Open Access Article
Open Access ArticleCharacterization of palladium species after γ-irradiation of a TBP–alkane–Pd(NO3)2 system†
Bénédicte Simon a,
Christine Bouyer*a,
Stéphanie De Siob,
Claude Berthona,
Nathalie Boubalsa,
Frédéric Miserquec,
Emmanuelle Brackxa,
Nicole Raymonda,
Alexandre Chagnes
a,
Christine Bouyer*a,
Stéphanie De Siob,
Claude Berthona,
Nathalie Boubalsa,
Frédéric Miserquec,
Emmanuelle Brackxa,
Nicole Raymonda,
Alexandre Chagnes d and
Laurence Berthon
d and
Laurence Berthon a
a
aCEA Marcoule, Nuclear Energy Division, Research Department on Mining and Fuel Recycling Processes (DMRC), BP17171, 30207 Bagnols-sur-Cèze Cedex, France. E-mail: christine.bouyer@cea.fr
bORANO Cycle, 1 Place Jean Millier, 92084 Paris La Défense, France
cDEN, Service de Corrosion et d’étude du Comportement des Matériaux dans leur Environnement (SCCME), CEA, Université Paris-Saclay, 91191, Gif-sur-Yvette, France
dUniversité de Lorraine, CNRS, GeoRessources, F-54000 Nancy, France
First published on 12th June 2018
Abstract
The γ-irradiation of a biphasic system composed of tri-n-butylphosphate in tetrapropylene hydrogen (TPH) in contact with palladium(II) nitrate in nitric acid aqueous solution led to the formation of two precipitates. A thorough characterization of these solids was performed by means of various analytical techniques including X-Ray Diffraction (XRD), Thermal Gravimetric Analysis coupled with a Differential Scanning Calorimeter (TGA-DSC), X-ray Photoelectron Spectroscopy (XPS), InfraRed (IR), RAMAN and Nuclear Magnetic Resonance (NMR) Spectroscopy, and ElectroSpray Ionization Mass Spectrometry (ESI-MS). Investigations showed that the two precipitates exhibit quite similar structures. They are composed at least of two compounds: palladium cyanide and palladium species containing ammonium, phosphorous or carbonyl groups. Several mechanisms are proposed to explain the formation of Pd(CN)2.
Introduction
In the PUREX process, 30%vol tri-n-butylphosphate (TBP) diluted in Tetra Propylene Hydrogen (TPH, a hydrocarbon diluent) is used to extract uranium and plutonium from fission products (ruthenium, molybdenum, cesium, palladium, etc.) and minor actinides contained in spent nuclear fuels.1,2 Due to the biphasic nature of the chemical system and the presence of numerous solutes (nitric acid, uranium, plutonium, fission products…), the solvent is subject to hydrolytic and radiolytic degradation. The resulting damage can modify the solution composition, alter the physico-chemical properties, modify the extraction kinetics, and modify the redox properties of the metallic ions to be extracted.3 Since the 1950s, many studies have been focused on the investigation of the stability of TBP and diluent under radioactive stress.3,4 In particular, the influence of the irradiation source (α-irradiation vs. γ-irradiation5–12), diluent,5,13–16 nitric acid concentration,17–22 and temperature14 have been studied. It appears that irradiation of TBP in alkane diluents in contact with aqueous nitric acid solution produces dibutyl phosphoric acid (HDBP), monobutylphosphoric acid (H2MBP), phosphoric acid (H3PO4), higher molecular weight dimers, acidic phosphates, hydroxylated and nitro-substituted phosphates, and low-molecular weight acid phosphates.23–28 Among these products, HDBP, H2MBP, H3PO4 and low-molecular weight acid phosphate species are generated by decomposition of the TBP radical cation produced by irradiation stresses or by hydrogen atom abstraction of TBP or the primary radiolysis products. Likewise, diluent decomposition leads to the formation of nitro-substituted alkanes in presence of nitrates and alkane oxidation products such as ketones and carboxylic acids while high-molecular weight products are formed by radical-addition reactions. Without treatment, accumulation of degradation products in the solvent could lead to (i) change of the physicochemical properties of the solutions (density and viscosity),29 (ii) crud formation,30–35 and (iii) changes in the extraction properties of the solvent (efficiency, selectivity, etc.).15,19,30,31,36–40In the literature, a particular interest has been focused on the complexing properties of the degradation products towards U(VI),9,39,41,42 Pu(IV),6,9,42–46 Zr(IV),30,32,35,45,47–55 Ln(III),56,57 Fe(III)58,59 as well as Ru.37 Few studies have reported the effect of irradiation on the palladium behaviour.12,19,36,40,58–60 Guedon et al.58,59 reported the formation of a precipitate containing palladium58 under accelerated degradation conditions (higher than 0.2 MGy) of 30%vol TBP diluted in n-dodecane in contact with 3 mol L−1 nitric acid. They mentioned that formation of palladium precipitate could be related to diluent degradation. Moreover, Nowak et al.19 showed that solvent degradation is more intense in the presence of palladium(II) during irradiation of 30%vol TBP diluted in n-dodecane in contact with nitric acid. In this case, palladium might be involved in the degradation mechanisms of the extraction solvent. Furthermore, degradation is accompanied by an increase of the distribution ratio of Pd(II) (DPd).40 In order to explain this behaviour, the effect of some degradation products on the distribution ratio of Pd(II) were investigated.40,60 The presence of alkenes and butyric aldehyde could be responsible for the increase of DPd. Conversely, no significant increase in the distribution ratio of palladium(II) was observed when HDBP, H2MBP, methyl-ethyl ketone, 2-butanol, or iodoethane were added to the extraction solvent. Beside the increase of the distribution ratio of palladium(II), the formation of a black precipitate was also observed. According to the authors, such a precipitate could result from the reduction of Pd(II) into Pd(0) during the oxidation of alkenes into carbonyl compounds.61
In the ORANO La Hague reprocessing plants, after two or three decades of industrial operations, it is essential to learn how to cope with aging equipment in order to avoid process dysfunctions. In particular, precipitates containing a high proportion of palladium (>50%w) and carbon (>25%w) were found to be responsible for partial equipment clogging.62 Because of the high radioactivity of these solids, a thorough characterization is difficult to conduct. Therefore, in order to investigate the phenomena responsible for precipitate formation in the presence of Pd(II) in nuclear fuel reprocessing plants, inactive precipitates were synthetized in the laboratory by contacting 30%vol TBP diluted in TPH with palladium(II) nitrate in 3 mol L−1 nitric acid solution under γ-irradiation with a 60Co source. X-Ray Diffraction (XRD) diagrams and InfraRed (IR) spectra of these inactive precipitates have demonstrated similarities between these precipitates and the solids observed in the ORANO plant.62 These solids have been thoroughly characterized in this work by X-Ray Diffraction (XRD), Thermal Gravimetric Analysis coupled with a Differential Scanning Calorimeter (TGA-DSC), X-ray Photoelectron Spectroscopy (XPS), InfraRed (IR), RAMAN and Nuclear Magnetic Resonance (NMR) spectroscopy as well as ElectroSpray Ionization Mass Spectrometry (ESI-MS). Finally, mechanisms responsible for precipitate formation have been discussed.
Results
A biphasic system composed of 30%vol TBP diluted in TPH in contact with nitric acid solution containing palladium(II) nitrate was irradiated under accelerated degradation conditions of the PUREX process. Such conditions lead to the formation of two solids as illustrated in Fig. 1.A thorough characterization of the two precipitates ΦS,1 and ΦS,2 was undertaken using complementary analytical techniques such as XRD, TGA, XPS and IR spectroscopy. Additional information has been deduced by dissolving the precipitates in appropriate solvents and by analysing them by NMR, IR and RAMAN spectroscopy as well as ESI-MS spectrometry.
Direct analysis of the precipitates
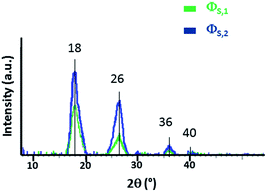
Samples were analysed by X-Ray Diffraction (XRD) to gain information about their structure. Fig. 2 shows XRD patterns of precipitate ΦS,1, located at the liquid–liquid interface (green line) and the precipitate ΦS,2 located at the bottom of the aqueous phase (blue line).Both precipitates have the same crystallographic structure as they exhibit four peaks located at 2θ = 18.1°, 26.5°, 36.2°, 40.8° as well as 18.1°, 26.2°, 36.1° and 41.2°, respectively. A comparison of XRD patterns with the literature data indicates that the crystallographic structure of these precipitates is close to that of Pd(CN)2·0.29H2O.63 Usually in an X-ray powder diffraction pattern, peak intensity and resolution (full-width half maximum) are related to the crystallinity state of the materials (crystallite size and micro-deformation of the crystal lattice). In Fig. 2, the width full-width half maximum of ΦS,1 and ΦS,2 are different: a finer and a higher intensity is observed for ΦS,2 because the precipitate ΦS,2 is probably better crystallized than the precipitate ΦS,1.
