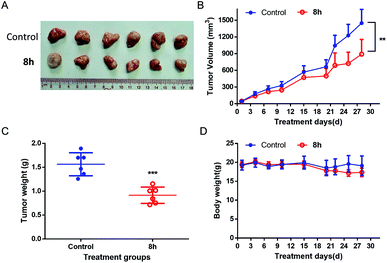
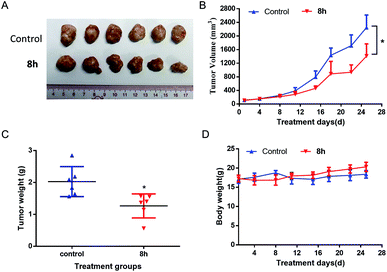
In this work, a set of structurally diverse synthetic carbazoles was screened for their anticancer activities. According to structure–activity relationship studies, carbazoles with an N-substituted sulfonyl group exhibited better anticancer activity. Moreover, compound 8h was discovered to show the most potent anticancer effects on Capan-2 cells by inducing apoptosis and cell cycle arrest in G2/M phase. Finally, the in vivo study demonstrated that 8h prevented the tumor growth in PANC-1 and Capan-2 xenograft models without apparent toxicity.
1. Introduction
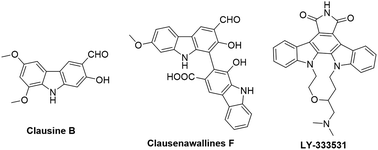
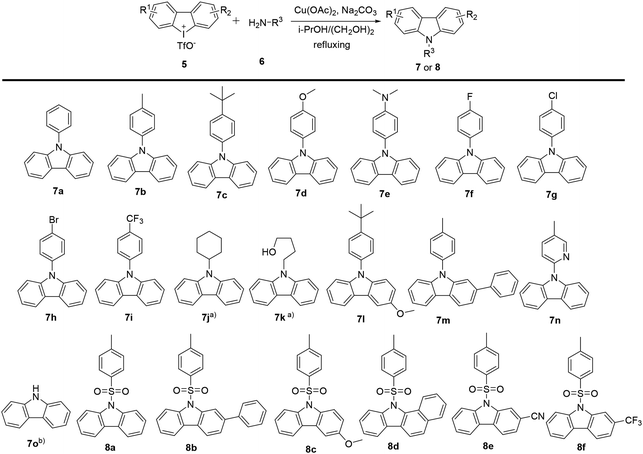
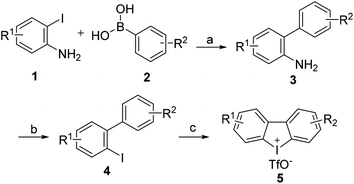
Carbazoles and their derivatives are of great interest in the research fields of synthetic chemistry and medicinal chemistry, due to their tricyclic structure and widespread pharmacological properties.1 In general, carbazoles constitute a useful class of polycyclic aromatic drugs displaying diverse activities including antibacterial,2 neuroprotective,3 anti-inflammatory,4 antioxidant,5 and anti-HIV functions.6 More importantly, carbazoles are found to have effective antiproliferative activity on tumor cells (Fig. 1). Clausine B, a naturally occurring carbazole alkaloid, was found to be active (IC50 < 30 μg mL−1) against four human cancer cell lines including MDA-MB-231 (breast cancer), HeLa (cervical cancer), CAOV3 (ovarian cancer) and HepG2 (hepatic cancer).7 Clausenawallines F showed cytotoxicity against oral cavity cancer (KB) and small-cell lung cancer (NCI-H187) with IC50 values of 10.2 μM and 4.5 μM, respectively.8 Due to the important pharmacological activities of the reported natural occurring carbazoles, an array of compounds containing carbazole motifs has been designed and synthesized for biological evaluation. For example, LY-333531 was reported as an anticancer agent by inhibiting Pim-1 kinase with IC50 value of 0.2 μM.9,10In the recent years, other research groups and ours have demonstrated that cyclic diphenyliodoniums (CDIs) are useful synthetic synthons for complex polycyclic arenes.11–20 Due to the structural similarity of carbazoles and CDIs, it was envisioned that the insertion of nitrogen into the CDIs would be able to afford carbazoles. Indeed, we previously developed a synthetic strategy for carbazoles via the reaction of CDIs with various amines which was mediated by Cu(OAc)2 at low cost.11 With this strategy, an in-house chemical library of carbazoles with structural diversity and complexity was successfully built. The previous biological neuroprotective screening of the carbazole library found that some N-phenyl carbazoles