DOI:
10.1039/C8RA02729C
(Paper)
RSC Adv., 2018,
8, 16802-16814
Diastereoselective synthesis of novel spiro indanone fused pyrano[3,2-c]chromene derivatives following hetero-Diels–Alder reaction and in vitro anticancer studies†
Received
29th March 2018
, Accepted 23rd April 2018
First published on 8th May 2018
Abstract
The development of concise methods for the synthesis of small functionalised spirocyclic molecules is important in the search of new bioactive molecules. To contribute this, here we represent a diastereoselective oxa-hetero-Diels–Alder reaction for the synthesis of novel spiro indanone fused pyrano[3,2-c]chromene derivatives and studied their in vitro anticancer activities. Using previously less explored cyclic ketone i.e. indane-1,3-dione and 3-vinyl-2H-chromene derivatives, we obtained novel spiro-heterocyclic frameworks at the interphase between “drug-like” molecules and natural products. Various spiro indanone fused pyrano[3,2-c]chromene derivatives were synthesized regiospecifically bearing a quaternary stereocenter in high yields (up to 85%) with excellent diastereoselectivity in toluene using 4 Å MS as additive under reflux condition at 120 °C. In vitro cytotoxic studies of these compounds against MCF-7 (breast cancer), HCT-116 (colon cancer), H-357 (oral cancer), MD-MB-231(Breast cancer) cell lines were evaluated by MTT {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide} assay in vitro. The screening results revealed that many of the compounds are showing moderate to high levels of anticancer activities against the tested cancer cell lines and some displayed potent inhibitory activities in comparison to the commercial anticancer drug 5-fluorouracil (5-FU). Among the series, compound 3′c showed most potent cytotoxicity (15.0–27.5 μM) in three cancer cell lines (MCF-7, HCT-116 and MD-MB-231).
Introduction
Chiral indane frameworks, particularly indanone subunits, constitute a unique group of desirable targets in organic synthesis and attracted the attention of organic chemists and biologists because of their broad distribution in biologically active natural products and pharmaceutically active compounds such as fredericamycin, colephomone-D, raddenone and yenhusomidine.1,2 Due to their synthetic utility and application in pharmaceuticals, a variety of synthetic protocols have been developed for the synthesis of optically pure indanone skeleton.3 Of particular interest are methods for stereoselective formation of medicinally important spirocyclic indanone frameworks, such as those that enantioselectively generate a quaternary stereocenter, considered a challenging transformation.4–8 Organocatalyst mediated synthesis of oxa spirocyclic indanone scaffold was reported in the literature in 2013 by Peng et al.1 following Morita–Baylis–Hillman reaction. Although good stereoselectivity was observed in product formation, however the synthetic method suffers from drawbacks in reaction conditions and yields. Efficient cost effective synthesis methods of the oxa spirocyclic indanone backbone are needed in order to expand the usefulness of this intriguing combination of pharmacologically interesting pyran and indanone motifs.
On the other hand, the pyran ring is among one of the most widely investigated heterocycles.9 It is the core unit of benzopyran, chromene, flavonoids, coumaroin, xanthones and naphthoquinones, which exhibit diverse pharmacological activities.10–15 The benzopyran or chromene cores are prevalent in many natural products show fascinating therapeutic activities.16–22 In recent years, chromeno fused pyran based heterocyclic natural products such as Ethuliacoumarion and its derivatives, spiro oxindole fused pyranochromene, dihydropyranochromene etc.23,24 have attracted tremendous interest among researchers because they possess both a chromene and pyran moiety which has potential applications in medicinal chemistry and exhibit significant biological activities, such as anticancer, anthelmintic and molluscicidal activity (Fig. 1).
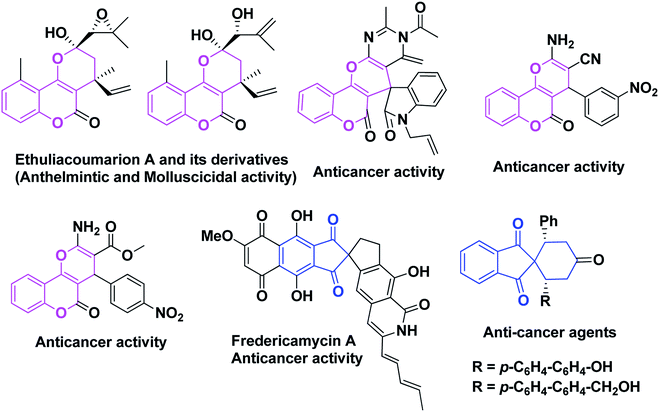 |
| | Fig. 1 Various natural and synthetic products based on biologically active indanone and pyranochromene scaffolds. | |
Due to the biomedical importance of chromeno fused pyran and spiro-indane-1,3-dione skeletons, combination of these two units in one molecule may benefit diversity of structure and drug discovery. The hetero-Diels–Alder (HDA) reaction is arguably the most powerful way of constructing six-membered heterocycles bearing a chiral tertiary or quaternary carbon centre in a single step from commercially available substrates.25 Although asymmetric HDA reactions of aldehydes as dienophile have been extensively studied,23 ketone substrates, in contrast, are much less reactive in HDA reactions due to electronic and steric properties, and are still challenging substrates for chemists.26
As a part of our continuing efforts for the synthesis of heterocycles, to combine the interesting and remarkable biological activities of both indanone and chromeno fused pyran derivatives we sought a synthesis of spiro indanone fused pyrano[3,2-c]chromene skeleton. Literature survey disclosed number of reports describing the synthesis of spiro pyran derivatives using isatin backbone.26–29 However, to the best of our knowledge only a very few reports are there for the synthesis of oxa spirocyclic indanone scaffold.30 In this study, we present our contribution to the successful discovery of new potent anticancer candidates through concise construction of novel chiral spiro indanone fused pyrano[3,2-c]chromene exhibiting a unique profile of biological activities via hetero Diels Alder reaction. Furthermore, we hope this new type of spiro pyrano chromene can serve as a potential anticancer agent.
A novel synthetic approach for the regiospecific and diastereoselective synthesis of spiro indanone fused pyrano[3,2-c]chromene 3(a–h) by the reaction of indane-1,3-dione 1 and 3-vinyl-2H-chromene 2(a–h) and 2′(a–h) in toluene via hetero Diels Alder reaction was reported (Scheme 1). It was interesting that regioisomer 3(a–h) and 3′(a–g) were formed regiospecifically whereas regioisomers 4(a–h) and 4′(a–g) were not observed might be due to the non-interaction of *C orbital of the diene (lower orbital co-efficient) with carbonyl carbon orbital of the dienophile (Scheme 1).
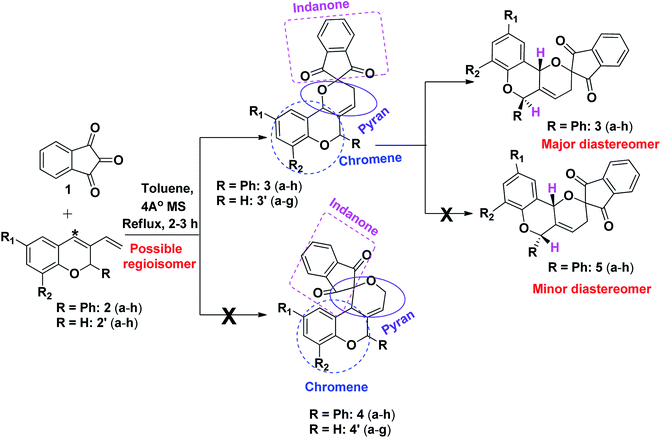 |
| | Scheme 1 Model for synthesis of spiro indanone fused pyrano chromene. | |
Results and discussion
Initially, 2H-chromene-3-carbaldehydes 8(a–h) and 10(a–g) were prepared by the reaction of salicylaldehyde 6(a–g) with cinnamaldehyde 7 or acrolein 9 following Oxa–Michael–Aldol reaction in good yields (Scheme 2).31,32 2H-Chromene-3-carbaldehyde derivatives 8(a–h) and 10(a–g) were completely characterised using their analytical and spectral data. For example, the 1H NMR spectrum of 8a and 10a exhibited a singlet at 9.66 and 9.59 ppm due to CHO, also the characteristic olefinic protons came at 7.42 and 7.26 ppm. The 2H-protons of chromene aldehyde appeared as singlet at 6.35 and 5.11 ppm in 8a and 10a which indicates the formation of chromene aldehydes.
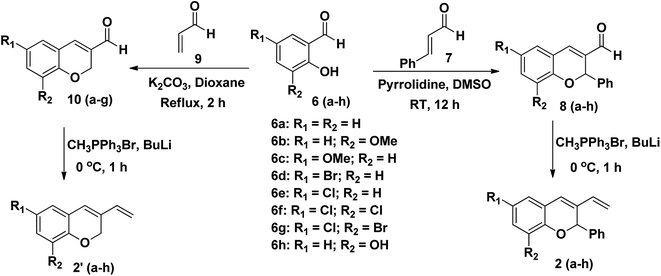 |
| | Scheme 2 Synthesis of 2H-chromene-3-carbaldehyde 8(a–h) and 10(a–g) and 3-vinyl-2H-chromene 2(a–h) and 2′(a–h). | |
Wittig reaction of 8(a–h) and 10(a–g) with methyl triphenyl phosphonium bromide in BuLi afforded 3-vinyl-2H-chromene derivatives 2(a–h) and 2′(a–h) in good yield.33 Compounds 2(a–h) and 2′(a–f) were successfully characterised by 1H, 13C NMR and IR. For example, the aldehydic proton which appears at 9.66 ppm for compound 8a vanishes in the 1H NMR of compound 2a. The olefinic protons of compound 2a appeared as double doublet at 6.52 ppm (J = 11.2 Hz, 17.6 Hz, CH) and 5.09 ppm (J = 11.2 Hz, 18.8 Hz, CH2) which indicates the formation of compound 2a.
Literature reports revealed that dienes can undergo hetero Diels Alder reaction with aldehyde or ketone to form an oxa spirocyclic scaffold. Prompted by the literature findings we planned to synthesize spiro indanone fused pyranochromene derivatives by the reaction of indane-1,3-dione and 3-vinyl-2H-chromene.
To optimise the reaction conditions, we screened the hetero Diels–Alder reaction of indane-1,3-dione 1 (1 mmol) and 3-vinyl-2H-chromene 2a (1 mmol) as a model. The effect of several solvents and additive were studied (Table 1). Using toluene as solvent at room temperature under additive free condition for 12 h, no product formation was observed (entry 1). When the reaction was put under reflux condition at 120 °C for 12 h, 40% conversion was observed and the starting material remains unreacted (entry 2). The same reaction was again repeated using 4 Å MS as additive. Gratifyingly, the addition of 4 Å MS increased the yield of the product to 80% and decreased the time of reaction to 2 h (entry 3). From this we come to the point that additives have significant influence to the yield of the product. The effect of other solvents such as benzene, CH3CN and DCM in the presence of 4 Å MS were also studied to improve the yield. No product formation was observed under room temperature stirring for 12 h in all cases (entries 4, 6 and 8). Also refluxing in various solvents such as benzene, CH3CN and DCM did not give significant influence to the yield of the product (entries 5, 7 and 9). So we conclude that the greatest reaction efficacy and stereocontrol in the product formation was obtained using toluene and 4 Å MS (entry 3). In all cases regiospecifically a single diastereoisomer was formed as major isomer.