The lattice structure of ΦS,1 and ΦS,2 is I41/amd and the lattice parameters are a = b = 4.949(29) Å and c = 8.486(68) Å (RBragg = 3.18, Rwp = 0.383) as well as a = b = 4.949(29) Å and c = 8.788(85) Å (RBragg = 3.35, Rwp = 0.336), respectively. The lattice parameters a, b and c are the same for both precipitates, but they are smaller than for the commercial compound Pd(CN)2·0.29H2O (a = b = 5.119 Å and c = 13.600 Å). Pd(CN)2·0.29H2O and Pd(CN)2·0.29NH3 contain water and NH3 molecules to terminate the periodic structure of the solid, respectively (see Fig. 3 for the sake of illustration in the case of Pd(CN)2·0.29H2O).63 The differences observed between the lattice parameter c for ΦS,1, ΦS,2 and Pd(CN)2·0.29H2O may be explained by difference in crystal network. For instance, it can be expected that water molecules located at the outer edges in the Pd(CN)2 network may be replaced by carboxylates groups in ΦS,1 and ΦS,2. Furthermore, the difference in lattice parameters may be explained by the presence of at least two compounds in the precipitate since inclusions of a second compound (in minor quantity) could modify the lattice parameter c.
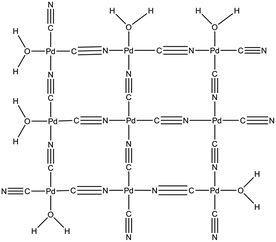 | ||
| Fig. 3 Chemical structure proposed for Pd(CN)2·xH2O based on Pt(CN)2·0.67H2O proposed by Hibble.63 | ||
In order to determine the surface elemental composition of the precipitate, X-ray photoelectron spectroscopy (XPS) was used. This technique provides information about surface composition of solids, their chemical environments and their oxidation states. The Pd-3d, C-1s and N-1s XPS spectra of ΦS,1 and ΦS,2 were recorded and compared with spectra of commercial compounds such as palladium acetate, palladium acetylacetonate, palladium pivalate and palladium cyanide. Atoms of Pd, C, O, N and P are present in both precipitates. Fig. 4 shows the Pd-3d core level spectra of the precipitates. It appears that the elemental composition and the nature of the chemical functions in ΦS,1 and ΦS,2 are similar. Table 1 depicts the binding energies of Pd-3d5/2, N-1s and C-1s contributions for both precipitates and the reference samples.
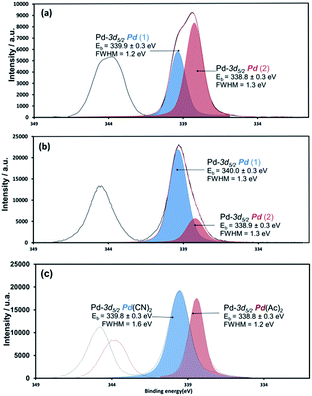 | ||
| Fig. 4 XPS core level spectra Pd-3d of (a) ΦS,1, (b) ΦS,2 and (c) commercial compounds (in red palladium acetate and in blue palladium cyanide). | ||
| Compound | Binding energy (eV) | ||
|---|---|---|---|
| Pd 3d5/2 | N-1s | C-1s | |
| a *Pd–NR4+: attributionbased on the ref. 64. | |||
| ΦS,1 | 338.8 ± 0.3 (Pd(OCO) | 399.8 ± 0.3 (Pd(CN)2) | 286.4 ± 0.3 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
| 339.9 ± 0.3 (Pd(CN)) | 401.9 ± 0.3 (*) | 289.0 ± 0.3 (C–COO) | |
| ΦS,2 | 338.9 ± 0.3 (PdOCO) | 399.8 ± 0.3 (Pd(CN)2) | 286.3 ± 0.3 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
| 340.0 ± 0.3 (Pd(CN)) | 401.9 ± 0.3 (*) | 289.0 ± 0.3 (C–COO) | |
 |
338.8 ± 0.3 (Pd(OCOCH3)) | — | 285.6 ± 0.3 (C–COO) |
| 286.5 ± 0.3 (C–O) | |||
 |
338.7 ± 0.3 (Pd(OCOC(CH3)3) | — | 289.0 ± 0.3 (C–COO) |
| 285.8 ± 0.3 (C–COO) | |||
 |
339.0 ± 0.3 (Pd(OC5H7O)) | — | 288.7 ± 0.3 (C–COO) |
| 285.8 ± 0.3 (C–CO) | |||
 |
339.8 ± 0.3 (Pd(CN)2) | 399.6 ± 0.3 (Pd(CN)2) | 286.1 ± 0.3 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
Two different palladium species may be present in the precipitates since XPS spectra exhibit two contributions for Pd-3d5/2. The comparison between reference samples and precipitates indicates the presence of Pd(II) bonded to –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and O
N and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O– groups. The presence of these two functions is confirmed by the C-1s core level spectra (Fig. S1, in ESI†): two contributions are observed at 286.3 eV for C
C–O– groups. The presence of these two functions is confirmed by the C-1s core level spectra (Fig. S1, in ESI†): two contributions are observed at 286.3 eV for C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group and 289.0 eV for O
N group and 289.0 eV for O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O group, respectively. In addition, NR4+ compound is likely present in the precipitates (R = hydrocarbon chain). Indeed, the presence of a peak located at a binding energy of 401.9 eV in the N-1s XPS data is consistent with the presence of ammonium type compound in the precipitates.64 These results confirm the presence of cyanide groups in close interaction with palladium but also show the presence of other interactions between palladium and carboxylate and/or ammonium groups. The calculation of the Pd/N atomic ratios by considering Pd and N bounded to cyano functions demonstrates that CN groups coordinate only palladium in ΦS,1 and ΦS,2, since Pd/N ratio are the same for the precipitates and commercial Pd(CN)2 (Table 2). Finally, by assuming that Pd is only surrounding by two different environments (Pd–CN and Pd–O–C
C–O group, respectively. In addition, NR4+ compound is likely present in the precipitates (R = hydrocarbon chain). Indeed, the presence of a peak located at a binding energy of 401.9 eV in the N-1s XPS data is consistent with the presence of ammonium type compound in the precipitates.64 These results confirm the presence of cyanide groups in close interaction with palladium but also show the presence of other interactions between palladium and carboxylate and/or ammonium groups. The calculation of the Pd/N atomic ratios by considering Pd and N bounded to cyano functions demonstrates that CN groups coordinate only palladium in ΦS,1 and ΦS,2, since Pd/N ratio are the same for the precipitates and commercial Pd(CN)2 (Table 2). Finally, by assuming that Pd is only surrounding by two different environments (Pd–CN and Pd–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), the proportion of Pd in Pd-cyano and Pd–O
O), the proportion of Pd in Pd-cyano and Pd–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O can be calculated for ΦS,1 and ΦS,2 (Table 3). The composition of the two precipitates is slightly different, i.e. ΦS,1 contains less Pd linked to CN− than ΦS,2.
C–O can be calculated for ΦS,1 and ΦS,2 (Table 3). The composition of the two precipitates is slightly different, i.e. ΦS,1 contains less Pd linked to CN− than ΦS,2.
| Ratio | Commercial compound Pd(CN)2 | ΦS,1 | ΦS,2 |
|---|---|---|---|
| Pd/N | 0.64 | 0.38 | 0.67 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ratios in ΦS,1 and ΦS,2 deduced by XPS
O ratios in ΦS,1 and ΦS,2 deduced by XPS
| Sample | Pd–CN | Pd–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O C–O |
|---|---|---|
| ΦS,1 | 41 | 59 |
| ΦS,2 | 80 | 20 |
IR spectroscopy was then used to identify the functional groups involved in the precipitates. The IR spectra of (a) ΦS,1 and (b) ΦS,2 precipitates were analyzed and compared with the IR spectra of (c) palladium acetylacetonate, (d) palladium cyanide, (e) palladium acetate and (f) palladium pivalate (Fig. 5). The different bands are assigned in Table 4.