showed good neuroprotection of neuronal cells HT22 against cell injury induced by glutamate or homocysteic acid.12 Pancreatic cancer is one leading cause of cancer-related death and remains a serious clinical problem due to poor prognosis and the limited drug options.21 In the continuation of our efforts to discover novel and potent anticancer agents, our in-house synthetic carbazoles were further screened for their potential antiproliferation of pancreatic cancer cells. The screening assay provided a structural starting point for further structure–activity study. In this work, we report the design and synthesis of structurally diversified carbazoles and their biological evaluation, leading to the discovery of novel N-arylsulfonyl carbazoles with both in vitro and in vivo antitumor therapeutic activities.
2. Results and discussion
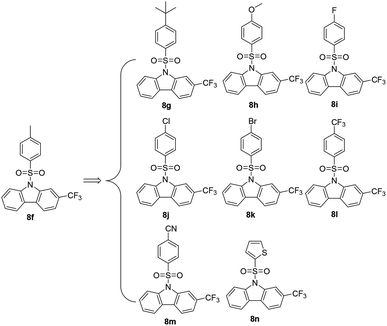
2.1 Chemistry
2.2 Biology
| Compound | Inhibitory rate of PANC-1 viability (%) | Inhibitory rate of Capan-2 viability (%) | ||
|---|---|---|---|---|
| 30 μM | 10 μM | 30 μM | 10 μM | |
| 7a | 25.02 | 20.38 | 31.48 | 25.25 |
| 7b | 12.59 | 16.42 | 21.01 | 23.82 |
| 7c | 22.8 | 18.61 | 20.65 | 15.85 |
| 7d | 25.9 | 17.65 | 34.8 | 12.91 |
| 7e | 43.5 | 24.4 | 49.29 | 20.56 |
| 7f | 11.75 | 34.27 | 22.55 | 28.82 |
| 7g | 3.8 | 3.81 | 20.32 | 11.75 |
| 7h | 71.1 | 19.05 | 38.12 | 18.58 |
| 7i | 54.31 | 25.61 | 14.53 | 20.05 |
| 7j | 19.78 | 18.18 | 15.93 | 32.61 |
| 7k | 44.34 | 36.68 | 35.65 | 33.72 |
| 7l | 5.4 | 23.76 | 35.98 | 30.36 |
| 7m | 9.61 | 13.16 | 42.79 | 38.41 |
| 7n | 34.73 | 33.84 | 24.94 | 20.91 |
| 7o | 35.9 | 36.27 | ND | 27.03 |
| 8a | 43.66 | 41.55 | 67.81 | 61.57 |
| 8b | 59.8 | 63.98 | 70.54 | 66.79 |
| 8c | 54.17 | 54.71 | 69.39 | 57.72 |
| 8d | 53.92 | 34.94 | 72.62 | 72.04 |
| 8e | 58.6 | 61.2 | 28.46 | 28.39 |
| 8f | 56.67 | 48.05 | 82.2 | 66.6 |
| Compound | Inhibitory rate of PANC-1 viability (%) | Inhibitory rate of Capan-2 viability (%) | ||
|---|---|---|---|---|
| 30 μM | 10 μM | 30 μM | 10 μM | |
| 8g | 35.5 | 25.1 | 36.1 | 44.1 |
| 8h | 72.2 | 68.2 | 86.8 | 89.4 |
| 8i | 5.6 | 13.42 | 19.55 | 14.4 |
| 8j | 36.8 | 16.5 | 58.7 | 52.4 |
| 8k | 53.9 | 54.8 | 84.9 | 73.3 |
| 8l | 43.3 | 8.49 | 29.7 | 31.41 |
| 8m | 76.9 | 59.9 | 80.2 | 64.0 |
| 8n | 61.6 | 57.3 | 40.4 | 49.1 |
| Compound | Cell line (IC50 in μM) | |||||
|---|---|---|---|---|---|---|
| PANC-1 | Capan-2 | SGC-7901 | HCT-116 | HL-60 | HFL-1 | |
| a NT: not tested. Values are expressed as mean ± standard deviation (SD) of three independent duplicate experiments. | ||||||
| 8h | 3.69 ± 0.30 | 1.30 ± 0.28 | 2.25 ± 0.21 | 1.90 ± 0.14 | 2.20 ± 0.70 | 20.04 ± 2.48 |
| 5-Fu | 19.31 ± 2.76 | 32.40 ± 3.70 | 16.56 ± 1.32 | 10.03 ± 0.88 | NT | NT |