Table 1 Optimisation for the synthesis of 3a
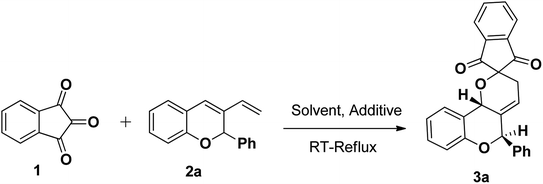
|
| Entry |
Solvent |
Additive |
Temp. (°C) |
Time (h) |
Yielda (%) |
| Indane-1,3-dione 1 (1 mmol) and 3-vinyl-2H-chromene 2a (1 mmol) at reflux in toluene for 3 h in the presence of 4 Å MS. |
| 1 |
Toluene |
_ |
RT |
12 h |
n.r |
| 2 |
Toluene |
_ |
Reflux |
12 h |
40% |
| 3 |
Toluene |
4 Å MS |
Reflux |
2 h |
80% |
| 4 |
Benzene |
4 Å MS |
RT |
12 h |
n.r |
| 5 |
Benzene |
4 Å MS |
Reflux |
4 h |
50% |
| 6 |
CH3CN |
4 Å MS |
RT |
12 h |
n.r |
| 7 |
CH3CN |
4 Å MS |
Reflux |
6 h |
40% |
| 8 |
DCM |
4 Å MS |
RT |
12 h |
n.r |
| 9 |
DCM |
4 Å MS |
Reflux |
6 h |
20% |
Results of experiments under the optimised conditions for probing the scope of the reaction are summarized in Scheme 3. A range of 3-vinyl-2H-chromene 2(a–h) and 2′(a–h) derivatives were examined with indane-1,3-dione 1 in toluene using 4 Å MS as additives under reflux condition at 120 °C for the construction of spiro indanone fused pyranochromene derivatives 3(a–h) and 3′(a–g) (Scheme 3).
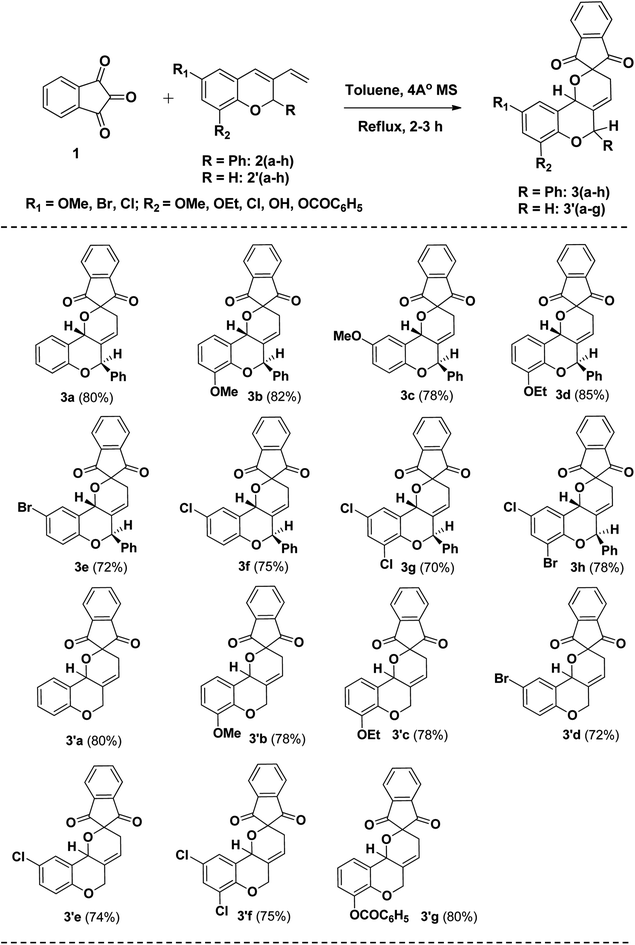 |
| | Scheme 3 Synthesis of spiro indanone fused pyrano chromene 3(a–h) and 3′(a–g). | |
The results showed that, in general variation of the electronic properties of the substituent at different positions of the 3-vinyl-2H-chromene 2(a–h) and 2′(a–g) was well tolerated giving the spiro indanone fused pyranochromene derivatives 3(a–h) and 3′(a–g) with excellent yields with diastereoselectivity albeit with a slight low yield for 3g. Additionally, dienes substituted with halogen groups took a slight longer reaction time might be due to the negative inductive effect of halogens. Dienes containing benzoate group 2′g also underwent the reaction smoothly affording good yield of the product with excellent diastereoselectivity. Unexpectedly, dienes containing hydroxyl groups 2′h and nitro group when treated with indane-1,3-dione do not provide the Diels Alder adduct. Presence of –I effecting group (NO2, –OH) in diene might be decreasing HOMO energy level causing higher HOMO–LUMO energy gap. The applied thermal energy is not sufficient enough to crossover the energy gap, causing no interaction between diene and dienophile to give the product. The structures of the products 3(a–h) and 3′(a–g) were established on the basis of their spectral data (1H and 13C NMR) as well as HRMS analysis. The regiospecific formation of the products 3(a–h) and 3′(a–g) were confirmed by 1H NMR. For instance, the allylic –CH2 of newly formed pyran ring in all cases comes at 2–3 ppm which indicates the formation of 3(a–h) and 3′(a–g). The regiospecific product formation 3a might be obtained due to the flow of electron from oxygen atom of the chromene ring to the olefinic *C of the diene which results the interaction of highest negative charge density *C of the diene with the lowest positive charge density carbonyl carbon of the dienophile. The stereochemistry of the major isomer 3a was determined by 2D NMR (NOESY). In the NOESY spectra of compound 3a (Major) the benzylic proton and 2H-chromene proton do not show interaction with each other whereas in case of minor isomer both the protons shows interaction from which we come to the point that the trans-isomer is major where as syn- is minor. Only in two cases we are able to separate out the minor cis-isomer whereas in other cases we failed. Diastereoselective trans-isomer is formed as the major isomer in all cases.
A plausible mechanism for the hetero-Diels–Alder reaction of indane-1,3-dione 1 and 3-vinyl-2H-chromene 2a is shown in Scheme 4. The stereochemistry of the product depends on the endo-orientation of the dienophile as well as by the stereochemistry of the diene in the transition state. Here we assume that the cycloadducts 3a could form via an endo-transition state A i.e. the dienophile approaches from the opposite face to the Ph group giving the anti product (major isomer), where Ha and Hb show no NOESY interaction, and the dienophile approaches from the same face to the Ph group i.e. more hindered side giving syn product i.e. 5a (minor isomer) as represented in Scheme 4. The diastereoisomeric ratio remains high throughout the course of the reaction.
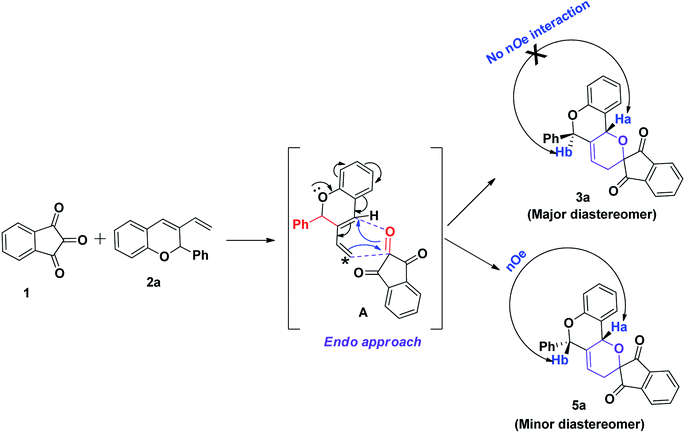 |
| | Scheme 4 A plausible mechanism for the formation of compound 3a. | |
Literature report reveals that indanone based natural products such as Fredericamycin and related synthetic derivatives are showing anticancer activity. Similarly pyranochromene based molecules also shows potent anticancer activity. The synthesized spiro indanone fused pyrano[3,2-c]chromene molecules 3(a–h) and 3′(a–g) were explored for in vitro cytotoxicity activity in various human cancer cell lines such as MCF-7 (breast cancer), HCT-116 (colon cancer), H-357 (oral cancer), MD-MB-231(breast cancer) using MTT assay as shown in Table 2. The results exhibited that compounds 3′c shows most potent cytotoxic activity [IC50 (fifty percent cell death in culture) 15.0–27.5 μM] against MCF-7 (breast cancer), HCT-116 (colon cancer), MD-MB-231 (breast cancer) cell lines. Compounds 3a, 3d, 3e, 3f, 3g, 3′f (IC50: 31.5–40.1 μM) shows better activity in MCF7 cell line comparison to standard drug 5-FU. Compounds 3a, 3b, 3c, 3d, 3e, 3h, 3′b, 3′e, (IC50: 35.5–41.5 μM) shows better activity in HCT-116 cell line comparison to standard drug 5-FU. Compounds 3a, 3d, 3e, 3f, 3g, 3′f, 3′g (IC50: 19.0–38.0 μM) shows better activity in MD-MB-231 cell line comparison to standard drug 5-FU. However none of the compounds in the series shows better activity in H-357 cancer cell line. SAR showed that possibly, the ethoxy group present in 3′c could be responsible for more cytotoxic activity. Though compound 3′c and 3d, both having ethoxy group but absence of phenyl group in 3′c might increase the cytotoxic activities.
Table 2 IC50 values of compound 3(a–h) and 3′(a–g) in MCF-7, HCT-116, H-357 and MD-MB-231 cell linea
| Compound name |
MCF-7 (μM) |
HCT-116 (μM) |
H-357 (μM) |
MD-MB-231 (μM) |
| The data was presented as mean ± SD of 4 different experiments. |
| 3a |
31.5 ± 2.0 |
35.5 ± 3.0 |
64.5 ± 10.0 |
38 ± 1.0 |
| 3b |
52.5 ± 4.0 |
41.5 ± 3.6 |
68 ± 10.0 |
63 ± 5.6 |
| 3c |
91 ± 6.2 |
41 ± 3.0 |
66 ± 8.0 |
78 ± 8.0 |
| 3d |
32 ± 3.2 |
36 ± 1.0 |
67 ± 6.0 |
19 ± 1.0 |
| 3e |
34 ± 2.2 |
40 ± 4.0 |
65.5 ± 2.8 |
37.5 ± 2.0 |
| 3f |
40 ± 1.0 |
50 ± 8.2 |
54.5 ± 8.0 |
36 ± 1.2 |
| 3g |
32.5 ± 3.0 |
38 ± 2.0 |
63.5 ± 6.0 |
33.5 ± 1.8 |
| 3h |
48 ± 2.2 |
35 ± 1.0 |
69 ± 6.0 |
51 ± 4.0 |
| 3′a |
65 ± 7.2 |
43 ± 1.0 |
76.5 ± 7.0 |
72.5 ± 6.2 |
| 3′b |
41 ± 2.1 |
40 ± 4.0 |
74 ± 11.3 |
52.5 ± 4.0 |
| 3′c |
20 ± 1.0 |
27.5 ± 1.0 |
55 ± 4.0 |
15 ± 1.0 |
| 3′d |
41.5 ± 6.0 |
44 ± 3.0 |
73 ± 6.3 |
47 ± 4.0 |
| 3′e |
65 ± 4.8 |
39.5 ± 1.8 |
71 ± 5.8 |
69 ± 6.0 |
| 3′f |
38.5 ± 2.1 |
50 ± 8.0 |
75 ± 7.4 |
31 ± 2.0 |
| 3′g |
50 ± 8.0 |
54 ± 8.0 |
70 ± 8.1 |
27 ± 1.0 |
| 5-FU |
40 ± 6.0 |
42 ± 1.0 |
48 ± 1.0 |
40 ± 2.0 |
Conclusion
In summary, we have developed a novel protocol for regiospecific and diastereoselective synthesis of spiro indanone fused pyrano chromene frameworks containing indanone, pyran and chromene moieties through oxa-hetero Diels–Alder approach by the reaction of indane-1,3-dione and 3-vinyl-2H-chromene in good to excellent yields with a wide substrate scope. Several advantages associated with this protocol such as cost effective, high yields, easy accessibility, wide substrate scope and short reaction time. The synthesized products were evaluated for their potential anticancer activities. Most of these compounds showed potent activities. Compound 3′c is significantly more potent. The possible mechanism of this kind of spiro indanone fused pyrano[3,2-c]chromene as an anticancer agent will be studied in future.