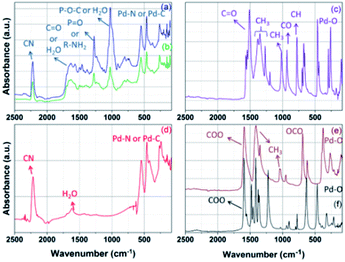 | ||
| Fig. 5 IR spectra of (a) ΦS,1 (b) ΦS,2, (c) palladium acetylacetonate, (d) palladium cyanide, (e) palladium acetate and (f) palladium pivalate. | ||
| Assignment | Wavenumber (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| ΦS,1 | ΦS,2 | ΦS,3 | Palladium cyanide63 | Palladium acetate65 | Palladium acetylacetonate66 | Palladium pivalate | |
| H2O or amine + CH3, as stretch | 3680 to 2999 (m) | 3679 to 2991 (m) | 3378 to 3015 (w) | — | 2937 (w) | 2921 (w) | |
| CH–CHalkane | 2960 to 2870 (w) | 2956 to 2870 (w) | 2975 to 2827 (w) | — | |||
C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N N |
2221 (m) | 2221 (m) | — | 2218 (m) | |||
H2O or O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1610 (m) | 1610 (m) | 1610 (s) | 1616 (w) | 1593 (s) | 1565 (m) | 1595 (s) |
| P–O–C or H2O | 1028 (s) | 1028 (s) | 1022 (s) | — | |||
| Pd–N or Pd–C | 551 (m) | 551 (m) | — | 547 (s) | |||
| Pd–N or Pd–C, Pd–O | 464 (m) | 464 (m) | — | 462 (s) | 462 (s) | 466 (s) | |
| Pd–N, Pd–O, OPdO | 266 (w) | 264 (w) | — | 266 (s) | 268 (m) | 262 (m) | |
ΦS,1 and ΦS,2 exhibit the same vibration bands. The latter are very similar to those observed in the Pd(CN)2 commercial compound. In particular, the vibration bands at 2220 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 551 cm−1 (Pd–N or Pd–C), 464 cm−1 (Pd–N or Pd–C) and 264 cm−1 (Pd–N) observed for the three compounds seem to confirm that ΦS,1 and ΦS,2 contain Pd(CN)2. The vibration band around 1610 cm−1 may indicate the presence of O–C
N), 551 cm−1 (Pd–N or Pd–C), 464 cm−1 (Pd–N or Pd–C) and 264 cm−1 (Pd–N) observed for the three compounds seem to confirm that ΦS,1 and ΦS,2 contain Pd(CN)2. The vibration band around 1610 cm−1 may indicate the presence of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functions in ΦS,1 and ΦS,2 as this band is also observed in the spectrum of commercial palladium acetate, palladium pivalate and palladium acetylacetonate, which contain O–C
O functions in ΦS,1 and ΦS,2 as this band is also observed in the spectrum of commercial palladium acetate, palladium pivalate and palladium acetylacetonate, which contain O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group (Fig. 5). The presence of the O–C
O group (Fig. 5). The presence of the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group is in agreement with the previous XPS results, which showed the presence of carboxylate groups in both precipitates. A comparison of the spectra of the precipitate, palladium acetate, palladium pivalate and palladium acetylacetonate shows a slight shift of the band located at 1610 cm−1. However, IR can only confirm the presence of carboxylate group in the compound without giving its exact composition.
O group is in agreement with the previous XPS results, which showed the presence of carboxylate groups in both precipitates. A comparison of the spectra of the precipitate, palladium acetate, palladium pivalate and palladium acetylacetonate shows a slight shift of the band located at 1610 cm−1. However, IR can only confirm the presence of carboxylate group in the compound without giving its exact composition.
ΦS,1, ΦS,2 and the commercial compound Pd(CN)2 were analysed by thermogravimetric analysis coupled with a differential scanning calorimeter (TGA-DSC) (Fig. 6). The thermal behaviour of ΦS,1 and ΦS,2 are quite close. Two endothermic peaks observed between 25 °C and 110 °C, and between 350 °C and 650 °C as well as an exothermic peak located between 110 °C and 350 °C accompanied by weight loss were observed. The reaction between 25 °C and 110 °C, and between 110 °C and 350 °C can be attributed to water vaporization63 and release of organic functions,67,68 respectively. The weight loss between 350 °C and 650 °C may correspond to the release of (CN)2(g).63 TGA analyses are consistent with the previous results: the precipitates may be composed of palladium cyanide and organic compounds.
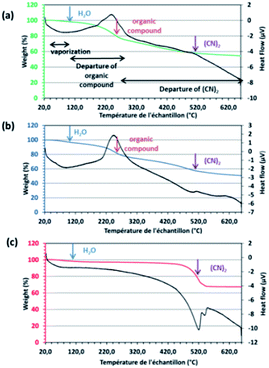 | ||
| Fig. 6 TGA thermograms of (a) ΦS,1 (in green), (b) ΦS,2 (in blue) and (c) Pd(CN)2,0.29H2O (in red). Black line: heat flow (μV); measurements performed in nitrogen atmosphere. | ||
TGA analyses allow calculating moisture content in the precipitates (% Moisture). Likewise, it is also possible to calculate the weight percentage of palladium in the precipitates (%w Pd) by considering a complete degradation of the product when samples are heated at 650 °C in nitrogen atmosphere. At this temperature, there is no additional organic matter in the precipitate and the weight of the sample corresponds to the weight of palladium. Moreover, the weight of organic (%w organic) and cyanogen (%w (CN)2) can also be calculated. Finally, the weight percentage of Pd-cyano groups (%w Pd(CN)2) and Pd-carboxylate groups (%w Pd(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)) in ΦS,1, ΦS,2 and Pd(CN)2 can be deduced from TGA experiments by using the calculation method reported in the experimental part (eqn (13) and (14)). Table 5 reports these values.
O)) in ΦS,1, ΦS,2 and Pd(CN)2 can be deduced from TGA experiments by using the calculation method reported in the experimental part (eqn (13) and (14)). Table 5 reports these values.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) corresponds to palladium coordinated to carboxylate group
O) corresponds to palladium coordinated to carboxylate group
| Sample | %w Moisture (between 25 °C and 110 °C) | %w Pd (at 650 °C) | %W (CN)2 (between 350 and 650 °C) | %W Organic (between 110 and 350 °C) | %w Pd(CN)2 | %w Pd(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|---|---|---|---|---|---|---|
| ΦS,1 | 3 | 54 | 10 | 33 | 39 | 61 |
| ΦS,2 | 3 | 57 | 16 | 24 | 55 | 45 |
| Pd(CN)2·xH2O | 2 | 68 | 30 | — | 94 | — |
The moisture contents are similar in the precipitates (2–3% for water). Moreover, total palladium contents are in the same order of magnitude in the solids ΦS,1 and ΦS,2 (around 54–57% for Pd). The major difference in composition between ΦS,1 and ΦS,2 is the distribution between the CN− group and COO− group. ΦS,2 contains more palladium coordinated to CN− than ΦS,1 (55% vs. 39%). Conversely, more palladium coordinated to carboxylate group is observed in ΦS,1 than ΦS,2. Although the distribution of Pd species determined by TGA-DSC is slightly different from that calculated from XPS data (see Table 3), both techniques show that more Pd is linked to CN− in ΦS,2 than in ΦS,1. This difference could be due to the difficulty by TGA-DSC to quantify precisely the (CN)2(g) release between 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 650
°C and 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C in ΦS,1 and ΦS,2. In the commercial compound Pd(CN2), the loss in mass of (CN)2(g) is well defined in TGA-DSC data, whereas a broad signal is observed for the precipitates. Moreover, the XPS analysis allows analysing the extreme surface of the powder only. Some errors can come from the choice of the background, the recombination of the spectra and a different depth of analysis from one sample to another.
°C in ΦS,1 and ΦS,2. In the commercial compound Pd(CN2), the loss in mass of (CN)2(g) is well defined in TGA-DSC data, whereas a broad signal is observed for the precipitates. Moreover, the XPS analysis allows analysing the extreme surface of the powder only. Some errors can come from the choice of the background, the recombination of the spectra and a different depth of analysis from one sample to another.
Analysis of the precipitates after solubilisation
Direct analyses of the precipitates by IR, Raman and XPS showed that Pd is coordinated to CN− and COO− while NR4+ groups may be present in the precipitates. However, it is impossible to confirm the exact number of palladium and organic complexes present in the precipitates. In order to deeply characterize the precipitates, they were dissolved in several solvents (DMSO (dimethyl sulfoxide), NH3 (1 mol L−1), pyridine) and the obtained solutions were analysed. It was expected that NH3 would be able to dissolve the precipitates as Pd(CN)2 can be dissolved in NH3 by forming the soluble complex Pd(CN)2(NH3)2.69 After 15 minutes of stirring of 20 mg of precipitate in 10 mL of 1 mol L−1 NH3, a filtrate (ΦF1) and an insoluble fraction (ΦS,3) were obtained. The insoluble fraction ΦS,3 and the filtrate ΦF1 were then analysed by IR and Raman spectroscopy (Fig. 7).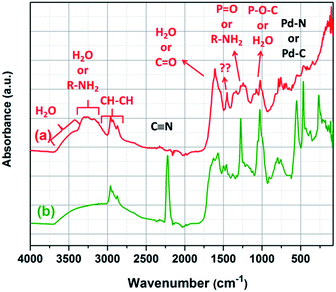 | ||
| Fig. 7 IR spectra of (a) ΦS,3 (solid residue after dissolution of the ΦS,1 in 1 mol L−1 NH3) and (b) ΦS,1. | ||
The corresponding vibration bands are reported in Table 4. These spectra show the presence of vibration bands at 2218 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 551 cm−1, 464 cm−1, and 266 cm−1 (Pd–N or Pd–C) in ΦS,1. These bands have not been observed in ΦS,3. Therefore, this study confirms that 1 mol.L−1 NH3 allows solubilizing Pd(CN)2. Moreover, further XPS analyses performed on the ΦS,3 fraction confirm that NH3 totally dissolved Pd(CN)2 since the Pd contribution located at 339.3 eV has disappeared (Fig. S2, ESI†). This conclusion was also confirmed by Raman spectroscopy on the filtrate ΦF1, since two vibration bands located at 2149 and 2140 cm−1 attributed to cyano groups were observed (Fig. 8).