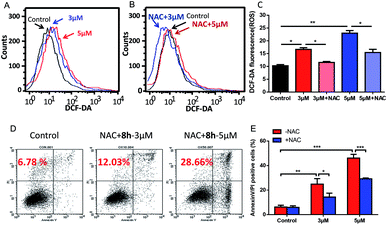 | ||
| Fig. 5 Induction of ROS increase by compound 8h and its partial reversion by an antioxidant NAC. (A) DCF-DA staining showed the effect of 3 μM (blue line) and 5 μM (red line) of 8h on cellular ROS levels after 24 h treatment. (B and C) Pretreatment with NAC effectively reversed the ROS accumulation. Capan-2 cells were pre-treated with 3 mM NAC for 1 h and then incubated with compound 8h (3 or 5 μM) for 24 h. Error bars represent mean ± SD. (D and E) Compound 8h-induced apoptosis in Capan-2 cells was partially prevented by pre-treatment with NAC compared to Fig. 4. Capan-2 cells were pre-treated with 3 mM NAC for 1 h, and then incubated with the indicated concentrations (3 or 5 μM) of 8h for 24 h. Error bars represent mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001. | ||
3. Conclusions
In conclusion, a chemical library of N-substituted carbazoles was synthesized conveniently, and all the compounds were screened for a potential anticancer activity. Results demonstrated that the N-arylsulfonyl substituted carbazoles showed significant antiproliferative ability. A preliminary SAR study found that the synthetic compound 8h displayed the most potent cytotoxic activity against PANC-1, Capan-2, SGC-7901, HCT-116, HL-60 cancer cells. Moreover, compound 8h exhibited promising in vivo anticancer therapeutic efficacy in PANC-1 and Capan-2 xenograft mice models without an apparent side effect. The following preliminary investigation of its action model mechanism implied that the ROS mediated-apoptosis as well as the G2/M cell cycle arrest might contribute to the anticancer activity of 8h. Environmentally benign and easily accessible synthesis and the potent anticancer activity may make these N-arylsulfonyl substituted carbazoles to be promising drug candidates to kill cancer cells.4. Experiments
4.1 Chemistry
The general procedure for the preparation of these carbazoles was exemplified by the synthesis of 8h:
To a stirred solution of 3-(trifluoromethyl)dibenzo[b,d]iodol-5-ium trifluoromethanesulfonate (100 mg, 0.2 mmol, 1 eq.) in i-PrOH (1.8 mL) and ethylene glycol (0.2 mL), was added 4-methoxybenzenesulfonamide (151 mg, 0.8 mmol, 4 equiv.), sodium carbonate (64 mg, 0.6 mmol, 3 equiv.), Cu(OAc)2 (8 mg, 0.04 mmol, 0.2 equiv.). The reaction proceeded at a reflux for 16 h under argon atmosphere before i-PrOH was removed by a rotary evaporation. The remained mixture was partitioned between water and EtOAc, and the aqueous phase was extracted with EtOAc. The combined organic phases were washed with H2O and brine, dried over anhydrous Na2SO4, evaporated in a vacuo. The residue was purified by column chromatography on a silica gel (PE/EtOAc = 15/1–5/1) to provide carbazole 8h.