Experimental section
General methods
1H NMR spectra were recorded on 400 MHz (100 MHz for 13C NMR) JEOL NMR spectrometer with CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. Chemical shifts were reported in parts per million (ppm, δ scale) downfield from TMS at 0.00 ppm and referenced to the CDCl3 at 7.26 ppm (for 1H NMR) or 77.0 ppm (for 13C NMR). Melting points are uncorrected and were determined with SMP10 digital melting point apparatus using open capillary tubes. All reagents and solvents used in this study were commercially available (from Sigma-Aldrich) and were used without further purification.
General procedure for the synthesis of 8(a–h)
Salicylaldehyde (1 mmole) and cinnamaldehyde (1.1 mmole) were taken in a round bottom flask and 3 ml of dry DMSO was added to it followed by pyrrolidine (0.2 mmol). Then it was stirred at room temperature in argon atmosphere. The progress of the reaction was monitored by TLC and was found to be completed after 12 h. After completion of reaction, 50 ml of water was added to it and then extracted with ethyl acetate. The combined organic layers were washed with brine (30 mL). The organic layers were dried over Na2SO4 and concentrated in rotavapor. The crude product was crystallised in isopropanol at 0 °C provided aldehyde compound in pure form. In some cases column chromatography has been done in order to purify the compound. Synthesized chromene aldehydes were successfully characterised by 1H, 13C NMR, IR and mass analysis.
2-Phenyl-2H-chromene-3-carboxaldehyde (8a). Yellow solid (88%); mp 72–74 °C. IR (KBr) (νmax/cm−1): 3048, 2820, 2707, 1670, 1570, 1457, 1216, 1102, 996, 768, 612, 520. 1H NMR (400 MHz, CDCl3): δH 9.66 (1H, s, CHO), 7.42 (1H, s, CH), 7.37–7.35 (2H, m, H–Ar), 7.30–7.25 (5H, m, H–Ar), 6.96–6.87 (2H, m, H–Ar), 6.35 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δC 190.1, 154.9, 140.8, 139.1, 133.7, 129.5, 128.7, 126.8, 121.8, 120.0, 117.1, 74.2. ESI-HRMS [M + Na]+: calcd for C16H12O2: 259.0730, found: 259.0730. Anal. calcd for C16H12O2: C, 81.34; H, 5.12. Found: C, 81.35; H, 5.15.
8-Methoxy-2-phenyl-2H-chromene-3-carboxaldehyde (8b). Yellow solid (85%); mp 117–119 °C. IR (KBr) (νmax/cm−1): 3051, 2908, 2811, 2720, 1657, 1631, 1573, 1378, 1255, 1210, 1093, 964, 892, 763, 723, 691, 581, 510. 1H NMR (400 MHz, CDCl3): δH 9.67 (1H, s, CHO), 7.40 (1H, s, CH), 7.38–7.36 (2H, m, H–Ar), 7.28–7.24 (3H, m, H–Ar), 6.94–6.88 (3H, m, H–Ar), 6.44 (1H, s, CH), 3.84 (3H, s, OCH3); 13C NMR (100 MHz, CDCl3): δC 190.3, 148.6, 144.2, 141.1, 139.1, 134.1, 128.7, 128.6, 126.7, 121.7, 121.4, 120.9, 116.2, 74.3, 56.4. ESI-HRMS [M + Na]+: calcd for C17H14O3: 289.0835, found: 289.0837. Anal. calcd for C17H14O3: C, 76.68; H, 5.30. Found: C, 76.69; H, 5.33.
7-Methoxy-2-phenyl-2H-chromene-3-carboxaldehyde (8c). Yellow solid (75%); mp 121–123 °C. IR (KBr) (νmax/cm−1): 3051, 2908, 2811, 2720, 1657, 1631, 1573, 1378, 1255, 1210, 1093, 964, 892, 763, 723, 691, 581, 510. 1H NMR (400 MHz, CDCl3): δH 9.59 (1H, s, CHO), 7.40 (1H, s, CH), 7.38–7.36 (2H, m, H–Ar), 7.32–7.25 (5H, m, H–Ar), 6.53 (1H, dd, J = 8.0 Hz, 4.0 Hz, H–Ar), 6.34 (1H, s, CH), 3.80 (3H, s, OCH3); 13C NMR (100 MHz, CDCl3): δC 189.7, 164.5, 156.7, 141.0, 139.3, 131.0, 128.5, 126.7, 113.4, 108.9, 105.8, 102.0, 98.0, 74.6, 55.5. ESI-HRMS [M + Na]+: calcd for C17H14O3: 289.0835, found: 289.0837. Anal. calcd for C17H14O3: C, 76.68; H, 5.30. Found: C, 76.72; H, 5.34.
8-Ethoxy-2-phenyl-2H-chromene-3-carboxaldehyde (8d). Yellow solid (85%); mp 98–100 °C. IR (KBr) (νmax/cm−1): 2974, 2807, 1748, 1664, 1627, 1609, 1469, 1376, 1256, 1218, 1098, 1005, 902, 754, 689, 642, 615, 521. 1H NMR (400 MHz, CDCl3): δH 9.70 (1H, s, CHO), 7.41 (1H, s, CH), 7.40–7.37 (2H, m, H–Ar), 7.30–7.26 (3H, m, H–Ar), 6.96–6.88 (3H, m, H–Ar), 6.46 (1H, s, CH), 4.08 (2H, q, J = 8.0 Hz, CH2), 1.40 (3H, t, J = 8.0 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 190.4, 147.9, 144.7, 141.3, 139.1, 134.1, 128.6, 128.5, 126.6, 121.7, 121.5, 121.2, 118.1, 73.9, 65.1, 14.9. ESI-HRMS [M + Na]+: calcd for C18H16O3: 303.0992, found: 303.0985. Anal. calcd for C18H16O3: C, 77.12; H, 5.75. Found: C, 77.15; H, 5.77.
6-Bromo-2-phenyl-2H-chromene-3-carboxaldehyde (8e). Pale yellow solid (80%); mp 137–139 °C. IR (KBr) (νmax/cm−1): 3058, 2934, 2824, 2714, 1891, 1819, 1677, 1631, 1586, 1560, 1482, 1411, 1384, 1307, 1203, 1158, 1132, 1067, 957, 814, 691, 626, 522. 1H NMR (400 MHz, CDCl3): δH 9.66 (1H, s, CHO), 7.39–7.27 (8H, m, CH, H–Ar), 6.78 (1H, d, J = 8.0 Hz, H–Ar), 6.34 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δC 189.9, 153.9, 139.2, 138.6, 136.2, 134.7, 131.6, 129.1, 128.8, 126.9, 121.9, 119.2, 113.8, 74.6. ESI-HRMS [M + Na]+: calcd for C16H11BrO2: 336.9835, found: 336.9833. Anal. calcd for C16H11BrO2: C, 60.98; H, 3.52. Found: C, 61.01; H, 3.54.
6-Chloro-2-phenyl-2H-chromene-3-carboxaldehyde (8f). Faint yellow solid (81%); mp 129–131 °C. IR (KBr) (νmax/cm−1): 3058, 2934, 2824, 2714, 1891, 1819, 1677, 1631, 1586, 1560, 1482, 1411, 1384, 1307, 1203, 1158, 1132, 1067, 957, 814, 691, 626, 522. 1H NMR (400 MHz, CDCl3): δH 9.67 (1H, s, CHO), 7.36 (1H, s, CH), 7.34–7.22 (7H, m, H–Ar), 6.82 (1H, d, J = 8.0 Hz, H–Ar), 6.34 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δC 189.8, 153.3, 139.3, 138.5, 134.6, 133.1, 128.9, 128.7, 128.5, 126.8, 126.7, 121.2, 118.6, 74.5. ESI-HRMS [M + Na]+: calcd for C16H11ClO2: 293.0340, found: 293.0338. Anal. calcd for C16H11ClO2: C, 70.99; H, 4.10. Found: C, 71.02; H, 4.13.
6,8-Dichloro-2-phenyl-2H-chromene-3-carboxaldehyde (8g). Golden yellow solid (82%); mp 136–138 °C. IR (KBr) (νmax/cm−1): 3071, 2811, 1683, 1631, 1462, 1398, 1333, 1242, 1171, 1086, 88, 883, 723, 652, 555, 451. 1H NMR (400 MHz, CDCl3): δH 9.73 (1H, s, CHO), 7.35–7.27 (7H, m, H–Ar), 7.17 (1H, s, CH), 6.48 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δC 189.8, 149.3, 138.7, 138.0, 135.4, 133.0, 129.1, 128.8, 127.1, 126.6, 123.3, 122.4, 74.8. ESI-HRMS [M + Na]+: calcd for C16H10Cl2O2: 326.9950, found: 326.9947. Anal. calcd for C16H10Cl2O2: C, 62.97; H, 3.30. Found: C, 62.99; H, 3.28.
6-Bromo-8-chloro-2-phenyl-2H-chromene-3-carboxaldehyde (8h). Yellow solid (81%); mp 132–134 °C. IR (KBr) (νmax/cm−1): 3071, 2811, 1683, 1631, 1462, 1398, 1333, 1242, 1171, 1086, 88, 883, 723, 652, 555, 451. 1H NMR (400 MHz, CDCl3): δH 9.75 (1H, s, CHO), 7.53 (1H, s, CH), 7.36–7.31 (6H, m, H–Ar), 7.22 (1H, d, J = 4.0 Hz, H–Ar), 6.51 (1H, s, CH); 13C NMR (100 MHz, CDCl3): δC 189.9, 149.3, 138.7, 138.0, 135.4, 133.0, 131.6, 129.5, 126.9, 121.9, 119.2, 117.1, 113.8, 74.5. Anal. calcd for C16H10BrClO2: C, 54.97; H, 2.88. Found: C, 54.95; H, 2.89.
General procedure for the synthesis of 10(a–f)
To a solution of salicylaldehyde (1 mmol) in dioxane (100 ml) was added K2CO3 (4–5 mmol) and acrolein (2 mmol). The reaction mixture was refluxed for 2 h. The progress of the reaction was monitored by TLC checking. After 2 h the reaction completed, the reaction mixture was then poured into water (100 ml). The solution was extracted with ethyl acetate (30 mL × 3). The combined organic layers were washed with brine (30 mL). Then the organic layers were dried over anhydrous Na2SO4 and evaporated under vaccum. The residue was crystallised from CHCl3/n-hexane to give 2H-chromene-3-carbaldehyde as solid. Synthesized chromene aldehydes were successfully characterised by 1H, 13C NMR, IR and mass analysis.