N), 551 cm−1, 464 cm−1, and 266 cm−1 (Pd–N or Pd–C) in ΦS,1. These bands have not been observed in ΦS,3. Therefore, this study confirms that 1 mol.L−1 NH3 allows solubilizing Pd(CN)2. Moreover, further XPS analyses performed on the ΦS,3 fraction confirm that NH3 totally dissolved Pd(CN)2 since the Pd contribution located at 339.3 eV has disappeared (Fig. S2, ESI†). This conclusion was also confirmed by Raman spectroscopy on the filtrate ΦF1, since two vibration bands located at 2149 and 2140 cm−1 attributed to cyano groups were observed (Fig. 8).
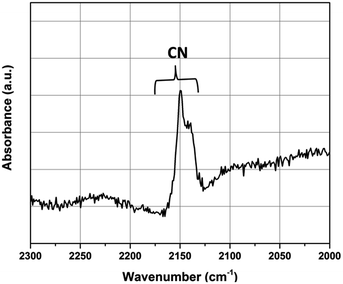 | ||
Fig. 8 Raman spectrum of ΦF1 obtained after dissolution of ΦS,1 in NH3 (1 mol L−1) at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 9 (mass of precipitate in g/volume of solvent in L). liquid ratio of 9 (mass of precipitate in g/volume of solvent in L). | ||
Furthermore, the presence of an insoluble fraction ΦS,3 (partial dissolution of ΦS,1 in NH3 1 mol L−1) seems to show that the precipitate is composed of at least of two different compounds. The second compound contains H2O molecules (vibration bands 3680–3379 cm−1 and 1610 cm−1), CH–CH groups (vibration bands at 2975 and 2827 cm−1 assigned to CH–CHalkane vibration), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functions (vibration band at 1610 cm−1), phosphate groups (vibration bands at 1274 cm−1, 1022 cm−1) or R–NH2 functions (vibration bands at 1274 cm−1).
O functions (vibration band at 1610 cm−1), phosphate groups (vibration bands at 1274 cm−1, 1022 cm−1) or R–NH2 functions (vibration bands at 1274 cm−1).
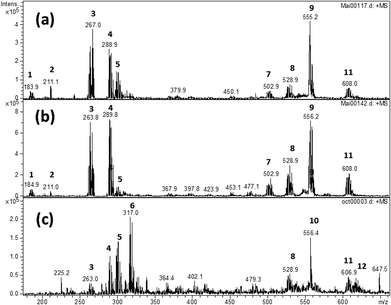
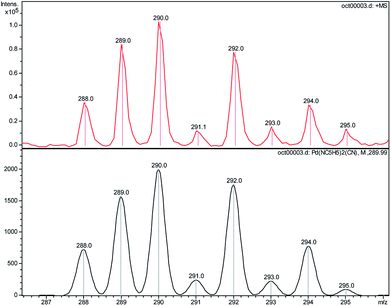
ESI-MS experiments were undertaken to characterize the precipitates after dissolution in pyridine. This technique allows the transfer of pre-existing ions from the solution into the gas phase in order to obtain speciation information. Fig. 9 shows ESI-MS spectra obtained after partial dissolution in pyridine of ΦS,1, ΦS,2 and the commercial compound Pd(CN)2. The main ions containing Pd were identified by comparison with a calculated isotopic pattern (Table 6).
| N° (Fig. 9) | m/z pattern | Identification | Pd(CN)2 | ΦS,1 | ΦS,2 |
|---|---|---|---|---|---|
| a *, observed species; —, non detected species; n.i.: non identified species. | |||||
| 1 | 180.4–188.9 | PdI(pyr)+ | — | * | * |
| 2 | 211.1 | (HDBP)H+ | — | * | * |
| 3 | 259.9–268.9 | PdI(pyr)2+ | * | * | * |
| 267.8 | (TBP)H+ | — | * | * | |
| 4 | 285.9–294.8 | PdI(pyr)2(CN)+ | * | * | * |
| 5 | 294.8–303.9 | PdI(pyr)2OH(H2O)+ | * | * | * |
| 6 | 313.1–322.0 | PdI(pyr)2OH(H2O)2+ | * | — | — |
| 7 | 496.0–509.0 | PdIPdII(pyr)3(CN)2+ | — | * | * |
| 8 | 523.0–533.9 | Pd2II(pyr)3(CN)3+ | * | * | * |
| 9 | 552.2–561.2 | PdI(pyr)2(CN)(TBP)+ | — | * | * |
| 555.2 | (TBP)2Na+ | — | * | * | |
| 10 | 556.4 | n.i. | * | — | — |
| 11 | 601.1–614.0 | Pd2II(pyr)4(CN)3+ | * | * | * |
| 12 | 610.9–622.9 | Pd2II(pyr)4(CN)2OH(H2O)+ | * | — | — |
Most ions contain pyridine indicating a strong solvation of Pd by pyridine during the solubilisation step. Moreover, the presence of ions of m/z = 180.4–188.9, 259.9–268.9, 285.9–294.8, 294.8–303.9, 313.1–322.0, 496.0–509.0 and 552.2–561.2 assigned to Pd(pyr)+ (pyr = C5H5N), Pd(pyr)2+, Pd(pyr)2(CN)+, Pd(pyr)2OH(H2O)+ Pd(pyr)2OH(H2O)2+, Pd2(pyr)3(CN)2+, and PdI(pyr)2(CN)(TBP)+ indicates the partial reduction of Pd(II) into Pd(I). Nevertheless, it is not possible to say if Pd reduction occured during the dissolution step of the solid in pyridine or during the de-solvation/ionization step. The ions of m/z = 259.9–268.9, 285.9–294.8, 294.8–303.9, 523.0–533.9 and 601.1–614.0 corresponding to Pd(pyr)2+, Pd(pyr)2(CN)+, Pd(pyr)2OH(H2O)+, Pd2(pyr)3(CN)3+ and Pd2(pyr)4(CN)3+, respectively, were present in both the commercial compound and the precipitates ΦS,1 and ΦS,2. The ions of m/z = 313.1–322.0 and 610.9–622.9 assigned to Pd(pyr)2OH(H2O)2+ and Pd2(pyr)4(CN)2OH(H2O)+, respectively, were only observed after dissolution of the Pd(CN)2 commercial compound whereas the ions of m/z equal to 259.9–268.9, 267.8, 523.0–533.9, 552.2–561.2, 555.2 and 601.1–614.0 assigned to Pd(pyr)2+, (TBP)H+, Pd2(pyr)3(CN)2+ PdI(pyr)2(CN)(TBP)+, (TBP)2Na+, and Pd2(pyr)4(CN)3+, respectively, were observed after dissolution of the precipitates in the pyridine. Thus, some differences are observed between the solution obtained after solubilisation of the commercial Pd(CN)2 compound and the precipitates. Moreover, the ion of m/z equal to 556.4 observed in the commercial compound does not contain palladium and may be due to the presence of an organic impurity because of the very high sensitivity of the technique.
In the Pd(CN)2 commercial compound, each palladium atom is surrounded by four CN groups. However, some water molecules can terminate the periodic structure (see Fig. 3). During the solubilisation step, pyridine molecules solvate the palladium and replace some CN group to produce Pd2(pyr)4(CN)3+ and Pd2(pyr)4(CN)2OH(H2O)+. Then, during the transfer from the solution to the gas phase in the mass spectrometer, these ions can be modified by release of a pyridine, CN group or water molecules and Pd(II) could be reduced so that Pd2II(pyr)4(CN)3+; Pd2II(pyr)3(CN)3+. PdI(pyr)2OH(H2O)2+; PdI(pyr)2OH(H2O)+; PdI(pyr)2CN+ and PdI(pyr)2+ can be formed.
After solubilisation of the precipitates, the ions Pd2(pyr)4(CN)2OH(H2O)+ and PdI(pyr)2OH(H2O)2+ were not observed. However, the ions PdI(pyr)2(CN)(TBP)+, (TBP)2Na+ and (TBP)H+ were observed and indicate the presence of the TBP in the solution. The presence of HDBP was also confirmed since an ion of m/z = 211.1 which corresponds to (HDBP)-H+ is observed. Comparing to the commercial compound in which water molecules terminate the periodic structure, it is possible that in the precipitate some TBP molecules terminate the structure. Thus, although not conclusive in providing structural information for the solid, the ESI-MS results provide evidence that the presence of TBP in the precipitates.
In order to get information about the chemical structure of the precipitates 1D and 2D 1H NMR spectra were recorded after ΦS,2 dissolution in DMSO-D6 (deuterated DMSO). Pyridine was not used for NMR analyses in spite of the high solubilization power of pyridine because of the two following drawbacks: (i) pyridine peak overlap 1H signal in the aromatic area (about 7 ppm) and (ii) pyridine can easily dissociate Pd species. The 1D 1H NMR spectrum of ΦS,2 exhibits the reference peak at 0 ppm (TMS) and a peak located at 2.5 ppm attributed to residual protons of DMSO-D6 (Fig. 10). The triplet and the three multiplets located at 0.85, 1.31, 1.53 and 3.82 ppm, respectively, correspond to the butyl protons in TBP (for more information ESI – Fig. S3 and S4†). The three symmetric peaks of same intensity centred at 7.05 ppm indicate protons coupling with a nucleus of spin 1 (Fig. 10). Among the nuclei in the sample, only the nitrogen has a spin 1. The corresponding J14N1H coupling constant of 104 Hz is in the magnitude order of 1JNH (i.e. 1H one bond apart from 14N) and consequently could be due to the presence of an amine function.
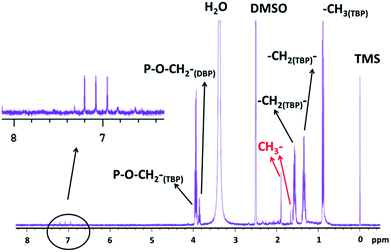 | ||
| Fig. 10 1D 1H NMR spectrum of ΦS,2 partially dissolved in DMSO-D6 m(ΦS,2) = 9.8 mg in 650 μL of DMSO-D6, i.e. S/L = 15.07 g of precipitate/L of solvent. | ||
In order to confirm the presence of amine functions in the precipitate ΦS,2, a biphasic system composed of 30%vol TBP diluted in TPH contacted with 3 mol L−1 nitric acid solution containing palladium nitrate (5 g L−1 Pd) and NaNO3 (66 g L−1) enriched with 98% atomic 15N was irradiated and the aqueous phase was analyzed. The 15N NMR spectrum shows a triplet centred on −360 ppm (15N scale is referenced to CH3–NO2) that is characteristic of NH2 functions (Fig. S5, ESI†). The 2D 15N–1H spectrum displayed in Fig. S7 (ESI†) shows a correlation spot (1J15N1H = 72 Hz) between the triplet at −360 ppm observed in the 15N NMR spectrum (Fig. S7†) and the peak located at 6.1 ppm observed in the 1H NMR spectrum (Fig. S6 in ESI†). Therefore, protons observed at 6.1 ppm corresponds to NH2 functions. Likewise, the peaks centred at 7.05 ppm in the 1H NMR spectrum of ΦS,2 can be assigned to an amine function.
To complete 1H NMR peak assignments for ΦS,2, gHSQCAD and gHMBCAD pulse sequences were performed. These 2D pulse sequences allow 13C assignments through 1H detections and the corresponding coupling constants: (1JCH for gHSQCAD) and (nJCH with n = 2 or 3 for gHMBCAD) (Fig. 11 and 12). In Fig. 11, positive red crosspeaks belong to CH– and –CH3 groups whereas negative blue peaks belong to –CH2– groups. The two red cross-peaks at (1.6 ppm; 13.8 ppm) (1H scale; 13C scale) and (1.9 ppm; 13.8 ppm) are two unshielded –CH3 groups. At 0.85, 1.31, 1.53 and 3.82 ppm (1H scale), the set of large and small cross peaks are assigned to the CH3–, two –CH2– and –CH2–O–P groups at 13.8, 18.6, 32.2 and 67.0 ppm (13C scale) of the butyl chains bearing on TBP (main compound) and HDBP peaks arising from TBP degradation. The presence of TBP or HDBP in the precipitate is consistent with IR and ESI-MS data. On the 1H–13C gHMBCAD (Fig. 12), the –CH3 chemical shift located at 1.9 ppm on the 1H scale is correlated to carbon peaks two or three bonds apart at 172 and 150 ppm which are assigned to carbonyl and cyano groups respectively. Such information allows to suggest the presence of methyl-oxo-cyanide in ΦS,2 (CH3–CO–CN). NMR analyses for ΦS,1 have not been undertaken. However, the same results could be expected.
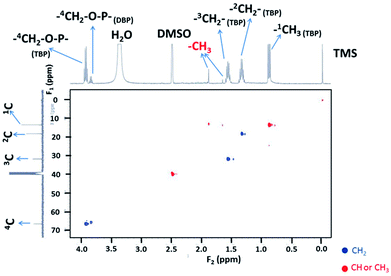 | ||
| Fig. 11 2D NMR spectrum of 1H and 13C (pulse sequence gHSQCAD) of ΦS,2 partially dissolved in DMSO-D6 with m(ΦS,2) = 9.8 mg in 650 μL of DMSO-D6 (S/L = 15.07 g of precipitate/L of solvent). | ||
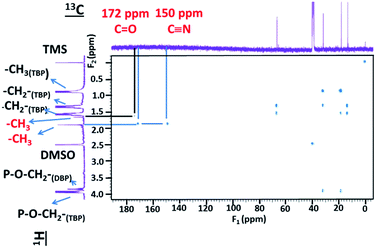 | ||
| Fig. 12 2D NMR spectrum of 1H and 13C (pulse sequence gHMBCAD) of ΦS,2 partially dissolved in DMSO-D6 with m(ΦS,2) = 9.8 mg in 650 μL of DMSO-D6 (S/L = 15.07 g of precipitate/L of solvent). | ||
Discussion
In this work, γ-irradiation of a biphasic system containing 30%vol TBP diluted in TPH (organic phase) in contact with palladium(II) nitrate dissolved in 3 mol L−1 nitric acid (aqueous phase) led to the formation of a precipitate located at the liquid–liquid interface (ΦS,1) and a precipitate that settled spontaneously (ΦS,2) (Fig. 1). Elemental analyses showed that both precipitates contain palladium. Solubilisation tests demonstrated that the precipitates do not contain only one compound but a mixture of at least two compounds. XRD, XPS, TGA-DSC, IR, ESI-MS and NMR analyses allowed for the identification of palladium cyanide in the precipitates. Other compounds such as palladium carboxylates, phosphates (i.e. TBP or HDBP), compounds with ammonium and/or amine functions may also be present to a lesser extent. In the following, the presence of the different compounds in the precipitate is discussed.The irradiation of a biphasic system leads to both radiolysis of nitric acid and water molecules in the aqueous phase, and radiolysis of the molecules present in the organic phase (TBP and TPH). Radiolysis of nitric acid primarily leads to the formation of HNO2 (ref. 23 and 70) and H2O2 (ref. 70) whereas radiolysis of TBP and TPH can generate many different degradation products such as alkenes, carbonyl compounds (R–CO–R′, R–CO–H), carboxylic acids (R–COOH), alcohols (R–OH), nitro compounds (R–NO2)23 and phosphorus compounds such as HBDP, H2MBP, H3PO4, etc.26 In addition, several authors mentioned the formation of secondary products, such as oximes (R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH), even though this molecule has never been formally identified.23,71 These oxime compounds have a great affinity for Pd.72
N–OH), even though this molecule has never been formally identified.23,71 These oxime compounds have a great affinity for Pd.72
NMR and ESI-MS analyses showed the presence of TBP and HDBP in ΦS,1 and ΦS,2 but these molecules may not be directly bonded to the Pd since XPS analyses showed only two different environments around Pd that are not characteristic of phosphorous compounds but rather C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and O–C
N and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. However, the presence of TBP and HDBP in the precipitate may also explained by the inclusion of TBP/HDBP molecule in the solid since the precipitate are obtained in the biphasic system containing both TBP and HDBP (due to the radiolytic degradation of the TBP).
O groups. However, the presence of TBP and HDBP in the precipitate may also explained by the inclusion of TBP/HDBP molecule in the solid since the precipitate are obtained in the biphasic system containing both TBP and HDBP (due to the radiolytic degradation of the TBP).
Besides, NMR analyses showed the presence of carboxylate and CN group. XPS indicated that these two groups are directly bonded to palladium. The formation of Pd-carboxylate compounds could be easily explained by the reaction of carboxylic acid (radiolytic degradation products of the TBP–alkane solution73–76) with the palladium leading to a precipitate.
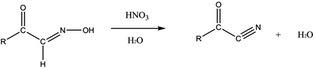
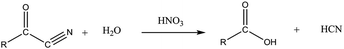
Regarding the palladium cyanide formation, an assumption would be the reaction of HCN with the palladium present in the organic/aqueous solutions. Ketones and oximes seem to be a valuable precursor to the formation of HCN among the degradation products formed during γ-irradiation.77,78 In particular, it is interesting to highlight that heating of aldehydes, ketones or alcohols in the presence of hydroxylamine (NH2OH) or nitrous acid (HNO2) in acidic media can be responsible for HCN formation.77 An oxo-oxime can be formed by the addition of HNO2 or NH2–OH to the carbon in α position of the carbonyl group (eqn (1)). This species becomes dehydrated and leads to the formation of an α-keto-cyanide (eqn (2)), which can be hydrolyzed into carboxylic acid (R–CO–OH) with the departure of HCN (eqn (3)).77
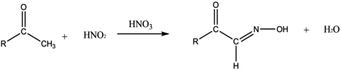 | (1) |
 | (2) |
 | (3) |
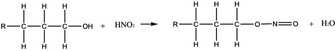
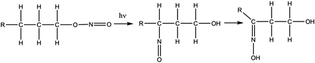
Other ways to produce oxime that are consistent with the experimental conditions of the PUREX process were also reported in the literature.77 In presence of HNO2, alcohols can and form nitrous acid ester by esterification (eqn (4)). A rearrangement can occur to produce a compound containing both an hydroxyl (–OH) and a nitroso (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, i.e. an oxime (eqn (5)). The oxime can be afterward hydrolysed into a ketone (eqn (6)). Finally, the ketone can form HCN by reacting with HNO2 or NH2OH according to eqn (1) to (3).
O) groups, i.e. an oxime (eqn (5)). The oxime can be afterward hydrolysed into a ketone (eqn (6)). Finally, the ketone can form HCN by reacting with HNO2 or NH2OH according to eqn (1) to (3).
 | (4) |
 | (5) |
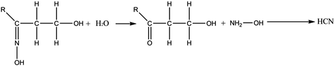 | (6) |
Another alternative pathway for oxime formation could be the reaction of CH3˙ radical with NO (eqn (7)) since NO radicals can be formed by irradiation of TBP or alkanes.79
CH3˙ + NO → H3C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⇄H2C O⇄H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH N–OH
| (7) |
In this particular case, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH can lead to the formation of HCN in the presence of Pd(0) according to Scheme 1. The presence of Pd(0) is likely and has already been reported by Vialard and Germain12 after radiolysis of TBP in alkane solution in contact with aqueous phase.
N–OH can lead to the formation of HCN in the presence of Pd(0) according to Scheme 1. The presence of Pd(0) is likely and has already been reported by Vialard and Germain12 after radiolysis of TBP in alkane solution in contact with aqueous phase.
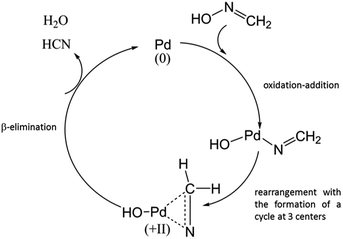 | ||
| Scheme 1 Proposition of catalytic mechanism for HCN formation in the presence of Pd0 and formaldehyde oxime. | ||
Finally, HCN can react with Pd2+ to form Pd(CN)2 as shown in eqn (8) since the stability constant is quite high (K = 10+41.8).80
| Pd2 + 2HCN = Pd(CN)2 + 2H+ | (8) |
Several formation pathways for Pd(CN)2 have been proposed. An organic degradation product, such as alcohol, carbonyl compound or methyl radical, in the presence of HNO2 or NH2OH allows the formation of HCN. Then, in presence of Pd2+ in solution and HCN, Pd(CN)2 can be formed with a thermodynamic constant of 10+41.8 (Scheme 2).
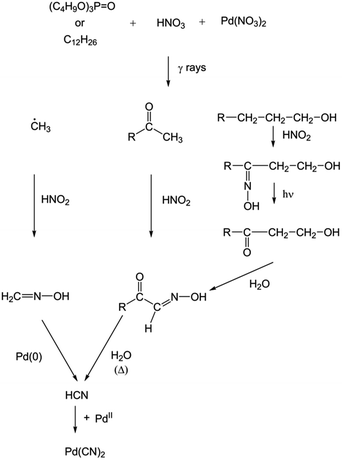 | ||
| Scheme 2 Proposition of a formation pathway for Pd(CN)2 after irradiation of a biphasic system composed of TBP in alkane in presence of nitric acid solution containing palladium(II) nitrate. | ||
Conclusion
XRD, XPS, TGA, IR, ESI-MS and NMR have been used to characterize the two precipitates (ΦS,1 and ΦS,2) produced by γ-irradiation at 500 kGy of 30% vol tri-n-butylphosphate diluted in TPH in contact with nitric acid containing palladium(II) nitrate. ΦS,1 and ΦS,2 exhibit similar structures and contain a mixture of Pd(CN)2 and other compounds (Pd-carboxylate, ammonium or amine functions and phosphorous compounds). The degradation products formed during irradiation of the two-phase biphasic system may be responsible for precipitates formation of palladium. Among the degradation products formed by irradiation, ketones and oximes could be responsible for precipitate formation.Future studies designed to validate the proposed Pd(CN)2 formation mechanism and to identify the other Pd compounds are currently under progress. Nitrate group arising from nitric acid or palladium nitrate may be responsible for the presence of CN− in the precipitates since only these two products contain nitrogen atoms in the biphasic system. Therefore, γ-irradiation in the presence of 15HNO3 may be very helpful to identify the different intermediates involved in HCN formation. Specifically, analyses of the organic and the aqueous phases could provide information on the nitrogen species formed by γ-irradiation and allow for the confirmation or invalidation of the proposed mechanism for palladium cyanide formation. Biphasic systems containing an organic phase either of pure TBP or pure dodecane in contact with a nitric acid aqueous solution containing palladium(II) nitrate will be irradiated under the same conditions as the biphasic system {TBP–TPH–HNO3–Pd} described in this study in order to determine which organic molecule is responsible for the precipitate formation.
Experimental
Reagent
Palladium nitrate (purity = 99.9%, Sigma Aldrich), 68.5%wt nitric acid (purity = 99.8%, VWR), TBP (purity ≥ 99.9%, VWR) and tetrapropylene hydrogen (TPH, petroleum fraction, Novasep) were used without further purification. The extraction solvent was prepared by mixing appropriate amounts of TBP and TPH (30%vol TBP diluted in TPH). Palladium(II) nitrate was weighted in order to obtain a concentration of 0.02 mol L−1 (or 2 g L−1) of Pd in 3 mol L−1 nitric acid aqueous solution prepared by dilution of 68.5%wt nitric acid in Milli-Q deionized water. The solution was filtered to remove traces of metallic palladium.Palladium(II) cyanide (purity = 99.9%), palladium(II) acetate (purity ≥ 99.9%) palladium(II) pivalate (purity = 97%), palladium(II) acetylacetonate (purity = 99%) were purchased from Sigma Aldrich and used without further purification.
Dimethyl sulfoxide (DMSO, purity = 99.5%, VWR), pyridine (pyr, purity = 99.7%, VWR), ammonia (purity = 99.8%, VWR), dichloromethane (purity = 99.9%, Sigma Aldrich), tetrahydrofuran (THF, purity ≥ 99.9%, Acros Organic), methanol (purity = 99.8%, VWR), acetone (purity = 99.9%, VWR) were used without further purification.
An aqueous solution of 1 mol L−1 sodium hydroxide was purchased from VWR and used for potentiometric titrations.
A palladium solution of 1000 μg mL−1 in 10%wt HCl provided by Fischer Scientific was used to prepare the standard solutions for ICP-AES measurement.
Synthesis of the palladium precipitates
A 3 mol L−1 nitric acid aqueous phase containing 0.02 mol L−1 palladium(II) nitrate (2 g L−1 of Pd) was contacted with an organic phase containing 30%vol TBP diluted in TPH with a volume ratio organic to aqueous phase of 1. The organic phase was not pre-equilibrated before extraction of Pd(II). The biphasic system was shaken for 5 minutes at ambient temperature before γ-irradiation to 500 kGy by using a 60Co source at the Synergy Health company (Marseille, France) with a dose rate of about 5 kGy h−1. The integrated dose was chosen to accelerate the degradation process and to simulate long term behaviour of the solvent under irradiation stress. After γ-irradiation of the biphasic system, four phases were observed: an organic phase (Φorg), an aqueous phase (Φaq), a light precipitate at the liquid–liquid interface (ΦS,1) and a heavy precipitate (ΦS,2) at the bottom of the flask as illustrated in Fig. 1. Precipitates were recovered and filtered under vacuum. Afterwards, they were washed twice with 2 mol L−1 nitric acid and dried at 40 °C for 24 hours. The palladium concentration and the acidity of the liquid phases, before and after irradiation are reported Table 7. The acidity of the organic and the aqueous phases scarcely decreased while the palladium concentration in the aqueous phase decreased drastically.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (γ-irradiation performed with 60Co)
1 (γ-irradiation performed with 60Co)
| [Pd]aq (mmol L−1) | [H+]aq (mol L−1) | [H+]orga (mol L−1) | |||
|---|---|---|---|---|---|
| Before irradiation | After irradiation | Before irradiation | After irradiation | Before irradiation | After irradiation |
| 18 ± 10−3 | 4 ± 10−3 | 2.63 ± 0.07 | 2.45 ± 0.07 | 0.62 ± 0.07 | 0.60 ± 0.07 |
The volumes of the aqueous and organic phases before γ-irradiation and the weight of the precipitates ϕS,1 and ϕS,2 recovered after irradiation are reported in Table 8.
| Volume of the solutions before irradiation | Weights of precipitates after irradiation | ||
|---|---|---|---|
| Vaq (mL) | Vorga (mL) | mϕS,1 (mg) | mϕS,2 (mg) |
| 200 | 170 | 264 | 312 |
In order to characterize the composition of the precipitates, dissolution tests were performed by using DMSO, CH2Cl2, THF, methanol, acetone, NH3 (1 mol L−1) and pyridine. No complete dissolution of ΦS,1 and ΦS,2 with a S/L of 2 (solid/liquid: mass of precipitate (g)/volume of the solvent (L)) for 15 minutes at room temperature was achieved with these solvents. DMSO, pyridine and 1 mol L−1 NH3 lead to the best dissolution of the precipitates. Table 9 shows the experimental conditions requested to dissolve partially the precipitates in DMSO, pyridine or 1 mol L−1 NH3 and the weight of the insoluble part (after the partial dissolution, a liquid phase (ΦF1) and a solid (ΦS,3) were obtained).
Table 10 gathers palladium concentration in these solvents after partial dissolution of the precipitates ΦS,1 and ΦS,2. The accuracy on the palladium concentration and the weight percent of palladium solubilized was 5%.
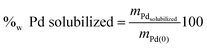 | (9) |
| Solvents used for the dissolution | %w Pd solubilizeda | [Pd] solubilized (mol L−1) | ||
|---|---|---|---|---|
| ΦS,1 | ΦS,2 | ΦS,1 | ΦS,2 | |
| a The weight percent of palladium solubilized in each solvent DMSO, pyridine and NH3 is calculated as shown in eqn (9). | ||||
| DMSO | 10 | 9 | 9.2 × 10−4 | 1.3 × 10−3 |
| Pyridine | 80 | 81 | 6.8 × 10−3 | 1.0 × 10−2 |
| NH3 | 99 | 83 | 9.1 × 10−3 | 9.6 × 10−3 |
The calculation of mPd(0) is described in the TGA-DSC analysis, it corresponds to the mass remaining at 650 °C.
Equipment and analysis
The weight loss observed around 80 °C attributed to the H2O vaporization allows calculating the moisture content in the precipitates according to eqn (10)
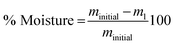 | (10) |
By considering that a complete degradation is achieved when the samples are heated at 650 °C, the weight percent of palladium in the precipitates can thus be calculated as follows eqn (11).
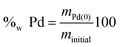 | (11) |
The weight loss observed between 25 °C and 110 °C, from 110 °C to 350 °C, and between 350 °C and 650 °C are attributed to H2O vaporization, organic compounds, and (CN)2 respectively. The weight percent calculations are detailed below. Based on the TGA analyses, a quantification of each loss in mass can be deduced.
By measuring the loss in mass of (CN)2 produced between 300 °C and 650 °C, TGA analysis allows the determination of the mass associated to gaseous (CN)2 thanks to eqn (12).
| m(CN)2 = m2 − mPd(0) | (12) |
By assuming all the (CN)2 is in palladium cyanide form, it is possible to calculate the fraction of Pd linked to cyano functions present in the total amount of the Pd in the precipitate as shown in eqn (13).
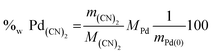 | (13) |
The weight percent of palladium linked to carboxylate present in the total amount of the Pd in the precipitate can be deduced as follows by considering that only carboxylate and CN functions are linked to palladium in the precipitates (eqn (14)).
%w Pd(O−C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 100 − %w Pd(CN)2 O) = 100 − %w Pd(CN)2
| (14) |
At 350 °C, the loss in mass is attributed to organic compounds. The weight percent of organic compound can be deduced thanks to eqn (15).
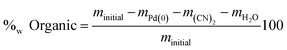 | (15) |
In general, it may be considered that the TGA measurement uncertainties lead to an error of approximatively 10% for the difference weight percent calculations.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O for ΦS,1 and ΦS,2 can be calculated based on XPS analysis. By calculating the maximum intensity ratio of Pd linked to CN function and Pd linked to carboxylate function and by considering only two kinds of palladium are present in the precipitates, it is possible to obtain the palladium proportion of the two kinds of palladium. For XPS analysis, there are several sources of error in the weight percent calculated by this technique. First of all, the XPS analysis only gives information to relative quantifications. The errors can come from the choice of the background, the recombination of the spectra and a different depth of analysis from one sample to another. Finally, this technique allows analysing the extreme surface of the powder only and does not allow going deep into the sample. All this errors can explain the large uncertainty error in the value obtain by calculated of the proportions of Pd linked to cyano functions (C
C–O for ΦS,1 and ΦS,2 can be calculated based on XPS analysis. By calculating the maximum intensity ratio of Pd linked to CN function and Pd linked to carboxylate function and by considering only two kinds of palladium are present in the precipitates, it is possible to obtain the palladium proportion of the two kinds of palladium. For XPS analysis, there are several sources of error in the weight percent calculated by this technique. First of all, the XPS analysis only gives information to relative quantifications. The errors can come from the choice of the background, the recombination of the spectra and a different depth of analysis from one sample to another. Finally, this technique allows analysing the extreme surface of the powder only and does not allow going deep into the sample. All this errors can explain the large uncertainty error in the value obtain by calculated of the proportions of Pd linked to cyano functions (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) and Pd linked to carboxylate functions (O–C
N) and Pd linked to carboxylate functions (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).The precipitates were analyzed by Rietveld refinement, using TOPAS 4.2 software and Le Bail pattern matching.81 The profile parameters (cell dimension, peak shape, background, sample displacement correction and asymmetry) were defined. The peak shape was described by pseudo-Voigt function with the formulation of Caglioti.82
The Rietveld method83 was used to determine the crystallographic structure of solid samples. The refinement program minimizes the residual between experimental and calculated XRD patterns by the method of least squares. The quality of a refinement was determined by comparing the experimental XRD pattern and the calculated one by means of the following parameters, which must be as low as possible:
- The RBragg factor associated with a given structure is based on the integrated intensities and is calculated by eqn (16). The structural model is assumed to be correct when the ratio RBragg value is lower than about 5.
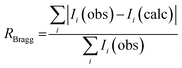 | (16) |
- The factor Rwp weighted profile is associated with the whole pattern and is calculated as follows in eqn (17):
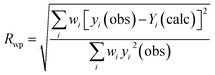 | (17) |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by ORANO Cycle and Commissariat à l’Énergie Atomique et aux Énergies Alternatives (CEA). The authors would like to thanks Ashleigh Kimberlin for helpful comments and editing assistance.References
- H. A. C. Mc Kay, in Science and Technology of Tributyl Phosphate, ed. W.W. Schulz and James D. Navratil, CRC Press, Boca Raton, Florida, 1984, vol. 1, pp. 1–12 Search PubMed
.
- R. Ruhela, A. K. Singh, B. S. Tomar and R. C. Hubli, RSC Adv., 2014, 4, 24344 RSC
.
- M. C. Charbonnel and L. Berthon, in Ion Exchange and Solvent Extraction, ed. B. Moyer, CRC Press Taylor & Francis Group, Boca Raton, London, New York, 2010, vol. 19, pp. 429–513 Search PubMed
.
- J. W. Davis, in Science and Technology of Tributyl Phosphate, ed. W.W. Schulz and James D. Navratil James, CRC Press, Boca Raton, Florida, 1984, vol. 1, pp. 221–266 Search PubMed
.
- G. S. Barney and D. G. Bouse, Alpha radiolysis of tributyl phosphate – Effect of diluents, Atlantic Richfield Hanford Company, ARH-ST-153, Washington, 1977 Search PubMed
.
- I. A. Kulikov, N. V. Kermanova, O. A. Sosnovskii, N. N. Shesterikov and M. V. Vladimirova, Sov. Radiochem., 1981, 23, 664–669 Search PubMed
.
- Y. Gao, W. Zheng, X. Cao and S. Chen, Nukleonika, 2014, 59, 123–128 CrossRef
.
- J. Pearson and M. Nilsson, Solvent Extr. Ion Exch., 2014, 32, 584–600 CrossRef
.
- I. A. Kulikov, N. V. Kermanova and M. V. Vladimirova, Sov. Radiochem., 1983, 25, 310–316 Search PubMed
.
- V. M. Adamov and V. I. Andrew, Kerntechnik, 1990, 55, 133–136 Search PubMed
.
- Z. Nowak, Nukleonika, 1977, 22, 155–172 Search PubMed
.
- E. Vialard and M. Germain, in Extraction 84: Symposium on Liquid-Liquid Extraction, ed. Yong Zhou, Pergamon Press, Dounreay (UK), 1984, vol. 88, pp. 19–30 Search PubMed
.
- E. S. Lane, Nucl. Sci. Eng., 1963, 17, 620–625 CrossRef
.
- O. K. Tallent, J. C. Mailen and K. D. Pannel, Nucl. Technol., 1985, 2, 417–425 CrossRef
.
- G. F. Egorov and O. P. Afanas’ev, At. Energy, 1983, 54, 347–355 Search PubMed
.
- S. C. Tripathi, P. Bindu and A. Ramanujam, Sep. Sci. Technol., 2001, 36, 1463–1478 CrossRef
.
- B. J. Mincher, S. P. Mezyk and L. R. Martin, J. Phys. Chem., 2008, 112, 6275–6280 CrossRef PubMed
.
- S. Mishra, C. Mallika, N. K. Pandey, U. Kamachi Mudali and R. Natarajan, Sep. Sci. Technol., 2015, 50, 1671–1676 CrossRef
.
- Z. Nowak, M. Nowak and A. Seydel, Radiochem. Radioanal. Lett., 1979, 38(5–6), 343–354 Search PubMed
.
- V. M. Adamov, V. I. Andreev, B. N. Belyaev, G. S. Markov, M. S. Polyakov, A. E. Ritori and A. Y. Yu Shil’nikov, Sov. Radiochem., 1991, 24, 153–158 Search PubMed
.
- M. V. Krishnamurthy and R. Sampathkumar, J. Radioanal. Nucl. Chem. Lett., 1992, 166(5), 421–429 CrossRef
.
- Z. Nowak and M. Nowak, Radiochem. Radioanal. Lett., 1973, 14(3), 161–168 Search PubMed
.
- D. Lesage, PhD thèse, Université de Paris VI, 1995
.
- D. Lesage, H. Virelizier, C. K. Jankowski and J. C. Tabet, J. Spectrosc., 1997, 13, 275–290 CrossRef
.
- D. Lesage, H. Virelizier, C. K. Jankowski and J. C. Tabet, Eur. Mass Spectrom., 1998, 4, 47–54 CrossRef
.
- M.-C. Charbonnel and L. Berthon, Ion Exch. Solvent Extr., 2009, 19, 17–24 Search PubMed
.
- B. J. Mincher, G. Modolo and S. P. Mezyk, Solvent Extr. Ion Exch., 2009, 27, 1–25 CrossRef
.
- V. Huss, PhD thesis, University of Aix-Marseille, 1991
.
- S. C. Tripathi and A. Ramanujam, Sep. Sci. Technol., 2003, 38, 2307–2326 CrossRef
.
- C. J. Hardy and D. Scargill, J. Inorg. Nucl. Chem., 1961, 17, 337–349 CrossRef
.
- J. C. Neace, Sep. Sci. Technol., 1983, 18, 1581–1594 CrossRef
.
- C. Miyake, M. Hirose, T. Yoshimura, M. Ikeda, S. Imoto and M. Sano, J. Nucl. Sci. Technol., 1990, 27, 157–166 CrossRef
.
- D. N. Smith, H. G. M. Edwards, M. A. Hughes and B. Courtney, Sep. Sci. Technol., 1997, 32, 2821–2849 CrossRef
.
- E. Zimmer and J. Bochardt, Nucl. Technol., 1986, 75, 332–337 CrossRef
.
- C. Miyake, M. Hirose, T. Yoshimura, M. Ikeda, S. Imoto and M. Sano, J. Nucl. Sci. Technol., 1990, 27, 256–261 CrossRef
.
- K. P. Lunichkina, E. V. Renard and V. B. Shevchenko, Russ. J. Inorg. Chem., 1974, 19, 110–113 Search PubMed
.
- H. X. Huang, G. H. Zhu and S. B. Hou, Radiochim. Acta, 1989, 46, 159–162 Search PubMed
.
- S. Tachimori, H. Nakamura and A. Sato, J. Radioanal. Nucl. Chem., 1979, 50, 143–151 CrossRef
.
- B. A. Powell, J. D. Navratil and M. C. Thompson, Solvent Extr. Ion Exch., 2003, 21, 347–368 CrossRef
.
- P. A. Zagorets, Sov. Radiochem., 1982, 24, 43–48 Search PubMed
.
- L. L. Burger, in Progress in Nuclear Energy, ed. F. R. Bruce, J. M. Fletcher and H. H. Hyman, Pergamon Press, New York, 1958, vol. 2, p. 307 Search PubMed
.
- M. V. Vladimirova, D. A. Fedoseev, I. A. Kulikov, I. A. Boikova, A. S. Milovanova and N. V. Kermanova, Sov. Radiochem., 1984, 26, 84–89 Search PubMed
.
- M. V. Vladimirova, D. A. Fedoseev, I. A. Kulikov, A. S. Milovanova, I. A. Boikova, O. A. Sosnovskii, N. V. Kermanova and B. I. Bulkin, Sov. Radiochem., 1981, 24, 33–38 Search PubMed
.
- M. V. Vladimirova, D. A. Fedoseev, I. A. Boikova and A. S. Milovanova, Sov. Radiochem., 1984, 26, 29–36 Search PubMed
.
- L. P. Sokina, A. S. Solovkin, E. G. Teterin, F. A. Bogdanov and N. N. Shesterikov, Sov. Radiochem., 1978, 20, 19–24 Search PubMed
.
- P. Zhang, C. L. Song, J. F. Liang and R. X. Xin, Solvent Extr. Ion Exch., 2001, 19, 79–89 CrossRef
.
- C. A. Blake Jr, W. Davis Jr and J. M. Schmitt, Nucl. Sci. Eng., 1963, 17, 626–637 CrossRef
.
- A. M. Rochon, Z. Nowak and Z. P. Zagorski, Radiochem. Radioanal. Lett., 1976, 27, 1–8 Search PubMed
.
- C. Lamouroux, C. Moulin, J. C. Tabet and C. K. Jankowki, Rapid Commun. Mass Spectrom., 2000, 14, 1869–1877 CrossRef PubMed
.
- E. V. Barelko, I. P. Solyanina and G. S. Babakina, Sov. Radiochem., 1976, 18, 573–577 Search PubMed
.
- E. G. Teterin, N. N. Shesterikov, P. G. Krutikov and A. S. Solovkin, Russ. J. Inorg. Chem., 1971, 16, 77–80 Search PubMed
.
- N. Uetake, J. Nucl. Sci. Technol., 1989, 26, 329–338 CrossRef
.
- J. H. Mesisenhelder and A. A. Siczek, Radiochim. Acta, 1980, 27, 223–227 Search PubMed
.
- H. G. M. Edwards, M. A. Hughes, D. N. Smith and B. Courtney, J. Mol. Struct., 1995, 351, 65–76 CrossRef
.
- H. Sugai and K. Munakata, Nucl. Technol., 1992, 99, 235–241 CrossRef
.
- G. Modolo and S. Seekamp, Solvent Extr. Ion Exch., 2002, 20, 195–210 CrossRef
.
- B. Weaver and F. A. Kappelmann, J. Inorg. Nucl. Chem., 1968, 30, 263–272 CrossRef
.
- V. Guedon, PhD thèse, Université de Grenoble I, 1993
.
- V. Guedon, J. C. Thieblemont, Y. Revel and A. Vandrot, J. Nucl. Sci. Technol., 1994, 31, 48–61 CrossRef
.
- J. Ly, PhD thèse, Université de Paris VI, 1984
.
- E. V. Renard, Sov. Radiochem., 1976, 18, 560–571 Search PubMed
.
- S. De Sio, I. Klur, E. Tison, C. Bouyer, D. Lebeau, F. Goutelard, L. Séjourné, C. Eysseric and N. Vigier, Procedia Chem., 2016, 21, 17–23 CrossRef
.
- S. J. Hibble, A. M. Chippindale, E. J. Bilbé, E. Marelli, P. J. F. Harris and A. C. Hannon, Inorg. Chem., 2011, 50, 104–113 CrossRef PubMed
.
- G. Beamson and D. Briggs, High Resolution XPS of Organic Polymers: the Scienta Esca 300 Database, John Wiley and Sons Ltd, UK, 1992 Search PubMed
.
- L. Soptrajanova and B. Soptrajanov, Spectrosc. Lett., 1992, 25, 1131–1139 CrossRef
.
- B. Bulkin and R. Rose, Appl. Spectrosc., 1978, 32, 151–157 CrossRef
.
- P. K. Gallagher and M. E. Gross, J. Therm. Anal., 1986, 31, 1231–1241 CrossRef
.
- L. Huang, Y. Wang, Z. Wang and F. Chen, Physical Chemistry, 2012, 2, 27–34 CrossRef
.
- A. G. Sharpe, in The Chemistry of Cyano Complexes of the Transition Metals, Academic Press, London, New York, 1976, vol. 11, pp. 243–248 Search PubMed
.
- F. P. Miner, A. R. Kajanjian, A. K. Brown, P. G. Hagan and J. W. Berry, Radiation chemistry of nitric acid solutions – Technical Report RFP–1299, 1969 Search PubMed
.
- A. J. Huggard and B. F. Warner, Nucl. Sci. Eng., 1963, 17, 638–650 CrossRef
.
- A. Dakshinamoorthy, P. S. Dhami, P. W. Naik, N. L. Dudwadkar, S. K. Munshi, P. K. Dey and V. Venugopal, Desalination, 2008, 232, 26–36 CrossRef
.
- N. Getoff, Radiat. Phys. Chem., 2006, 75, 514–523 CrossRef
.
- V. M. Adamov, Radiokhimia, 1987, 29, 822–829 Search PubMed
.
- Y. Tashiro, R. Kodama, H. Sugai, K. Suzuki and S. Matsuoka, Nucl. Technol., 2000, 129, 93–100 CrossRef
.
- J. Kuruc, A. Petrů, R. Čech and P. Rajec, J. Radioanal. Nucl. Chem., 1996, 208, 351–368 CrossRef
.
- H. Modler and M. Nonomura, Toxicol. Environ. Chem., 1995, 48, 155–175 CrossRef
.
- A. Costagliola, L. Venault, A. Deroche, G. Garaix, J. Vermeulen, R. Omnee, F. Duval, G. Blain, J. Vandenborre, M. Fattahi-Vanani and N. Vigier, Radiat. Phys. Chem., 2016, 119, 186–193 CrossRef
.
- B. Braithwate, Chem. Commun., 1969, 22, 1329 RSC
.
- J. M. Harrington, S. B. Jones and R. D. Hancock, Inorg. Chim. Acta, 2005, 358, 4473–4480 CrossRef
.
- AXS BRUKER, Software TOPAS – version 4.2, 2009 Search PubMed
.
- G. Caglioti, A. Paelotti and F. P. Ricci, Nucl. Instrum., 1958, 3, 223–228 CrossRef
.
- H. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02695e |
| This journal is © The Royal Society of Chemistry 2018 |