4.1.3.1 9-((4-(tert-Butyl)phenyl)sulfonyl)-2-(trifluoromethyl)-9H-carbazole (8g). A white solid, 63% yield. 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 8.37 (d, J = 8.4 Hz, 1H), 8.00 (dd, J = 16.8, 7.9 Hz, 2H), 7.77 (d, J = 8.7 Hz, 2H), 7.63 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 7.9 Hz, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.37 (d, J = 8.7 Hz, 2H), 1.21 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 158.3, 139.3, 137.9, 134.9, 128.8, 126.6, 126.5, 125.2, 124.3, 120.8, 120.5, 115.3, 112.5, 35.4, 31.0 ppm. HRMS calcd for C23H20F3NO2S [M + H]+: 432.1245, found: 432.1239.
4.1.3.2 9-((4-Methoxyphenyl)sulfonyl)-2-(trifluoromethyl)-9H-carbazole (8h). A white solid, 69% yield. 1H NMR (400 MHz, CDCl3) δ 8.63 (s, 1H), 8.36 (d, J = 8.5 Hz, 1H), 7.98 (dd, J = 17.1, 8.0 Hz, 2H), 7.76 (d, J = 8.9 Hz, 2H), 7.62 (d, J = 8.0 Hz, 1H), 7.57 (t, J = 7.4 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 6.79 (d, J = 8.9 Hz, 2H), 3.74 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 164.2, 139.3, 137.9, 129.4, 129.2, 128.8, 128.7, 125.9, 125.2, 124.3, 123.1, 120.8, 120.5, 115.3, 114.5, 112.6, 112.5, 55.7 ppm. HRMS calcd for C20H14F3NO3S [M + H]+: 406.0724, found: 406.0721.
4.1.3.3 9-((4-Fluorophenyl)sulfonyl)-2-(trifluoromethyl)-9H-carbazole (8i). A white solid, 46% yield. 1H NMR (400 MHz, CDCl3) δ 8.61 (s, 1H), 8.34 (d, J = 8.3 Hz, 1H), 7.99 (dd, J = 17.7, 7.9 Hz, 2H), 7.89–7.78 (m, 2H), 7.65 (d, J = 7.9 Hz, 1H), 7.59 (t, J = 7.9 Hz, 1H), 7.44 (t, J = 7.3 Hz, 1H), 7.03 (t, J = 7.8 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 167.3, 164.8, 139.2, 137.8, 133.6, 133.6, 129.7, 129.5, 129.4, 129.0, 125.8, 125.4, 124.7, 123.1, 120.9, 120.6, 116.9, 116.7, 115.3, 112.6, 112.5 ppm. HRMS calcd for C19H11F4NO2S [M − H]−: 392.0369, found: 392.0371.
4.1.3.4 9-((4-Chlorophenyl)sulfonyl)-2-(trifluoromethyl)-9H-carbazole (8j). A white solid, 52% yield. 1H NMR (400 MHz, CDCl3) δ 8.60 (s, 1H), 8.33 (d, J = 8.4 Hz, 1H), 7.99 (dd, J = 18.1, 8.0 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.65 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 7.8 Hz, 1H), 7.44 (t, J = 7.5 Hz, 1H), 7.32 (d, J = 8.6 Hz, 2H). 13CNMR (100 MHz, CDCl3) δ 141.1, 139.2, 137.8, 136.0, 129.7, 129.4, 129.0, 128.0, 125.4, 124.8, 121.3, 121.3, 121.0, 120.7, 115.3, 112.6, 112.6 ppm. HRMS calcd for C19H11ClF3NO2S [M + H]+: 410.0029, found: 410.0028.