2H-chromene-3-carbaldehyde (10a). Yellow solid (90%); mp 64–66 °C. IR (KBr) (νmax/cm−1): 3045, 2818, 2708, 2312, 1683, 1631, 1462, 1398, 1242, 1171, 1086, 886, 833, 723, 652. 1H NMR (400 MHz, CDCl3): δH 9.59 (1H, s, CHO), 7.33–7.29 (2H, m, CH, H–Ar), 7.22 (1H, dd, J = 8.0 Hz, 4.0 Hz, H–Ar), 6.97 (1H, td, J = 8.0 Hz, 2.0 Hz, H–Ar), 6.88 (1H, d, J = 8.0 Hz, H–Ar), 5.04 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 189.9, 156.1, 141.3, 133.3, 131.8, 129.4, 122.0, 120.5, 116.6, 63.3. ESI-HRMS [M + H]+: calcd for C10H8O2: 161.0558, found: 161.0594. Anal. alcd for C10H8O2: C, 74.99; H, 5.03. Found: C, 75.02; H, 5.06.
8-Methoxy-2H-chromene-3-carbaldehyde (10b). Yellow solid (87%); mp 82–84 °C. IR (KBr) (νmax/cm−1): 2974, 2883, 2818, 2733, 2364, 2325, 1663, 1560, 1475, 1339, 1216, 1093, 1002, 886, 782, 723, 704, 594, 490. 1H NMR (400 MHz, CDCl3): δH 9.59 (1H, s, CHO), 7.26 (1H, s, CH), 6.97–6.92 (2H, m, H–Ar), 6.86 (1H, dd, J = 8.0 Hz, 4.0 Hz, H–Ar), 5.11 (2H, s, CH2), 3.90 (3H, s, OCH3); 13C NMR (100 MHz, CDCl3): δC 189.9, 148.1, 145.0, 141.3, 131.8, 121.7, 121.3, 121.1, 115.5, 63.7, 56.2. GCMS m/z: calcd for C11H10O3: 190.0, found: 190.2. Anal. calcd for C11H10O3: C, 69.46; H, 5.30. Found: C, 69.49; H, 5.32.
8-Ethoxy-2H-chromene-3-carbaldehyde (10c). Yellow solid (85%); mp 89–91 °C. IR (KBr) (νmax/cm−1): 2974, 2883, 2818, 2733, 2364, 2325, 1663, 1560, 1475, 1339, 1216, 1093, 1002, 886, 782, 723, 704, 594, 490. 1H NMR (400 MHz, CDCl3): δH 9.60 (1H, s, CHO), 7.26 (1H, s, CH), 6.96 (1H, dd, J = 8.0 Hz, 4.0 Hz, H–Ar), 6.91 (1H, t, J = 8.0 Hz, H–Ar), 6.85 (1H, dd, J = 4.0 Hz, 4.8 Hz, H–Ar), 5.11 (2H, s, CH2), 4.12 (2H, q, J = 4.0 Hz, CH2), 1.47 (3H, t, J = 4.8 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 189.9, 147.4, 145.4, 141.5, 131.7, 121.6, 121.3, 117.0, 64.7, 63.6, 14.8. MS m/z: calcd for C12H12O3: 204.0, found: 204.1. Anal. calcd for C12H12O3: C, 70.57; H, 5.92. Found: C, 70.59; H, 5.95.
6-Bromo-2H-chromene-3-carbaldehyde (10d). Straw yellow solid (79%); mp 104–106 °C. IR (KBr) (νmax/cm−1): 3058, 2934, 2824, 2714, 1891, 1819, 1677, 1631, 1586, 1560, 1482, 1411, 1384, 1307, 1203, 1158, 1132, 1067, 957, 814, 691, 626, 522. 1H NMR (400 MHz, CDCl3): δH 9.58(1H, s, CHO), 7.37–7.35(1H, m, H–Ar), 7.31(1H, d, J = 1.6 Hz, H–Ar), 7.16(1H, s, CH), 6.75(1H, d, J = 6.8 Hz, H–Ar), 5.02(2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 189.8, 155.2, 139.7, 135.8, 132.7, 131.7, 122.4, 118.6, 114.0, 63.7. ESI-HRMS [M + H]+: calcd for C10H7BrO2: 238.9702, found: 239.9736. Anal. calcd for C10H7BrO2: C, 50.24; H, 2.95. Found: C, 50.22; H, 2.97.
6-Chloro-2H-chromene-3-carbaldehyde (10e). Yellow solid (82%); mp 93–95 °C. IR (KBr) (νmax/cm−1): 3058, 2934, 2824, 2714, 1891, 1819, 1677, 1631, 1586, 1560, 1482, 1411, 1384, 1307, 1203, 1158, 1132, 1067, 957, 814, 691, 626, 522. 1H NMR (400 MHz, CDCl3): δH 9.52(1H, s, CHO), 7.15(1H, dd, J = 8.0 Hz, 4.0 Hz, H–Ar), 7.10(2H, m, CH, H–Ar), 6.74(1H, d, J = 8.0 Hz, H–Ar), 4.95(2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 189.6, 154.5, 139.6, 132.7, 132.6, 128.5, 126.7, 121.7, 118.0, 63.5. GCMS m/z: calcd for C10H7ClO2: 194.0, found: 194.0. Anal. calcd for C10H7ClO2: C, 61.72; H, 3.63. Found: C, 61.73; H, 3.65.
6,8-Dichloro-2H-chromene-3-carbaldehyde (10f). Yellow solid (80%); mp 127–129 °C. IR (KBr) (νmax/cm−1): 3077, 2857, 1683, 1631, 1553, 1456, 1339, 1210, 1152, 1093, 970, 866, 847, 717, 645, 568. 1H NMR (400 MHz, CDCl3): δH 9.55(1H, s, CHO), 7.27 (1H, s, CH), 7.09 (1H, s, H–Ar), 7.03(1H, s, H–Ar), 5.06(2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 189.3, 150.3, 138.6, 133.0, 132.7, 127.1, 126.6, 122.6, 122.4, 64.3. GCMS m/z: calcd for C10H6Cl2O2: 227.9, found: 228.0. Anal. calcd for C10H6Cl2O2: C, 52.43; H, 2.64. Found: C, 52.45; H, 2.67.
6-Hydroxy-2H-chromene-3-carbaldehyde (10g). Straw yellow solid (68%); mp 170–172 °C. IR (KBr) (νmax/cm−1): 3206, 2954, 2837, 2714, 1650, 1573, 1482, 1405, 1346, 1294, 1287, 1216, 1145, 1106, 1021, 898, 814, 717, 626, 568. 1H NMR (400 MHz, CDCl3): δH 9.58 (1H, s, CHO), 7.19 (1H, s, CH), 6.82–6.71 (3H, m, H–Ar), 4.97 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 189.9, 150.3, 150.1, 141.2, 132.6, 121.3, 120.2, 117.4, 115.0, 63.2. GCMS m/z: calcd for C10H8O3: 176.0, found: 176.0. Anal. calcd for C10H8O3: C, 68.18; H, 4.58. Found: C, 68.19; H, 4.61.
General procedure for the synthesis of 2(a–h) and 2′(a–h)
Wittig salt methyl triphenylphosphonium bromide (3 mmol) dissolved in dry THF was taken in a round bottom flask and stirred at 0 °C in argon atmosphere. To it BuLi(1.6 M) (3 mmol) was added in a drop wise manner. After half an hour the previously prepared 2-phenyl-2H-chromene aldehyde (1 mmol) in dry THF was added slowly at −20 °C and stirred for 1 h. The progress of the reaction was monitored by TLC checking. After completion of the reaction, the reaction mixture was quenched by saturated ammonium chloride and extracted with ethyl acetate. The organic layers were washed with brine, dried over anhydrous Na2SO4 and evaporated in vaccum. The crude product was purified by column chromatography to afford the pure product. Most of the purified compounds are amorphous solid.
2-Phenyl-3-vinyl-2H-chromene (2a). White solid (70%); IR (KBr) (νmax/cm−1): 3058, 2824, 2727, 1663, 1573, 1462, 1333, 1314, 1152, 1093, 996, 879, 763, 691, 607, 522. 1H NMR (400 MHz, CDCl3): δH 7.40–7.38 (2H, m, H–Ar), 7.28–7.26 (3H, m, H–Ar), 7.06–7.03 (2H, m, H–Ar), 6.85–6.81 (1H, t, J = 8.0 Hz, H–Ar), 6.73 (1H, d, J = 8.0 Hz, H–Ar), 6.66 (1H, s, CH), 6.52 (1H, dd, J = 11.2 Hz, 17.6 Hz, CH), 6.10 (1H, s, CH), 5.09 (2H, dd, J = 11.2 Hz, 18.8 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 152.0, 138.6, 135.2, 132.4, 129.5, 128.5, 127.6, 126.1, 124.4, 122.3, 121.3, 116.4, 114.9, 76.3. EI-HRMS m/z: calcd for C17H14O: 234.1045, found: 234.9634. Anal. calcd for C17H14O: C, 87.15; H, 6.02. Found: C, 87.12; H, 6.05.
8-Methoxy-2-phenyl-3-vinyl-2H-chromene (2b). White solid (68%); IR (KBr) (νmax/cm−1): 2928, 1627, 1488, 1442, 1311, 1209, 1153, 1014, 884, 819, 763, 689, 568. 1H NMR (400 MHz, CDCl3): δH 7.44–7.42 (2H, m, H–Ar), 7.24–7.16 (3H, m, H–Ar), 6.75–6.71 (1H, m, H–Ar), 6.66–6.59 (3H, m, H–Ar, CH), 6.49 (1H, dd, J = 11.2 Hz, 17.6 Hz, CH), 6.18 (1H, s, CH), 5.07 (2H, dd, J = 6.4 Hz, 11.2 Hz, CH2), 3.65 (3H, s, CH3); 13C NMR (100 MHz, CDCl3): δC 148.0, 140.8, 138.2, 135.0, 132.4, 128.3, 128.2, 127.3, 124.1, 123.0, 120.8, 119.0, 114.7, 112.6, 75.8, 55.9. EI-HRMS m/z: calcd for C18H16O2: 264.1150, found: 264.9737. Anal. calcd for C18H16O2: C, 81.79; H, 6.10. Found: C, 81.80; H, 6.14.
6-Methoxy-2-phenyl-3-vinyl-2H-chromene (2c). White solid (70%); IR (KBr) (νmax/cm−1): 2928, 1627, 1488, 1442, 1311, 1209, 1153, 1014, 884, 819, 763, 689, 568. 1H NMR (400 MHz, CDCl3): δH 7.31–7.29 (2H, m, H–Ar), 7.21–7.14 (3H, m, H–Ar), 6.59–6.50 (4H, m, H–Ar, CH), 6.45 (1H, dd, J = 10.8 Hz, 17.2 Hz, CH), 5.97 (1H, s, CH), 5.04 (2H, dd, J = 10.8 Hz, 16.4 Hz, CH2), 3.65 (3H, s, CH3); 13C NMR (100 MHz, CDCl3): δC 154.0, 145.8, 138.4, 135.1, 133.3, 128.4, 127.6, 124.4, 122.9, 116.9, 115.0, 114.8, 111.5, 76.0, 55.6. Anal. calcd for C18H16O2: C, 81.79; H, 6.10. Found: C, 81.75; H, 6.09.
8-Ethoxy-2-phenyl-3-vinyl-2H-chromene (2d). Orange liquid (67%); IR (νmax/cm−1): 2928, 1627, 1488, 1442, 1311, 1209, 1153, 1014, 884, 819, 763, 689, 568. 1H NMR (400 MHz, CDCl3): δH 7.45 (2H, d, J = 8.8 Hz, H–Ar), 7.25–7.23 (3H, m, H–Ar), 6.77–6.63 (3H, m, H–Ar), 6.55 (2H, dd, J = 11.2 Hz, 18.0 Hz, CH), 6.19 (1H, s, CH), 5.13–5.06 (2H, m, CH2), 3.95 (2H, q, J = 11.2 Hz, CH2), 1.29 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 147.3, 141.5, 138.4, 135.3, 132.6, 128.3, 128.2, 127.5, 124.4, 123.5, 121.0, 119.4, 115.0, 114.8, 75.6, 65.0, 14.8. Anal. calcd for C19H18O2: C, 81.99; H, 6.52. Found: C, 81.97; H, 6.53.
6-Bromo-2-phenyl-3-vinyl-2H-chromene (2e). White solid (70%); IR (KBr) (νmax/cm−1): 3018, 2915, 1631, 1579, 1475, 1417, 1249, 1210, 1106, 1080, 989, 898, 814, 704, 626, 542. 1H NMR (400 MHz, CDCl3): δH 7.35–7.27 (5H, m, H–Ar), 7.19–7.13 (2H, m, H–Ar), 6.61–6.58 (2H, m, H–Ar, CH), 6.49 (1H, dd, J = 11.2 Hz, 17.6 Hz, CH), 6.09 (1H, s, CH), 5.15 (2H, dd, J = 10.8 Hz, 24.8 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 150.9, 137.9, 134.7, 133.5, 131.9, 129.0, 128.7, 128.5, 127.6, 124.2, 123.0, 118.2, 115.9, 113.2, 76.4. EI-HRMS [M + H]+: calcd for C17H13BrO: 313.1885, found: 314.3597. Anal. calcd for C17H13BrO: C, 65.19; H, 4.18. Found: C, 65.17; H, 4.21.
6-Chloro-2-phenyl-3-vinyl-2H-chromene (2f). White solid (66%); IR (KBr) (νmax/cm−1): 3018, 2915, 1631, 1579, 1475, 1417, 1249, 1210, 1106, 1080, 989, 898, 814, 704, 626, 542. 1H NMR (400 MHz, CDCl3): δH 7.37–7.35 (2H, m, H–Ar), 7.32–7.28 (3H, m, H–Ar), 7.03–6.97 (2H, m, H–Ar), 6.66 (1H, d, J = 8.4 Hz, H–Ar), 6.59 (1H, s, CH), 6.50 (1H, dd, J = 10.8 Hz, 16.8 Hz, CH), 6.09 (1H, s, CH), 5.15 (2H, dd, J = 11.2 Hz, 24.0 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 150.4, 138.0, 134.7, 133.6, 129.0, 128.7, 128.5, 128.4, 127.6, 126.1, 126.0, 123.7, 123.2, 117.7, 115.9, 76.4. EI-HRMS m/z: calcd for C17H13ClO: 268.0655, found: 268.9242. Anal. calcd for C17H13ClO: C, 75.98; H, 4.88. Found: C, 75.95; H, 4.88.
6,8-Dichloro-2-phenyl-3-vinyl-2H-chromene (2g). White solid (65%); IR (KBr) (νmax/cm−1): 3071, 2928, 1819, 1637, 1612, 1560, 1462, 1262, 1210, 1048, 976, 905, 873, 840, 723, 685, 658, 588. 1H NMR (400 MHz, CDCl3): δH 7.42–7.27 (5H, m, H–Ar), 7.09 (1H, d, J = 2.0 Hz, H–Ar), 6.93 (1H, d, J = 2.8 Hz, H–Ar), 6.58–6.50 (2H, m, CH), 6.24 (1H, s, CH), 5.24 (2H, dd, J = 11.2 Hz, 26.4 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 146.5, 137.4, 134.6, 134.5, 129.0, 128.8, 128.5, 127.4, 126.0, 125.0, 124.7, 122.7, 122.2, 116.9, 76.4. Anal. calcd for C17H12Cl2O: C, 67.35; H, 3.99. Found: C, 67.38; H, 3.97.
8-Bromo-6-chloro-2-phenyl-3-vinyl-2H-chromene (2h). Orange liquid (69%); IR (KBr) (νmax/cm−1): 3071, 2928, 1819, 1637, 1612, 1560, 1462, 1262, 1210, 1048, 976, 905, 873, 840, 723, 685, 658, 588. 1H NMR (400 MHz, CDCl3): δH 7.42–7.40 (2H, m, H–Ar), 7.27–7.22 (4H, m, H–Ar), 6.94 (1H, d, J = 2.4 Hz, H–Ar), 6.56–6.49 (2H, m, CH), 6.24 (1H, s, CH), 5.21 (2H, dd, J = 11.2 Hz, 24.8 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 147.5, 137.3, 134.5, 134.4, 131.6, 128.8, 128.4, 127.4, 126.3, 125.3, 124.9, 122.8, 116.8, 111.0, 76.4. Anal. calcd for C17H12BrClO: C, 58.73; H, 3.48. Found: C, 58.75; H, 3.45.
3-Vinyl-2H-chromene (2′a). Orange liquid (65%); IR (νmax/cm−1): 2974, 2928, 1722, 1605, 1586, 1469, 1405, 1255, 1210, 1086, 976, 782, 730, 658. 1H NMR (400 MHz, CDCl3): δH 7.09–7.05 (1H, m, H–Ar), 6.98 (1H, d, J = 6.0 Hz, H–Ar), 6.86–6.78 (2H, m, H–Ar), 6.43 (1H, dd, J = 10.8 Hz, 26.8 Hz, CH), 6.35 (1H, s, CH), 5.14 (2H, dd, J = 10.8 Hz, 20.4 Hz, CH2), 4.94 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 153.7, 134.6, 130.7, 129.1, 127.0, 123.6, 122.3, 121.3, 115.3, 113.3, 65.1. Anal. calcd for C11H10O: C, 83.51; H, 6.37. Found: C, 83.53; H, 6.34.
8-Methoxy-3-vinyl-2H-chromene (2′b). Orange liquid (68%); IR (νmax/cm−1): 2974, 2928, 1722, 1605, 1586, 1469, 1405, 1255, 1210, 1086, 976, 782, 730, 658. 1H NMR (400 MHz, CDCl3): δH 6.83–6.75 (2H, m, H–Ar), 6.60–6.64 (1H, m, H–Ar), 6.58–6.36 (2H, m, CH), 5.16 (2H, dd, J = 9.2 Hz, 17.8 Hz, CH2), 5.02 (2H, s, CH2), 3.86 (3H, s, CH3); 13C NMR (100 MHz, CDCl3): δC 147.4, 142.3, 134.4, 130.7, 123.4, 123.0, 121.0, 119.3, 113.6, 112.0, 65.3, 56.0. Anal. calcd for C12H12O2: C, 76.57; H, 6.43. Found: C, 76.59; H, 6.40.
8-Ethoxy-3-vinyl-2H-chromene (2′c). Orange liquid (70%); IR (νmax/cm−1): 2974, 2928, 1722, 1605, 1586, 1469, 1405, 1255, 1210, 1086, 976, 782, 730, 658. 1H NMR (400 MHz, CDCl3): δH 6.78–6.74 (2H, m, H–Ar), 6.64–6.62 (1H, m, H–Ar), 6.45–6.35 (2H, m, CH), 5.15 (2H, dd, J = 10.8 Hz, 16.4 Hz, CH2), 5.01 (2H, s, CH2), 4.07 (2H, q, J = 14.0 Hz, CH2), 1.44 (3H, t, J = 8.8 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 146.6, 142.7, 134.4, 130.5, 123.5, 123.1, 120.8, 119.2, 113.6, 113.4, 65.2, 64.3, 14.7. Anal. calcd for C13H14O2: C, 77.20; H, 6.98. Found: C, 77.19; H, 6.97.
6-Bromo-3-vinyl-2H-chromene (2′d). White solid (65%); IR (KBr) (νmax/cm−1): 3018, 2915, 1631, 1579, 1475, 1417, 1249, 1210, 1106, 1080, 989, 898, 814, 704, 626, 542. 1H NMR (400 MHz, CDCl3): δH 7.10–7.02 (2H, m, H–Ar), 6.61 (1H, d, J = 9.2 Hz, H–Ar), 6.38–6.30 (1H, m, CH), 6.22 (1H, s, CH), 5.13 (2H, dd, J = 11.2 Hz, 26.0 Hz, CH2), 4.88 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 152.7, 134.2, 131.7, 131.5, 129.2, 124.2, 122.3, 117.1, 114.4, 113.3, 65.2. EI-HRMS [M + H]+: calcd for C11H9BrO: 237.0926, found: 238.9675. Anal. calcd for C11H9BrO: C, 55.72; H, 3.83. Found: C, 55.74; H, 3.85.
6-Chloro-3-vinyl-2H-chromene (2′e). White solid (67%); IR (KBr) (νmax/cm−1): 3018, 2915, 1631, 1579, 1475, 1417, 1249, 1210, 1106, 1080, 989, 898, 814, 704, 626, 542. 1H NMR (400 MHz, CDCl3): δH 7.10–6.98 (2H, m, H–Ar), 6.72 (1H, d, J = 9.2 Hz, H–Ar), 6.48–6.39 (1H, m, CH), 6.28 (1H, s, CH), 5.27 (2H, dd, J = 11.4 Hz, 26.2 Hz, CH2), 4.98 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 152.2, 134.3, 131.9, 128.9, 126.9, 126.2, 123.9, 122.8, 116.9, 114.4, 65.2. Anal. calcd for C11H9ClO: C, 68.58; H, 4.71; found: C, 68.57; H, 4.73.
6,8-Dichloro-3-vinyl-2H-chromene (2′f). White solid (70%); IR (KBr) (νmax/cm−1): 3071, 2928, 1819, 1637, 1612, 1560, 1462, 1262, 1210, 1048, 976, 905, 873, 840, 723, 685, 658, 588. 1H NMR (400 MHz, CDCl3): δH 7.14 (1H, d, J = 2.0 Hz, H–Ar), 6.88 (1H, d, J = 2.0 Hz, H–Ar), 6.45 (1H, dd, J = 11.2 Hz, 18.0 Hz, CH), 6.30 (1H, s, CH), 5.27 (2H, dd, J = 10.8 Hz, 29.6 Hz, CH2), 5.07 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 148.0, 133.9, 132.3, 128.8, 125.9, 125.0, 124.5, 121.9, 121.2, 115.4, 65.9. GCMS [M + H]+: calcd for C11H8Cl2O: 227.0, found: 228.0. Anal. calcd for C11H8Cl2O: C, 58.18; H, 3.55. Found: C, 58.20; H, 3.58.