4.1.3.5 9-((4-Bromophenyl)sulfonyl)-2-(trifluoromethyl)-9H-carbazole (8k). A white solid, 49% yield. 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H), 8.33 (d, J = 8.5 Hz, 1H), 7.99 (dd, J = 17.9, 8.0 Hz, 2H), 7.66 (d, J = 8.7 Hz, 3H), 7.58 (t, J = 7.8 Hz, 1H), 7.48 (d, J = 8.7 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 139.2, 137.8, 136.5, 132.7, 129.7, 129.5, 129.0, 128.0, 125.5, 124.8, 121.3, 121.0, 120.7, 115.4, 112.6 ppm. HRMS calcd for C19H11BrF3NO2S [M + H]+: 453.9724, found: 453.9718.
4.1.3.6 2-(Trifluoromethyl)-9-((4-(trifluoromethyl)phenyl)sulfonyl)-9H-carbazole (8l). A white solid, 57% yield. 1H NMR (400 MHz, CDCl3) δ 8.61 (s, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.00 (dd, J = 18.5, 7.9 Hz, 2H), 7.93 (d, J = 8.1 Hz, 2H), 7.67 (d, J = 8.1 Hz, 1H), 7.64–7.57 (m, 3H), 7.45 (t, J = 7.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 140.9, 139.1, 137.7, 136.0, 135.7, 129.9, 129.5, 129.1, 127.2, 126.6, 126.6, 125.7, 125.5, 125.0, 124.3, 123.0, 121.5, 121.0, 120.8, 115.3, 112.6, 112.5 ppm. HRMS calcd for C20H11F6NO2S [M − H]−: 442.0337, found: 442.0335.
4.1.3.7 4-((2-(Trifluoromethyl)-9H-carbazol-9-yl)sulfonyl)benzonitrile (8m). A white solid, 51% yield. 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.00 (dd, J = 18.6, 7.9 Hz, 2H), 7.89 (d, J = 8.6 Hz, 2H), 7.67 (d, J = 8.2 Hz, 1H), 7.63 (dd, J = 7.6, 5.0 Hz, 2H), 7.62–7.57 (m, 1H), 7.46 (t, J = 7.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 141.2, 138.9, 137.6, 133.2, 130.0, 129.6, 129.2, 127.2, 125.6, 125.2, 121.7, 121.7, 121.1, 120.8, 118.1, 116.8, 115.3, 112.6, 112.6 ppm. HRMS calcd for C20H11F3N2O2S [M − H]−: 399.0415, found: 399.0415.
4.1.3.8 9-(Thiophen-2-ylsulfonyl)-2-(trifluoromethyl)-9H-carbazole (8n). A white solid, 72% yield. 1H NMR (400 MHz, CDCl3) δ 8.61 (s, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.00 (dd, J = 16.7, 7.8 Hz, 2H), 7.66 (d, J = 8.1 Hz, 1H), 7.63–7.56 (m, 2H), 7.48–7.42 (m, 2H), 6.92 (dd, J = 5.0, 3.9 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 139.0, 137.6, 136.9, 133.4, 132.9, 129.5, 129.2, 128.8, 127.5, 125.7, 125.4, 124.7, 123.0, 121.1, 120.7, 120.4, 115.5, 112.8 ppm. HRMS calcd for C17H10F3NO2S2 [M + H]+: 382.0183, found: 382.0181.