3-Vinyl-2H-chromen-8-yl benzoate (2′g). Colour less liquid (65%); IR (νmax/cm−1): 3071, 1800, 1722, 1683, 1593, 1437, 1268, 1216, 1171, 983, 769, 685, 626, 555. 1H NMR (400 MHz, CDCl3): δH 8.23–8.20 (2H, m, H–Ar), 7.82–7.48 (3H, m, H–Ar), 7.28–6.82 (3H, m, H–Ar), 6.46–6.38 (2H, m, CH), 5.14 (2H, dd, J = 11.2 Hz, 31.6 Hz, CH2), 4.94 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 164.4, 145.6, 138.3, 134.3, 133.4, 131.3, 130.2, 129.4, 129.2, 128.4, 128.2, 124.4, 124.2, 123.1, 122.5, 121.1, 113.9, 65.3. Anal. calcd for C18H14O3: C, 77.68; H, 5.07. Found: C, 77.69; H, 5.09.
3-Vinyl-2H-chromen-8-ol (2′h). Yellow liquid (60%); IR (νmax/cm−1): 3206, 1650, 1573, 1482, 1405, 1346, 1294, 1287, 1216, 1145, 1106, 1021, 898, 814, 717, 626, 568. 1H NMR (400 MHz, CDCl3): δH 6.78–6.73 (2H, m, H–Ar), 6.59–6.56 (1H, m, H–Ar), 6.43 (1H, dd, J = 10.8 Hz, 18.4 Hz, CH), 6.37 (1H, s, CH), 5.65 (1H, broad singlet, CH), 5.15 (2H, dd, J = 10.8 Hz, 26.8 Hz, CH2), 4.97 (2H, s, CH2); 13C NMR (100 MHz, CDCl3): δC 143.7, 139.9, 134.5, 130.6, 123.5, 122.5, 121.5, 118.4, 115.4, 113.5, 65.4. Anal. calcd for C11H10O2: C, 75.84; H, 5.79. Found: C, 75.86; H, 5.77.
General procedure for the synthesis of 3(a–h) and 3′(a–f)
Ninhydrin (1 mmol) was taken in dry toluene (5 mL) to it 4 Å MS was added and stirred at 120 °C for 10 min till the colour change observed. Then 3-vinyl-2H-chromene (1 mmol) was added to it. The progress of the reaction was monitored by TLC checking. The reaction was completed in 2–3 h. The reaction mixture was cooled, 4 Å MS were filtered and the toluene was removed under vaccum. The crude reaction mixture was purified by flash column chromatography to get the pure product. After column purification most of the compounds obtained as amorphous solid.
(5′R,10b′R)-5′-Phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3a). White solid (80%); IR (KBr) (νmax/cm−1): 3058, 2921, 2863, 1735, 1715, 1573, 1482, 1287, 1229, 1035, 996, 892, 756, 704, 652, 594. 1H NMR (400 MHz, CDCl3) δ 8.04–8.02 (2H, m, H–Ar), 7.93–7.90 (2H, m, H–Ar), 7.58 (2H, d, J = 8.4 Hz, H–Ar), 7.40–7.36 (2H, m, H–Ar), 7.28–7.16 (3H, m, H–Ar), 7.00 (1H, d, J = 8.4 Hz, H–Ar), 6.86–6.82 (1H, m, H–Ar), 6.27–6.25 (1H, m, CH), 5.82 (1H, s, CH), 5.52 (1H, s, CH), 2.87–2.81 (1H, m, CH2), 2.33–2.26 (1H, m, CH2); 13C NMR (100 MHz, CDCl3) δ 197.9, 196.6, 152.8, 140.5, 140.4, 139.1, 136.6, 136.4, 132.5, 129.1, 128.5, 127.6, 127.4, 125.8, 124.3, 124.1, 121.8, 121.0, 119.8, 116.6, 79.8, 74.3, 66.1, 25.9. HRMS [M + H]+ calcd for C26H18O4: 394.4187, found: 395.1326. Anal. calcd for C26H18O4: C, 79.17; H, 4.60. Found: C, 79.19; H, 4.59.
(5′S,10b′R)-5′-Phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (5a). White solid (70%); IR (KBr) (νmax/cm−1): 3058, 2921, 2863, 1735, 1715, 1573, 1482, 1287, 1229, 1035, 996, 892, 756, 704, 652, 594. 1H NMR (400 MHz, CDCl3): δH 8.04–8.02 (2H, m, H–Ar), 7.95–7.91 (2H, m, H–Ar), 7.50–7.38 (6H, m, H–Ar), 7.22–7.17 (1H, m, H–Ar), 6.94–6.89 (2H, m, H–Ar), 6.07 (1H, s, CH), 5.71 (1H, s, CH), 5.28–5.25 (1H, m, CH), 2.65–2.59 (1H, m, CH2), 2.13–2.04 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.3, 196.8, 154.1, 140.1, 136.6, 136.5, 129.1, 128.4, 128.3, 127.9, 127.6, 124.2, 124.1, 121.1, 119.3, 116.5, 78.4, 74.3, 68.8, 26.1. Anal. calcd for C26H18O4: C, 79.17; H, 4.60. Found: C, 79.18; H, 4.57.
7′-Methoxy-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3b). White solid (82%); IR (KBr) (νmax/cm−1): 2921, 2863, 1761, 1696, 1586, 1475, 1346, 1255, 1210, 1086, 989, 924, 873, 710, 645. 1H NMR (400 MHz, CDCl3): δH 8.03–8.01 (2H, m, H–Ar), 7.91–7.87 (2H, m, H–Ar), 7.58 (2H, d, J = 7.6 Hz, H–Ar), 7.39–7.37 (2H, m, H–Ar), 7.28–7.23 (1H, m, H–Ar), 6.82–6.78 (3H, m, H–Ar), 6.28 (1H, d, J = 4.8 Hz, CH), 5.93 (1H, s, CH), 5.52 (1H, s, CH), 3.92 (3H, s, CH3), 2.85–2.80 (1H, m, CH2), 2.31–2.25 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.8, 196.4, 148.1, 142.2, 140.3, 140.2, 138.6, 136.5, 136.4, 132.1, 128.4, 127.6, 125.5, 124.1, 124.0, 122.4, 120.6, 119.9, 119.1, 110.7, 80.0, 74.3, 66.0, 56.0, 25.8. HRMS calcd for C27H20O5: 424.4447, found: 425.1440. Anal. calcd for C27H20O5: C, 76.40; H, 4.75. Found: C, 76.42; H, 4.73.
9′-Methoxy-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3c). White solid (78%); IR (KBr) (νmax/cm−1): 2921, 2863, 1761, 1696, 1586, 1475, 1346, 1255, 1210, 1086, 989, 924, 873, 710, 645. 1H NMR (400 MHz, CDCl3): δH 8.04–8.00 (2H, m, H–Ar), 7.92–7.87 (2H, m, H–Ar), 7.56–7.54 (2H, m, H–Ar), 7.38–7.29 (3H, m, H–Ar), 6.92–6.90 (1H, m, H–Ar), 6.76–6.70 (2H, m, H–Ar), 6.23–6.22 (1H, m, CH), 5.76 (1H, s, CH), 5.51 (1H, s, CH), 3.62 (3H, s, CH3), 2.84–2.79 (1H, m, CH2), 2.32–2.25 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.9, 196.6, 153.9, 146.8, 140.4, 140.3, 139.2, 136.6, 136.5, 132.7, 128.4, 127.5, 125.9, 124.2, 124.1, 122.2, 119.7, 119.5, 117.4, 115.9, 115.8, 111.2, 79.7, 74.3, 66.3, 55.6, 26.0. HRMS calcd for C27H20O5: 424.4447, found: 425.1480. Anal. calcd for C27H20O5: C, 76.40; H, 4.75. Found: C, 76.39; H, 4.76.
7′-Ethoxy-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3d). White solid (85%); IR (KBr) (νmax/cm−1): 2980, 2902, 1741, 1709, 1579, 1489, 1249, 1203, 1074, 1054, 892, 736, 710. 1H NMR (400 MHz, CDCl3): δH 8.06–8.02 (2H, m, H–Ar), 7.92–7.89 (2H, m, H–Ar), 7.59–7.56 (2H, m, H–Ar), 7.42–7.36 (2H, m, H–Ar), 7.32–7.28 (1H, m, H–Ar), 6.82–6.72 (3H, m, H–Ar), 6.28–6.22 (1H, m, CH), 5.94 (1H, s, CH), 5.51 (1H, s, CH), 4.18 (2H, q, J = 14.0 Hz, CH2), 2.86–2.81 (1H, m, CH2), 2.32–2.30 (1H, m, CH2), 1.50 (3H, t, J = 6.8 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 197.9, 196.6, 147.4, 142.6, 140.4, 140.3, 138.8, 136.6, 136.4, 132.2, 128.4, 127.6, 125.6, 124.2, 124.0, 122.5, 120.6, 119.9, 119.1, 112.2, 80.0, 74.4, 66.2, 64.4, 25.9, 14.8. HRMS calcd for C28H22O5: 438.4713, found: 439.1497. Anal. calcd for C28H22O5: C, 76.70; H, 5.06. Found: C, 76.72; H, 5.09.
9′-Bromo-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3e). White solid (72%); IR (KBr) (νmax/cm−1): 3064, 2941, 2853, 1761, 1709, 1586, 1469, 1405, 1287, 1203, 1132, 989, 866, 743, 691, 600. 1H NMR (400 MHz, CDCl3): δH 8.06–8.01 (2H, m, H–Ar), 7.93–7.90 (2H, m, H–Ar), 7.53–7.52 (2H, m, H–Ar), 7.42–7.36 (2H, m, H–Ar), 7.38–7.31 (3H, m, H–Ar), 6.88 (1H, d, J = 8.8 Hz, H–Ar), 6.28–6.27 (1H, m, CH), 5.82 (1H, s, CH), 5.47 (1H, s, CH), 2.86–2.80 (1H, m, CH2), 2.33–2.27 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.6, 196.4, 151.9, 140.3, 138.5, 136.7, 136.6, 132.0, 131.5, 130.1, 128.5, 127.8, 125.7, 124.3, 124.1, 123.8, 120.4, 118.5, 113.2, 79.9, 74.2, 65.7, 25.8. HRMS [M + H]+ calcd for C26H17BrO4: 472.0310, found: 473.0364. Anal. calcd for C26H17BrO4: C, 65.98; H, 3.62. Found: C, 65.96; H, 3.65.
9′-Chloro-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3f). White solid (75%); IR (KBr) (νmax/cm−1): 2910, 1739, 1701, 1600, 1469, 1414, 1274, 1218, 1098, 986, 884, 735, 698, 596, 466. 1H NMR (400 MHz, CDCl3): δH 8.06–8.01 (2H, m, H–Ar), 7.98–7.90 (2H, m, H–Ar), 7.53–7.52 (2H, m, H–Ar), 7.42–7.36 (2H, m, H–Ar), 7.38–7.21 (3H, m, H–Ar), 6.98 (1H, d, J = 8.8 Hz, H–Ar), 6.38–6.32 (1H, m, CH), 5.82 (1H, s, CH), 5.48 (1H, s, CH), 2.86–2.80 (1H, m, CH2), 2.38–2.26 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.7, 196.4, 151.4, 140.4, 140.3, 138.6, 136.7, 136.6, 131.7, 129.2, 128.6, 127.8, 127.2, 126.0, 125.9, 124.4, 124.1, 123.3, 120.4, 118.0, 79.9, 74.2, 65.8, 25.9. HRMS calcd for C26H17ClO4: 428.8638, found: 429.0905. Anal. calcd for C26H17ClO4: C, 72.82; H, 4.00. Found: C, 72.84; H, 4.03.
7′,9′-Dichloro-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3g). White solid (70%); IR (KBr) (νmax/cm−1): 2919, 2853, 1748, 1701, 1609, 1460, 1367, 1284, 1227, 1190, 1126, 995, 931, 745, 707, 587. 1H NMR (400 MHz, CDCl3): δ 8.07–8.03 (2H, m, H–Ar), 7.94–7.92 (2H, m, H–Ar), 7.56 (2H, d, J = 8.0 Hz, H–Ar), 7.43–7.39 (2H, m, H–Ar), 7.32–7.25 (2H, m, H–Ar), 7.11 (1H, d, J = 4.0 Hz, H–Ar), 6.37–6.31 (1H, m, CH), 5.98 (1H, s, CH), 5.51 (1H, s, CH), 2.88–2.82 (1H, m, CH2), 2.36–2.30 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.6, 196.2, 147.4, 140.3, 137.7, 136.8, 136.7, 130.7, 129.3, 128.7, 128.1, 126.0, 125.8, 125.6, 124.5, 124.4, 124.2, 122.3, 121.3, 80.6, 74.3, 65.8, 25.9. HRMS [M + H]+ calcd for C26H16Cl2O4: 462.0426, found: 463.0390. Anal. calcd for C26H16Cl2O4: C, 67.40; H, 3.48. Found: C, 67.42; H, 3.52.
9′-Bromo-7′-chloro-5′-phenyl-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3h). White solid (78%); IR (KBr) (νmax/cm−1): 2919, 2853, 1748, 1701, 1609, 1460, 1367, 1284, 1227, 1190, 1126, 995, 931, 745, 707, 587. 1H NMR (400 MHz, CDCl3): δH 7.99–7.96 (2H, m, H–Ar), 7.88–7.85 (2H, m, H–Ar), 7.49 (2H, d, J = 6.8 Hz, H–Ar), 7.36–7.32 (3H, m, H–Ar), 7.28–7.22 (1H, m, H–Ar), 7.08–7.07 (1H, m, H–Ar), 6.27–6.25 (1H, m, CH), 5.91 (1H, s, CH), 5.44–5.40 (1H, m, CH), 2.80–2.74 (1H, m, CH2), 2.28–2.22 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 197.6, 196.2, 148.2, 140.3, 137.7, 136.8, 136.7, 132.1, 130.7, 128.7, 128.0, 126.7, 126.2, 125.6, 124.4, 124.3, 124.2, 121.3, 111.2, 80.8, 74.3, 65.8, 25.9. HRMS [M + H]+ calcd for C26H16BrClO4: 507.7598, found: 508.9917. Anal. calcd for C26H16BrClO4: C, 61.50; H, 3.18. Found: C, 61.53; H, 3.17.
5′,10b′-Dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′a). White solid (80%); IR (KBr) (νmax/cm−1): 2974, 2908, 1754, 1709, 1579, 1469, 1262, 1210, 1086, 989, 879, 769, 710, 645. 1H NMR (400 MHz, CDCl3): δH 8.06–8.04 (2H, m, H–Ar), 7.96–7.91 (2H, m, H–Ar), 7.34–7.32 (1H, m, H–Ar), 7.19–7.13 (1H, m, H–Ar), 6.90–6.80 (2H, m, H–Ar), 6.08–6.05 (1H, m, CH), 5.88 (1H, d, J = 2.4 Hz, CH), 4.73–4.58 (2H, m, CH2), 2.78–2.73 (1H, m, CH2), 2.24–2.18 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.2, 196.6, 154.1, 140.3, 136.6, 136.5, 130.7, 128.9, 127.7, 124.3, 124.1, 122.1, 120.9, 118.4, 116.4, 74.5, 68.3, 67.6, 25.7. HRMS [M + H]+ calcd for C20H14O4: 318.3228, found: 319.1013. Anal. calcd for C20H14O4: C, 75.46; H, 4.43. Found: C, 75.47; H, 4.41.
7′-Methoxy-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′b). White solid (78%); IR (KBr) (νmax/cm−1): 2928, 2844, 1748, 1701, 1581, 1478, 1357, 1274, 1227, 1107, 1051, 1014, 874, 745, 707, 652. 1H NMR (400 MHz, CDCl3): δH 8.06–8.04 (2H, m, H–Ar), 7.94–7.93 (2H, m, H–Ar), 6.96 (1H, d, J = 8.0 Hz, H–Ar), 6.85–6.76 (2H, m, H–Ar), 6.07 (1H, s, CH), 5.88 (1H, s, CH), 4.77–4.70 (2H, m, CH2), 3.86 (3H, s, CH3), 2.78–2.73 (1H, m, CH2), 2.22–2.15 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.3, 196.4, 148.1, 143.9, 140.5, 136.8, 136.7, 130.5, 124.4, 124.2, 122.9, 120.7, 119.4, 118.7, 110.7, 78.8, 68.8, 68.2, 56.0, 25.9. HRMS calcd for C21H16O5: 348.3487, found: 349.1124. Anal. calcd for C21H16O5: C, 72.41; H, 4.63. Found: 72.43; H, 4.61.
7′-Ethoxy-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′c). White solid (78%); IR (KBr) (νmax/cm−1): 2974, 2908, 1754, 1709, 1579, 1469, 1262, 1210, 1086, 989, 879, 769, 710, 645. 1H NMR (400 MHz, CDCl3): δH 8.08–8.04 (2H, m, H–Ar), 7.96–7.93 (2H, m, H–Ar), 6.92–6.90 (1H, m, H–Ar), 6.87–6.79 (2H, m, H–Ar), 6.12 (1H, s, CH), 5.83 (1H, s, CH), 4.77 (2H, s, CH2), 4.12 (2H, q, J = 8.0 Hz, CH2), 2.80 (1H, d, J = 28.8 Hz, CH2), 2.25 (1H, d, J = 20.8 Hz, CH2), 1.48 (3H, t, J = 8.0 Hz, CH3); 13C NMR (100 MHz, CDCl3): δC 198.2, 196.6, 147.2, 144.0, 140.3, 136.6, 136.5, 130.5, 124.3, 124.1, 122.9, 120.5, 119.3, 118.8, 118.4, 112.1,74.5, 68.7, 67.7, 64.3, 25.7, 14.7. HRMS [M + H]+ calcd for C22H18O5: 362.3753, found: 363.1278. Anal. calcd for C22H18O5: C, 72.92; H, 5.01. Found: C, 72.90; H, 5.03.
9′-bBomo-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′d). White solid (72%); IR (KBr) (νmax/cm−1): 2921, 2843, 1748, 1715, 1593, 1469, 1411, 1287, 1229, 1177, 1048, 989, 853, 820, 691, 626, 548. 1H NMR (400 MHz, CDCl3): δH 8.00–7.97 (2H, m, H–Ar), 7.88–7.86 (2H, m, H–Ar), 7.36 (1H, d, J = 2.8 Hz, H–Ar), 7.29–7.12 (1H, m, H–Ar), 6.63 (1H, d, J = 8.8 Hz, H–Ar), 6.00 (1H, s, CH), 5.74 (1H, s, CH), 4.63–4.51 (2H, m, CH2), 2.71–2.65 (1H, m, CH2), 2.16–2.10 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.0, 196.4, 153.2, 140.3, 136.7, 136.6, 131.8, 130.2, 129.7, 124.4, 124.2, 124.0, 119.0, 118.2, 113.1, 74.4, 68.4, 67.2, 25.7. HRMS [M + H]+ calcd for C20H13BrO4: 397.2188, found: 398.9998. Anal. calcd for C20H13BrO4: C, 60.47; H, 3.30. Found: C, 60.49; H, 3.27.
9′-Chloro-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′e). White solid (74%); IR (KBr) (νmax/cm−1): 2915, 2857, 1741, 1722, 1579, 1469, 1417, 1287, 1229, 1190, 96, 866, 820, 704, 633, 561. 1H NMR (400 MHz, CDCl3): δH 8.06–8.02 (2H, m, H–Ar), 7.94–7.92 (2H, m, H–Ar), 7.28–7.27 (1H, m, H–Ar), 7.19–7.06 (1H, m, H–Ar), 6.72 (1H, d, J = 8.8 Hz, H–Ar), 6.07–6.06 (1H, m, CH), 5.81 (1H, d, J = 2.4 Hz, CH), 4.70–4.57 (2H, m, CH2), 2.77–2.71 (1H, m, CH2), 2.23–2.16 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.0, 196.4, 152.7, 140.3, 136.7, 136.6, 129.7, 128.9, 127.3, 125.7, 124.3, 124.1, 123.5, 118.9, 117.8, 74.4, 68.4, 67.3, 25.7. HRMS calcd for C20H13ClO4: 352.7678, found: 353.0492. Anal. calcd for C20H13ClO4: C, 68.09; H, 3.71. Found: C, 68.07; H, 3.74.
7′,9′-Dichloro-5′,10b′-dihydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromene]-1,3-dione (3′f). White solid (75%); IR (KBr) (νmax/cm−1): 3103, 2934, 2857, 1748, 1709, 1586, 1489, 1462, 1352, 1274, 1223, 1177, 1093, 976, 918, 860, 704, 626, 535. 1H NMR (400 MHz, CDCl3): δH 8.08–8.04 (2H, m, H–Ar), 7.96–7.93 (2H, m, H–Ar), 7.27–7.20 (2H, m, H–Ar), 6.12 (1H, s, CH), 5.83 (1H, s, CH), 4.77 (2H, s, CH2), 2.80 (1H, d, J = 28.8 Hz, CH2), 2.25 (1H, d, J = 20.8 Hz, CH2); 13C NMR (100 MHz, CDCl3): δC 197.9, 196.2, 148.6, 140.2, 136.8, 136.7, 129.2, 128.9, 125.9, 125.6, 124.7, 124.4, 124.2, 121.9, 119.6, 74.4, 68.9, 67.2, 25.7. HRMS [M + H]+ calcd for C20H12Cl2O4: 386.0113, found: 387.0100. Anal. calcd for C20H12Cl2O4: C, 62.04; H, 3.12. Found: C, 62.02; H, 3.15.
(R)-1,3-Dioxo-1,3,5′,10b′-tetrahydro-3′H-spiro[indene-2,2′-pyrano[3,2-c]chromen]-7′-yl benzoate (3′g). White solid (75%); IR (KBr) (νmax/cm−1): 2919, 2844, 1757, 1710, 1600, 1478, 1265, 1089, 986, 865, 773, 707, 642, 540. 1H NMR (400 MHz, CDCl3): δH 8.22–8.20 (2H, m, H–Ar), 8.06–8.04 (2H, m, H–Ar), 7.94–7.92 (2H, m, H–Ar), 7.64–7.60 (1H, m, H–Ar), 7.52–7.48 (2H, m, H–Ar), 7.28–7.26 (1H, m, H–Ar), 7.05–7.03 (1H, d, J = 8.0 Hz, H–Ar), 6.91 (1H, t, J = 8.0 Hz, H–Ar), 6.05 (1H, s, CH), 5.91 (1H, s, CH), 4.73 (1H, d, J = 12.0 Hz, CH2), 4.61 (1H, d, J = 12.0 Hz, CH2), 2.79–2.74 (1H, m, CH2), 2.21–2.16 (1H, m, CH2); 13C NMR (100 MHz, CDCl3): δC 198.1, 196.5, 164.6, 146.2, 140.3, 138.7, 136.6, 136.5, 133.4, 130.3, 130.3, 129.3, 128.4, 125.1, 124.3, 124.1, 124.0, 122.2, 120.6, 118.9, 74.5, 68.7, 67.6, 25.8. Anal. calcd for C27H18O6: C, 73.97; H, 4.14. Found: C, 73.99; H, 4.11.
Methodology for biological experiments
Cell culture and reagents
Breast cancer (MDA-MB-231, MCF-7), colon cancer (HCT-116) and oral squamous cell carcinoma (H357) cells were grown and cultured in DMEM supplemented with 10% FBS, 1.5 mM L-glutamine and 1% antibiotic (100 U ml−1 of penicillin, 10 mg ml−1 of streptomycin) at 37 °C in a humidified atmosphere of 5% CO2. Cell culture reagents and other growth supplements were procured from Himedia, India.
Cell viability assay
The anchorage dependant cell viability of the investigational compounds, parent compound and 5-FU (commonly used anti-cancer agent) were measured using MTT cell viability assay. In brief, 8000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well were seeded in 96 well flat bottom tissue culture plates and grown to 70–80% confluence. Then cells were treated with increasing concentrations of the compounds for 48 h prior to harvest. Then media was aspirated and washed once with 1XPBS. Then 0.05% MTT solution was added to each well and incubated in 37 °C for 5–6 h to allow formation of formazan crystals. The formazan crystals were dissolved by adding 100 μL of 0.2% NP-40 detergent solution and incubated in dark for 1 h. The colour intensity was measured spectrophotometrically at 570 nm by using microplate reader (Mithras LB 940, Berthold Germany). Each data point was calculated in triplicate and all the assays were performed at least thrice.
000 cells per well were seeded in 96 well flat bottom tissue culture plates and grown to 70–80% confluence. Then cells were treated with increasing concentrations of the compounds for 48 h prior to harvest. Then media was aspirated and washed once with 1XPBS. Then 0.05% MTT solution was added to each well and incubated in 37 °C for 5–6 h to allow formation of formazan crystals. The formazan crystals were dissolved by adding 100 μL of 0.2% NP-40 detergent solution and incubated in dark for 1 h. The colour intensity was measured spectrophotometrically at 570 nm by using microplate reader (Mithras LB 940, Berthold Germany). Each data point was calculated in triplicate and all the assays were performed at least thrice.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
SN and SRM are thankful to CSIR New Delhi [02(0218)/14/EMR-II] and DRDO New Delhi ERIP/ER/1203083/M/01 for providing research grant. SN thanks to CSIR, New Delhi for providing research grant. PP thanks to DST, Odisha for providing research fellowship. Authors are also thankful to DST, FIST, New Delhi for providing NMR facility in Ravenshaw University.
Notes and references
- X. Li, L. Yang, C. Peng, X. Xie, H.-J. Leng, B. Wang, Z.-W. Tang, G. He, L. Ouyang, W. Huang and B. Han, Chem. Commun., 2013, 49, 8692–8694 RSC.
-
(a) T. Chanda and M. S. Singh, Org. Biomol. Chem., 2016, 14, 8895–8910 RSC;
(b) D. Pizzirani, M. Roberti, S. Grimaudo, A. D. Cristina, R. M. Pipitone, M. Tolomeo and M. Recanatini, J. Med. Chem., 2009, 52, 6936–6940 CrossRef CAS PubMed;
(c) R. Misra and R. C. Pandey, J. Am. Chem. Soc., 1982, 104, 4478–4479 CrossRef CAS.
- N. C. Dige and D. M. Pore, Synth. Commun., 2015, 1532–2432 Search PubMed.
- Y. Yang, D. Philips and S. Pan, J. Org. Chem., 2011, 76, 1902–1905 CrossRef CAS PubMed.
- S. Mahajan, P. Chauhan, M. Blümel, R. Puttreddy, K. Rissanen, G. Raabe and D. Enders, Synthesis, 2016, 48, 1131–1138 CrossRef CAS.
- J. Duan, J. Cheng, Y. Cheng and P. Li, Asian J. Org. Chem., 2016, 5, 477–480 CrossRef CAS.
- S. A. Patil, R. Patil and S. A. Patil, Eur. J. Med. Chem., 2017, 138, 182–198 CrossRef CAS PubMed.
- S. Asadi and G. M. Ziarani, Mol. Diversity, 2016, 20, 111–152 CrossRef CAS PubMed.
- D. Kumar, P. Sharma, H. Singh, K. Nepali, G. K. Gupta, S. K. Jain and F. Ntie-Kang, RSC Adv., 2017, 7, 36977–36999 RSC.
- S. S. Mansoor, K. Logaiya, K. Aswin and P. N. Sudhan, J. Taibah Uni. Sci., 2015, 9, 213–226 CrossRef.
- J. Madda, A. Venkatesham, N. K. Bejjanki, N. Kommu, S. Pombala, C. G. Kumar, T. P. Rao and J. B. Nanubolu, Bioorg. Med. Chem. Lett., 2014, 24, 4428–4434 CrossRef CAS PubMed.
- N. Jain, R. M. Kanojia, J. Xu, G. Jian-Zhong, E. Pacia, M.-T. Lai, F. Du, A. Musto, G. Allan, D. W. Hahn, S. Lundeen and Z. Sui, J. Med. Chem., 2006, 49, 3056–3059 CrossRef CAS PubMed.
- A. Saha, S. Payra and S. Banerjee, RSC Adv., 2015, 5, 101664–101671 RSC.
- B. D. Rupnar, T. R. Kachave, P. D. Jawale, S. U. Shisodia and R. P. Pawar, Pharma Chem., 2017, 9, 120–124 CAS.
- S. Kanakaraju, B. Prasanna, S. Basavoju and G. V. P. Chandramouli, Arabian J. Chem., 2017, 10, 2705–2713 CrossRef.
- J. Liu, S.-S. Wen, J. Wang, J.-A. Xiao, S.-J. Huang and H. Yang, Tetrahedron, 2015, 71, 4629–4634 CrossRef CAS.
- N. J. Parmar, R. A. Patel, S. B. Teraiya, D. Sharma and V. K. Gupta, RSC Adv., 2012, 2, 3069–3075 RSC.
- S. Khodabakhshi, B. Karami, K. Eskandari and M. Farahi, Tetrahedron Lett., 2014, 55, 3753–3755 CrossRef CAS.
- M. S. Singh, G. C. Nandi and S. Samai, Green Chem., 2012, 14, 447–455 RSC.
- N. A. Al-Masoudi, H. H. Mohammed, A. M. Hamdy, O. A. Akrawi, N. Eleya, A. Spannenberg, C. Pannecouque and P. Langer, Z. Naturforsch., 2013, 68b, 229–238 Search PubMed.
- S. Ahadi, M. Zolghadr, H. R. Khavasi and A. Bazgir, Org. Biomol. Chem., 2013, 11, 279–286 CAS.
- A. Venkatesham, R. Srinivasa Rao, K. Nagaiah, J. S. Yadav, G. RoopaJones, S. J. Basha, B. Sridhar and A. Addlagatta, Med. Chem. Commun., 2012, 3, 652–658 RSC.
-
(a) M. M. Heravi, T. Ahmadi, M. Ghavidel, B. Heidari and H. Hamidi, RSC Adv., 2015, 5, 101999–102075 RSC;
(b) S. Vodnala, A. K. D. Bhavani, R. Kamutam, V. G. M. Naidu and P. C. Prabhakar, Bioorg. Med. Chem. Lett., 2016, 26, 3973–3977 CrossRef CAS PubMed;
(c) R. Rahimi, M. Mahdavi, S. Pejman, P. Zare and S. Balalaei, Acta Biochim. Pol., 2015, 62, 83–88 CrossRef CAS PubMed.
-
(a) P. Parenti, A. Pizzigoni, G. Hanozet, E. H. Hakim, L. Makmur, S. A. Achmad and B. Giordana, Biochem. Biophys. Res. Commun., 1998, 244, 445–448 CrossRef CAS PubMed;
(b) Y. ; Sun, J. Liu, X. Jiang, T. Sun, L. Liu, X. Zhang, S. Ding, J. Li, Y. Zhuang, Y. Wang and R. Wang, Sci. Rep., 2015, 5, 13699 CrossRef PubMed.
- L. Liu, H. Kim, Y. Xie, C. Farès, P. S. J. Kaib, R. Goddard and B. List, J. Am. Chem. Soc., 2017, 139, 13656–13659 CrossRef CAS PubMed.
- T. Liang, G. Li, L. Wojtas and J. C. Antilla, Chem. Commun., 2014, 50, 14187–14190 RSC.
- H.-L. Cui and F. Tanaka, Chem.–Eur. J., 2013, 19, 6213–6216 CrossRef CAS PubMed.
- H.-L. Cui, P. V. Chouthaiwale, F. Yin and F. Tanaka, Org. Biomol. Chem., 2016, 14, 1777–1783 CAS.
- T.-P. Gao, J.-B. Lin, X.-Q. Hu and P.-F. Xu, Chem. Commun., 2014, 50, 8934–8936 RSC.
- G. B. Gill, M. S. H. ldris and K. S. Kirollos, J. Chem. Soc., Perkin Trans. 1, 1992, 1, 2355–2365 RSC.
- S. Nayak, S. Chakroborty, S. Bhakta, P. Panda, S. Mohapatra, S. Kumar, P. K. Jena and C. Purohit, Lett. Org. Chem., 2015, 12, 352–358 CrossRef CAS.
- J. Zhang, C. Lou, Z. Hu and M. Yan, ARKIVOC, 2009, xiv, 362–375 Search PubMed.
- P. T. Parvatkar, P. S. Torney and S. G. Tilve, Curr. Org. Synth., 2013, 10, 288–317 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02729c |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Susanta Ku. Sahooa,
Seetaram Mohapatraa,
Deepika Nayakb,
Rajalaxmi Pradhanb and
Chanakya Nath Kundu*b
*a,
Susanta Ku. Sahooa,
Seetaram Mohapatraa,
Deepika Nayakb,
Rajalaxmi Pradhanb and
Chanakya Nath Kundu*b


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well were seeded in 96 well flat bottom tissue culture plates and grown to 70–80% confluence. Then cells were treated with increasing concentrations of the compounds for 48 h prior to harvest. Then media was aspirated and washed once with 1XPBS. Then 0.05% MTT solution was added to each well and incubated in 37 °C for 5–6 h to allow formation of formazan crystals. The formazan crystals were dissolved by adding 100 μL of 0.2% NP-40 detergent solution and incubated in dark for 1 h. The colour intensity was measured spectrophotometrically at 570 nm by using microplate reader (Mithras LB 940, Berthold Germany). Each data point was calculated in triplicate and all the assays were performed at least thrice.
000 cells per well were seeded in 96 well flat bottom tissue culture plates and grown to 70–80% confluence. Then cells were treated with increasing concentrations of the compounds for 48 h prior to harvest. Then media was aspirated and washed once with 1XPBS. Then 0.05% MTT solution was added to each well and incubated in 37 °C for 5–6 h to allow formation of formazan crystals. The formazan crystals were dissolved by adding 100 μL of 0.2% NP-40 detergent solution and incubated in dark for 1 h. The colour intensity was measured spectrophotometrically at 570 nm by using microplate reader (Mithras LB 940, Berthold Germany). Each data point was calculated in triplicate and all the assays were performed at least thrice.