4.2 Biological assay
| Inhibitory rate of cell viability (%) = 100 − cell viability (%) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, then incubated with secondary antibody at a concentration of 1
1000, then incubated with secondary antibody at a concentration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
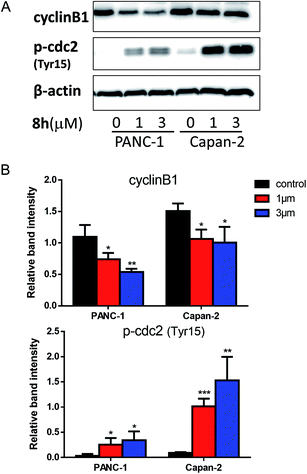
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and read using ECL detection system (Bio-Rad, Richmond, CA). β-Actin was used as a loading control. Anti-γ-H2AX (phospho-Ser139), anti-cyclinB1, anti-phospho-cdc2 (Tyr15), and anti-β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). The expression ratio of cyclinB1 and p-cdc2 (Tyr15) proteins was quantified against β-actin using ImageJ software. One-way ANOVA was used for statistical analysis.
000 and read using ECL detection system (Bio-Rad, Richmond, CA). β-Actin was used as a loading control. Anti-γ-H2AX (phospho-Ser139), anti-cyclinB1, anti-phospho-cdc2 (Tyr15), and anti-β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). The expression ratio of cyclinB1 and p-cdc2 (Tyr15) proteins was quantified against β-actin using ImageJ software. One-way ANOVA was used for statistical analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by National Natural Science Foundation of China (81672952, 81430060), and Guangdong Science and Technology Program (2017A020215198).References
- A. W. Schmidt, K. R. Reddy and H. J. Knolker, Chem. Rev., 2012, 112, 3193–3328 CrossRef CAS PubMed.
- P. Y. Wang, H. S. Fang, W. B. Shao, J. Zhou, Z. Chen, B. A. Song and S. Yang, Bioorg. Med. Chem. Lett., 2017, 27, 4294–4297 CrossRef CAS PubMed.
- A. A. Pieper, S. Xie, E. Capota, S. J. Estill, J. Zhong, J. M. Long, G. L. Becker, P. Huntington, S. E. Goldman, C. H. Shen, M. Capota, J. K. Britt, T. Kotti, K. Ure, D. J. Brat, N. S. Williams, K. S. MacMillan, J. Naidoo, L. Melito, J. Hsieh, J. De Brabander, J. M. Ready and S. L. McKnight, Cell, 2010, 142, 39–51 CrossRef CAS PubMed.
- A. D. Favia, D. Habrant, R. Scarpelli, M. Migliore, C. Albani, S. M. Bertozzi, M. Dionisi, G. Tarozzo, D. Piomelli, A. Cavalli and M. De Vivo, J. Med. Chem., 2012, 55, 8807–8826 CrossRef CAS PubMed.
- D. Pratico, Trends Pharmacol. Sci., 2008, 29, 609–615 CrossRef CAS PubMed.
- K. M. Meragelman, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2000, 63, 427–428 CrossRef CAS.
- W. N. Wan Mohd Zain, A. Rahmat, F. Othman and T. Y. Yap, Malays J Med Sci, 2009, 16, 29–34 Search PubMed.
- W. Maneerat, T. Ritthiwigrom, S. Cheenpracha, T. Promgool, K. Yossathera, S. Deachathai, W. Phakhodee and S. Laphookhieo, J. Nat. Prod., 2012, 75, 741–746 CrossRef CAS PubMed.
- R. Akue-Gedu, E. Rossignol, S. Azzaro, S. Knapp, P. Filippakopoulos, A. N. Bullock, J. Bain, P. Cohen, M. Prudhomme, F. Anizon and P. Moreau, J. Med. Chem., 2009, 52, 6369–6381 CrossRef CAS PubMed.
- D. Drygin, M. Haddach, F. Pierre and D. M. Ryckman, J. Med. Chem., 2012, 55, 8199–8208 CrossRef CAS PubMed.
- D. Zhu, Q. Liu, B. Luo, M. Chen, R. Pi, P. Huang and S. Wen, Adv. Synth. Catal., 2013, 355, 2172–2178 CrossRef CAS.
- D. Zhu, M. Chen, M. Li, B. Luo, Y. Zhao, P. Huang, F. Xue, S. Rapposelli, R. Pi and S. Wen, Eur. J. Med. Chem., 2013, 68, 81–88 CrossRef CAS PubMed.
- D. Zhu, Y. Wu, B. Wu, B. Luo, A. Ganesan, F. H. Wu, R. Pi, P. Huang and S. Wen, Org. Lett., 2014, 16, 2350–2353 CrossRef CAS PubMed.
- Z. Liu, D. Zhu, B. Luo, N. Zhang, Q. Liu, Y. Hu, R. Pi, P. Huang and S. Wen, Org. Lett., 2014, 16, 5600–5603 CrossRef CAS PubMed.
- M. Wang, S. Chen and X. Jiang, Org. Lett., 2017, 19, 4916–4919 CrossRef CAS PubMed.
- M. Wang, Q. Fan and X. Jiang, Org. Lett., 2018, 20, 216–219 CrossRef CAS PubMed.
- M. Wang, J. Wei, Q. Fan and X. Jiang, Chem. Commun., 2017, 53, 2918–2921 RSC.
- Q. Tan, D. Zhou, T. Zhang, B. Liu and B. Xu, Chem. Commun., 2017, 53, 10279–10282 RSC.
- S. Riedmuller and B. J. Nachtsheim, Beilstein J. Org. Chem., 2013, 9, 1202–1209 CrossRef PubMed.
- H. Xie, M. Ding, M. Liu, T. Hu and F. Zhang, Org. Lett., 2017, 19, 2600–2603 CrossRef CAS PubMed.
- A. P. Klein, Nat. Rev. Cancer, 2013, 13, 66–74 CrossRef CAS PubMed.
- In Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval, ed. B. A. Teicher and P. A. Andrews, Humana press, Totowa, NJ, 2004, pp. 41–62 Search PubMed.
- C. Asche and M. Demeunynck, Anti-Cancer Agents Med. Chem., 2007, 7, 247–267 CrossRef CAS PubMed.
- M. Laiho and L. Latonen, Ann. Med., 2003, 35, 391–397 CrossRef CAS PubMed.
- K. Ishikawa, H. Ishii and T. Saito, DNA Cell Biol., 2006, 25, 406–411 CrossRef CAS PubMed.
- I. A. Shaltiel, L. Krenning, W. Bruinsma and R. H. Medema, J. Cell Sci., 2015, 128, 607–620 CrossRef CAS PubMed.
- R. Y. Zhao and R. T. Elder, Cell Res., 2005, 15, 143–149 CrossRef CAS PubMed.
- L. Tang, Y. Zhang, H. Pan, Q. Luo, X. M. Zhu, M. Y. Dong, P. C. Leung, J. Z. Sheng and H. F. Huang, Reprod. Biol. Endocrinol., 2009, 7, 144 CrossRef PubMed.
- S. Lim and P. Kaldis, Development, 2013, 140, 3079–3093 CrossRef CAS PubMed.
- T. Miyazaki and S. Arai, Cell Cycle, 2007, 6, 1419–1425 CrossRef CAS PubMed.
- J. M. Mates, J. A. Segura, F. J. Alonso and J. Marquez, Arch. Toxicol., 2012, 86, 1649–1665 CrossRef CAS PubMed.
- K. Sinha, J. Das, P. B. Pal and P. C. Sil, Arch. Toxicol., 2013, 87, 1157–1180 CrossRef CAS PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- J. Wang, B. Luo, X. Li, W. Lu, J. Yang, Y. Hu, P. Huang and S. Wen, Cell Death Dis., 2017, 8, e2887 CrossRef CAS PubMed.
- D. Trachootham, Y. Zhou, H. Zhang, Y. Demizu, Z. Chen, H. Pelicano, P. J. Chiao, G. Achanta, R. B. Arlinghaus, J. Liu and P. Huang, Cancer Cell, 2006, 10, 241–252 CrossRef CAS PubMed.
- A. M. Schrand, J. B. Lin and S. M. Hussain, Methods Mol. Biol., 2012, 906, 395–402 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02939c |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |