Open Access Article
Open Access ArticleCuI nanoparticle-catalyzed synthesis of tetracyclic benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine heterocycles by SNAr-type C–S, C–N bond formation from isothiocyanatobenzenes and benzimidazoles†
Xiaolong Guo ,
Luyao Wang*,
Jing Hu and
Mengmeng Zhang
,
Luyao Wang*,
Jing Hu and
Mengmeng Zhang
Department of Chemistry, Capital Normal University, Beijing 100048, China. E-mail: xdwly@163.com
First published on 19th June 2018
Abstract
In this paper, a simple and practical synthesis of benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine tetracyclic heterocycles via a CuI nanoparticle-catalyzed intramolecular C(sp2)–S coupling reaction is presented. This strategy provides a straightforward method for synthesizing analogs of the anti-HIV drug 3,4-dihydro-2H,6H-pyrimido[1,2-c][1,3]benzothiazin-6-imine (PD 404182). The reaction rate and yield were increased by employing CuI nanoparticles.
Introduction
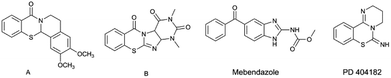
A major challenge in organic synthesis and pharmaceutical chemistry is to develop novel synthetic methods for accessing analogs of biologically active heterocyclic compounds.Benzo[1,3]thiazides and benzimidazoles are not only common in natural products but also important functional groups in many biologically active compounds. In particular, benzo[1,3]thiazides are widely used as sedatives, antibiotics, and cell growth inhibitors.1 Due to their chemical and biological properties, the synthesis of various benzo[1,3]thiazine derivatives has been intensely studied.2 On the other hand, benzimidazoles are important nitrogen heterocycles that are used in polymers and as enzyme inhibitors, drugs, and dyes.3 Some imidazo[2,1-b][1,3]thiazinones have shown promising antimicrobial activity against bacteria, yeast, and fungi (Fig. 1).4
A recent study found that 4-dihydro-2H,6H-pyrimido[1,2-c][1,3]benzothiazin-6-imine (PD 404182) was an effective anti-HIV and anti-HCV drug as well as having other therapeutic properties (Fig. 1).5 A structure–activity relationship (SAR) study on PD 404182 indicated that the 6-imino group and sulfur atom at the 7-position were essential for its activity.6
Despite many synthetic methods having been reported for the synthesis of 1,3-thiazines and benzimidazoles, to the best of our knowledge, the synthesis of compounds containing a 2-arylimino-1,3-thiazine unit fused to a benzimidazole core have not been reported. Thus, the synthesis of tetracyclic 2-arylimino-1,3-thiazinobenzimidazoles is not only a synthetical challenge but also potentially of biological interest. In recent years, great advancements have been made in copper-catalyzed Ullmann-type coupling reactions for the formation of C(sp2)–X (X = C, N, O, S, etc.) bonds because of the high efficiency, low cost and good functional group tolerance of this reaction.7 In addition, one-pot cascade strategies for synthesizing various fused heterocyclic compounds based on copper-catalyzed reactions are attracting more attention.8 Since nanoparticles have a larger specific surface area and greater number of active sites per unit area, copper iodide nanoparticles (CuI NPs) can react more rapidly, which allows better control and improves the yield of organic reactions.9 In view of the above advantages, using CuI NPs and 1,10-phenanthroline as the catalytic system, we intend to develop a simple and effective method for the synthesis of the tetracyclic heterocyclic system containing benzimidazole, benzothiazole and imino groups, benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine.
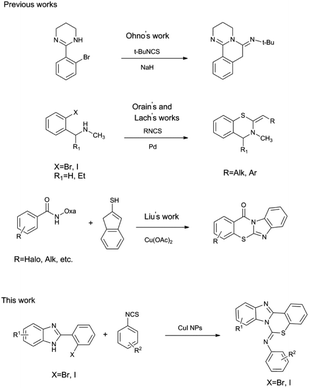
Although a few preparation methods have been reported, most of them have limitations such as low product yields, harsh reaction conditions, poor reaction scope and requiring conventional multistep protocols (Scheme 1). Ohno10 reported improvements were observed by using a bromoarene scaffold and substituted isothiocyanates as the starting materials in an intramolecular SNAr to form a C(sp2)–S bond. Regrettably, this method only tolerated substrates with σ- or π-acceptors in the ortho position, and the substituent at the C2 imine position had to be a tert-butyl moiety. The Orain group11 and the Lach group12 separately reported syntheses of 3,4-dihydro-2H-1,3-benzothiazine-2-imines from 2-halo-N-methyl benzylamine derivatives and substituted isothiocyanates. In this process, the key C–S bond was generated by a regioselective intramolecular Pd-catalyzed cyclization between a thiourea moiety and a haloarene. Although the method can be used to introduce different substituents at the C2 imine position, N1 in these reactions is not present in the final aromatic system. Liu and co-workers13 developed an efficient Cu-catalyzed tandem C(sp2)–H sulfenylation and annulation of arenes with 2-mercaptoimidazoles to provide fused imidazo[2,1-b][1,3]thiazinones. With this method, N1 is present in the aromatic system, but the C2 imine moiety is also in the imidazole ring system. It was therefore necessary to develop a highly efficient one-pot method for the synthesis of benzo[1,3]thiazin-6-imine derivatives in which N1 is present in the aromatic system and various substituents are tolerated at the C2 imine sites.
Herein, we propose a simple route that employs benzyl or aryl isothiocyanate and 2-(2-halophenyl)-1H-benzo[d]imidazole as substrates. The benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine was synthesized by an Ullmann-type coupling with CuI NPs to form the C(sp2)–S bonds.
Results and discussion
To investigate the proposed CuI nanoparticle-catalyzed C(sp2)–S bond formation protocol, we selected 2-(2-iodophenyl)-1H-benzo[d]imidazole and isothiocyanatobenzene as model substrates to optimize the reaction conditions, including catalysts, bases and solvents; the reactions were conducted at reflux under air, and the reaction time was maintained at 6 h (Table 1). Only a trace amount of the desired product was detected in the absence of catalysts (entry 1); therefore, different catalysts (10 mol%) were investigated when using 2 equiv. of Cs2CO3 as the base, 1,10-phenanthroline as the ligand, and acetonitrile as the solvent (compare entries 2–7). CuI NPs were found to provide the highest yield (entry 7). Other solvents (DMF, toluene and DMSO) were also tested (compare entries 7–10), and acetonitrile gave the best result (entry 7). The effect of bases was investigated (compare entries 7 and 11–13), and Cs2CO3 was found to be a suitable base (entry 7). The amount of CuI NPs was changed (compare entries 7, 14 and 15), and although 20 mol% CuI NPs provided the highest yield (entry 15), the increase was relatively small. When the amount of ligand was increased to 20 mol%, the yield was 52%, which is almost the same as what was observed with 10 mol% (entry 19). To our surprise, when KI was added to the reaction as an additive, the yield improved significantly to 83% (entry 16), but when the additive was replaced with TBAI, the yield dropped (entry 17). Further, in the absence of ligand, the yield was only 22% (entry 18). The yield was the same when the reaction was conducted under an Ar atmosphere (entry 20).| Entry | Cat. (mol%) | Solvent | Base | Additive | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: under air, 2-(2-iodophenyl)-1H-benzo[d]imidazole (1a) (0.5 mmol), isothiocyanatobenzene (2a) (0.6 mmol), catalyst (0.05 mmol), ligand (0.05 mmol), additive (0.5 mmol), base (1 mmol), solvent (3 mL), reaction time (6 h), ligand = 1,10-phenanthroline.b Isolated yields.c TBAI = tetra-n-butylammonium iodide.d The reaction was performed without ligand.e The reaction was performed with ligand (0.10 mmol).f The reaction was performed under an atmosphere of Ar. | |||||
| 1 | MeCN | Cs2CO3 | Trace | ||
| 2 | CuI (10) | MeCN | Cs2CO3 | 38 | |
| 3 | Cu(OAc)2 (10) | MeCN | Cs2CO3 | 34 | |
| 4 | CuSO4 (10) | MeCN | Cs2CO3 | 13 | |
| 5 | Pd(OAc)2 (10) | MeCN | Cs2CO3 | 31 | |
| 6 | NiCl2 (10) | MeCN | Cs2CO3 | Trace | |
| 7 | CuI NPs (10) | MeCN | Cs2CO3 | 51 | |
| 8 | CuI NPs (10) | DMF | Cs2CO3 | 13 | |
| 9 | CuI NPs (10) | Toluene | Cs2CO3 | 33 | |
| 10 | CuI NPs (10) | DMSO | Cs2CO3 | 26 | |
| 11 | CuI NPs (10) | MeCN | K2CO3 | 47 | |
| 12 | CuI NPs (10) | MeCN | Na3PO4 | 46 | |
| 13 | CuI NPs (10) | MeCN | NaOAc | 8 | |
| 14 | CuI NPs (5) | MeCN | Cs2CO3 | 38 | |
| 15 | CuI NPs (20) | MeCN | Cs2CO3 | 53 | |
| 16 | CuI NPs (10) | MeCN | Cs2CO3 | KI | 83 |
| 17 | CuI NPs (10) | MeCN | Cs2CO3 | TBAIc | 61 |
| 18 | CuI NPs (10) | MeCN | Cs2CO3 | KI | 22d |
| 19 | CuI NPs (10) | MeCN | Cs2CO3 | 52e | |
| 20 | CuI NPs (10) | MeCN | Cs2CO3 | KI | 82f |
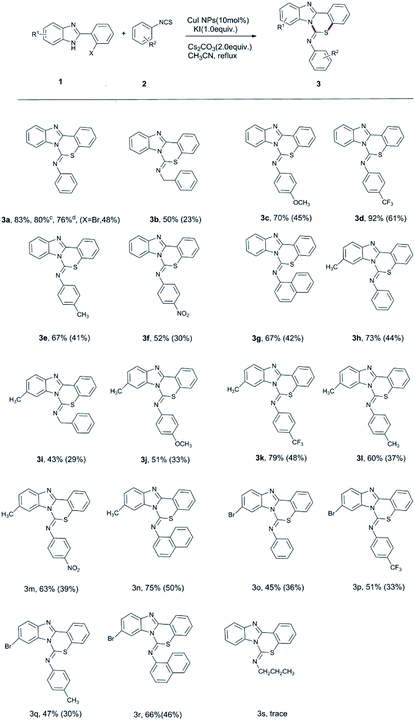
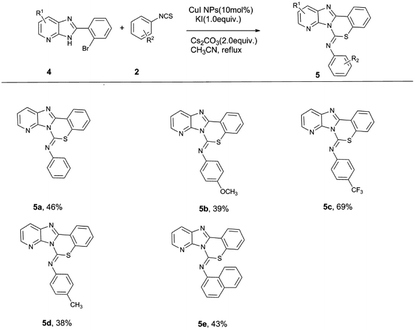
With the optimized conditions in hand, we started to explore the substrate scope of the reaction. Representative isothiocyanates (1) were chosen to react with typical 2-(2-halophenyl)-1H-benzo[d]imidazoles (2), and in all cases, the desired products were obtained in moderate to good yields. The reaction conditions and isolated yields are summarized in Scheme 2. The reaction proceeded smoothly with various electron-deficient and electron-rich isothiocyanates and benzo[d]imidazoles.
Compounds 1 and 2 bearing either an electron-donating group (EDG), such as a methyl or methoxy group, or an electron-withdrawing group (EWG), such as a bromo or trifluoromethyl group, on the phenyl ring were well-tolerated in this reaction. From these results, when the benzimidazole moiety has an electron-donating substituent, the yield is generally higher than when an electron-withdrawing group is present; when a nitro group is present, the yield is almost zero, which may be due to the strong electron-withdrawing nature of the nitro groups since this greatly reduces the nucleophilicity of the nitrogen in the imidazole ring. Meanwhile, when the isothiocyanate moiety has an electron-withdrawing substituent, the yield is generally higher than when an electron-donating substituent is used. It is possible that the electron density of the –NCS is reduced by the presence of an electron-withdrawing substituent, which favors nucleophilic attack. In addition, the yield of the reaction with the iodine-substituted benzimidazole substrate is significantly higher than that of the analogous bromine-substituted substrate. Finally, it was found that 1-naphthyl isothiocyanate could also participate in this reaction and smoothly gave the corresponding products. Unfortunately, these reaction conditions were not suitable for aliphatic isothiocyanates (propyl, cyclohexyl, allyl, etc.).
Imidazopyridine compounds are a class of nitrogen-containing fused heterocyclic compounds that have a wide range of applications in biomedicine, agriculture and the dye industry.14 Because of their structural similarities to indoles and azole indoles, imidazopyridines often have physiological activities, which are attracting more and more attention. Moreover, these compounds have a conjugated π-electron system, their aromatic heterocyclic pentacyclic systems have large dipole moments, and these compounds contain a certain number of nitrogen atoms that, when combined with the appropriate parent ring, enhance the selectivity of the drug. Hence, we further extended the scope of our methodology to include imidazopyridine analogs (Scheme 3). The reaction successfully provided the desired products with various isothiocyanates.
We recycled the CuI NPs to re-catalyze the reaction of substrates 1a and 2a. The yield of 3a was 76% after two cycles, having no significant decrease (Scheme 2). We believe that this reaction has undergone a heterogeneous catalytic process.15 Further, we conducted a leaching experiment of the catalyst to verify whether the CuI NPs or the copper ions leached from the CuI NPs surface played a real catalytic role. First, we performed an AAS test on the organic phase of the reaction and found that the concentration of copper ions in the organic phase was only 14.8 mg L−1. Then, according to the step of catalytically SNAr-Type C–S, C–N bond formation with CuI NPs, it was allowed to react for 1 h, the catalyst in the reaction tube was removed by centrifugation, and allowed to continue reacting for 5 h. After the catalyst was removed by centrifugation, TLC showed that there was no further conversion to product, indicating that the reaction was not catalyzed by the copper ions in the solution, but a heterogeneous reaction occurred on the surface of the CuI NPs.
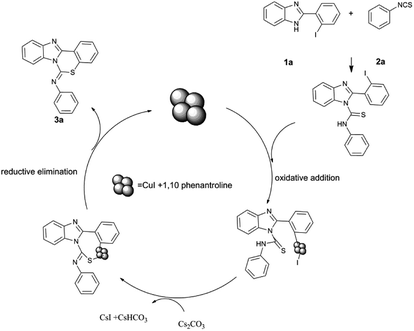
The proposed mechanism for the reaction using CuI NPs chelated to 1,10-phenanthroline is shown in Scheme 4. The reaction proceeds via nucleophilic addition of the amidine moiety of the benzo[d]imidazole to the isothiocyanate followed by an intramolecular SNAr reaction of the resulting adduct; C–S coupling occurs by oxidative addition followed by reductive elimination. These processes are carried out on the nano copper surface. CuI nanoparticles have high catalytic activity due to their high surface area.
Experimental
General information
All reagents and solvents were used directly as received from commercial sources without further purification. Column chromatography was performed using silica gel (200–300 mesh size) with the indicated solvents. Thin-layer chromatography (TLC) was conducted with silica gel GF254 precoated plates (0.25 mm) and visualized with UV light. All 1H NMR spectra were recorded on 600 MHz spectrometers, and the data are reported in ppm using solvents as internal standards (CDCl3 at 7.26 ppm, DMSO-d6 at 2.50 ppm). All proton-decoupled 13C NMR spectra were recorded at 151 MHz, and the data are reported in ppm using solvents as internal standards (CDCl3 at 77.2 ppm, DMSO-d6 at 39.5 ppm). HRMS analyses of the compounds were conducted on a Thermo Q Exactive mass spectrometer using electrospray ionization in the positive ion mode. All the compounds were solid, and melting points were measured on a micromelting point apparatus. Powder X-ray diffraction (XRD) of the CuI NPs was carried out on a Philips diffractometer (X'pert Company) with monochromatized Cu Kα radiation (λ = 1.5406 Å). Microscopic morphologies were visualized using an SEM (LEO 1455VP).General procedure for the synthesis of benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine derivatives
To a mixture of 2-(2-bromophenyl)-1H-benzo[d]imidazole or 2-(2-iodophenyl)-1H-benzo[d]imidazole (0.5 mmol, 1.0 equiv.) with KI (0.5 mmol, 1.0 equiv.), 1,10-phenanthroline (0.05 mmol, 10 mol%), CuI NPs (0.05 mmol, 10 mol%), and Cs2CO3 (1.0 mmol, 2.0 equiv.) in dry acetonitrile (3 mL) was added isothiocyanate (0.6 mmol, 1.2 equiv.) at room temperature. The reaction mixture was stirred at reflux for 6 h, and then, after the reaction appeared complete by TLC, it was quenched with H2O. The aqueous layer was extracted with ethyl acetate (4 × 10 mL). The combined organic layers were washed with water (3 × 10 mL), dried over anhydrous Na2SO4, and concentrated. The crude product was purified by silica gel column chromatography with a gradient of 10–20% ethyl acetate in petroleum ether to obtain the desired product.Conclusions
In summary, we developed a simple and practical method to prepare the tetracyclic heterocyclic system benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine using a CuI nanoparticle-catalyzed intramolecular C(sp2)–S coupling. This reaction provides divergent access to several related heterocycles under mild conditions without a powerful activating group. Furthermore, the reaction is very effective due to the high surface-to-volume ratio of nanoparticles. We have also examined the viability of this protocol with different substrates. We believe that this practical strategy will be useful for synthesizing analogs of the anti-HIV drug PD 404182 in good yields. Further explorations of related processes are underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Beijing Natural Science Foundation of China (No. 2144045) for their financial support.Notes and references
-
(a) S. T. Hilton, W. B. Motherwell, P. Potier, C. Pradet and D. L. Selwood, Bioorg. Med. Chem. Lett., 2005, 15, 2239 CrossRef PubMed
; (b) Z.-C. Song, G.-Y. Ma, P. C. Lv, H. Q. Li, Z. P. Xiao and H. L. Zhu, Eur. J. Med. Chem., 2009, 44, 3903 CrossRef PubMed
; (c) A. T. Mavrova, D. Vuchev, K. Anichina and N. Vassilev, Eur. J. Med. Chem., 2010, 45, 5856 CrossRef PubMed
; (d) S. P. Kaur, R. Rao and S. Nanda, Int. J. Pharm. Pharm. Sci., 2011, 3, 30 Search PubMed
; (e) D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, A. Gzella and R. Lesyk, Med. Chem., 2012, 55, 8630 CrossRef PubMed
.
-
(a) N. Hucher, B. Decroix and A. Daïch, J. Org. Chem., 2001, 66, 4695 CrossRef PubMed
; (b) W. P. Unsworth, C. Kitsiou and R. J. Taylor, Org. Lett., 2013, 15, 258 CrossRef PubMed
; (c) X. Li, Z. Qin, T. Yang, H. Zhang, S. Wei, C. Li, H. Chen and M. Meng, Bioorg. Med. Chem. Lett., 2012, 22, 2712 CrossRef PubMed
; (d) C. L. Jarvis, M. T. Richers, M. Breugst, K. N. Houk and D. Seidel, Org. Lett., 2014, 16, 3556 CrossRef PubMed
; (e) J. Liu, A. Xue, Z. Zeng, Y.-X. Chen and G. Chen, Adv. Synth. Catal., 2016, 358, 3694 CrossRef
.
-
(a) N. H. Hauel, H. Nar, H. Priepke, U. Ries, J. Stassen and W. J. Wienen, Med. Chem., 2002, 45, 1757 CrossRef
; (b) J. VelIk, V. Baliharova, J. Fink-Gremmels, S. Bull, J. Lamka and L. Skalova, Res. Vet. Sci., 2004, 76, 95 CrossRef PubMed
; (c) J. A. Asensio and P. Gomez-Romero, Fuel Cells, 2005, 5, 336 CrossRef
; (d) M. Hranjec, I. Piantanida, M. Kralj, L. Suman, K. Pavelic and G. K. Zamola, J. Med. Chem., 2008, 51, 4899 CrossRef PubMed
; (e) R. P. Ortiz, H. Herrera, R. Blanco, H. Huang, A. Facchetti, T. J. Marks, Y. Zheng and J. L. Segura, J. Am. Chem. Soc., 2010, 132, 8440 CrossRef PubMed
; (f) D. V. Ferraris, J. Med. Chem., 2010, 53, 4561 CrossRef PubMed
; (g) T. W. Moore, K. Sana, D. Yan, S. A. Krumm, P. Thepchatri, J. P. Snyder, J. Marengo, R. F. Arrendale, A. J. Prussia, M. G. Natchus, D. C. Liotta, R. K. Plemper and A. Sun, ACS Med. Chem. Lett., 2013, 4, 762 CrossRef PubMed
; (h) K. Fahsi, S. G. Dutremez, A. Vioux and L. Viau, J. Mater. Chem., 2013, 1, 4451 RSC
; (i) H.-F. Chen, L.-C. Chi, W.-Y. Hung, W.-J. Chen, T.-Y. Hwu, Y.-H. Chen, S.-H. Chou, E. Mondal, Y.-H. Liu and K.-T. Wong, Org. Electron., 2013, 13, 2671 CrossRef
; (j) P. S. Hameed, A. Raichurkar, P. Madhavapeddi, S. Menasinakai, S. Sharma, P. Kaur, R. Nandishaiah, V. Panduga, J. Reddy, V. K. Sambandamurthy and D. Sriram, ACS Med. Chem. Lett., 2014, 5, 820 CrossRef PubMed
; (k) M. K. Kim, H. Shin, K. Park, H. Kim, J. Park, K. Kim, J. Nam, H. Choo and Y. Chong, J. Med. Chem., 2015, 58, 7596 CrossRef PubMed
.
-
(a) M. A. Salama and L. A. Almotabacani, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 305 CrossRef
; (b) V. H. Bhaskar, K. Puli, B. Sangameswaran and B. Jayakar, Indian J. Heterocycl. Chem., 2006, 15, 299 Search PubMed
.
-
(a) M. R. Birck, T. P. Holler and R. W. Woodard, J. Am. Chem. Soc., 2000, 122, 9334 CrossRef
; (b) A. Golebiowski, S. R. Klopfenstein and D. E. Portlock, Curr. Opin. Chem. Biol., 2001, 5, 273 CrossRef PubMed
; (c) C. Sansom, Drug Discovery Today, 2001, 6, 499 CrossRef PubMed
; (d) B. P. Duckworth and C. C. Aldrich, Anal. Biochem., 2010, 403, 13 CrossRef PubMed
; (e) T. L. Foley, A. Yasgar, C. J. Garcia, A. Jadhav, A. Simeonov and M. D. Burkart, Org. Biomol. Chem., 2010, 8, 4601 RSC
; (f) T. Mizuhara, S. Oishi, H. Ohno, K. Shimura, M. Matsuoka and N. Fujii, Bioorg. Med. Chem., 2013, 21, 2079 CrossRef PubMed
; (g) A. M. Chamoun, M. Bobardt, B. Moncla, M. K. Mankowski, R. G. Ptak, P. Gallay and Z. Chen, Antimicrob. Agents Chemother., 2014, 58, 687 CrossRef PubMed
.
-
(a) K. Chockalingam, R. L. Simeon, C. M. Rice and Z. Chen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 3764 CrossRef PubMed
; (b) A. M. Chamoun, K. Chockalingam, M. Bobardt, R. Simeon, J. Chang, P. Gallay and Z. Chen, Antimicrob. Agents Chemother., 2012, 56, 672 CrossRef PubMed
.
-
(a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef PubMed
; (b) I. P. Beletskaya and A. V. Cheprakov, Organometallics, 2012, 31, 7753 CrossRef
; (c) J. Lin, I. N. Houpis, R. Liu, C. Wang and J. Zhang, Org. Process Res. Dev., 2014, 18, 205 CrossRef
; (d) Q. Liao, X. Yang and C. Xi, J. Org. Chem., 2014, 79, 8507 CrossRef PubMed
.
-
(a) C. Wang, S. Li, H. Liu, Y. Jiang and H. Fu, J. Org. Chem., 2010, 75, 7936 CrossRef PubMed
; (b) D. Ma, Q. Geng, H. Zhang and Y. Jiang, Angew. Chem., Int. Ed., 2010, 49, 1291 CrossRef PubMed
; (c) H. Xu, Y. Zhang, J. Huang and W. Chen, Org. Lett., 2010, 12, 3704 CrossRef PubMed
; (d) J. Zhu, H. Xie, Z. Chen, S. Li and Y. Wu, Chem. Commun., 2009, 2338 RSC
; (e) A. K. Verma, T. Kesharwani, J. Singh, V. Tandon and R. C. Larock, Angew. Chem., Int. Ed., 2009, 48, 1138 CrossRef PubMed
; (f) Z. Tan, H. Zhao, C. Zhou, H. Jiang and M. Zhang, J. Org. Chem., 2016, 81, 9939 CrossRef PubMed
; (g) S. Mai, Y. Zhao and Q. Song, Org. Biomol. Chem., 2016, 14, 8685 RSC
.
-
(a) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef PubMed
; (b) H. Zhang, Q. Cai and D. Ma, J. Org. Chem., 2005, 70, 5164 CrossRef PubMed
; (c) B. Sreedhar, R. Arundhathi, P. L. Reddy and M. L. Kantam, J. Org. Chem., 2009, 74, 7951 CrossRef PubMed
; (d) H.-J. Xu, Y. -Feng Liang, Z.-Y. Cai, H.-X. Qi, Ch.-Y. Yang and Y.-S. Feng, J. Org. Chem., 2011, 76, 2296 CrossRef PubMed
; (e) M. B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722 CrossRef PubMed
.
- T. Mizuhara, S. Oishi, N. Fujii and H. Ohnon, J. Org. Chem., 2010, 75, 265 CrossRef PubMed
.
- D. Orain, A. Blumstein, E. Tasdelen and S. Haessig, Synlett, 2008, 16, 2433 CrossRef
.
- F. Lach, Synlett, 2012, 23, 2639 CrossRef
.
- J. Liu, Z. Xue, Z. Zeng, Y.-X. Chen and G. Chen, Adv. Synth. Catal., 2016, 358, 3694 CrossRef
.
-
(a) S. L. Coiietti, C. J. Frie, E. C. Dixon, S. B. Singh and B. K. Choi, J. Med. Chem., 2003, 46, 349 CrossRef PubMed
; (b) S. Das, R. Froehlich and A. Pramanik, Org. Lett., 2006, 8, 4263 CrossRef PubMed
; (c) A. M. Palmer, B. Grobbel, C. Jecke, C. Brehm and P. J. Zimmermann, J. Med. Chem., 2007, 50, 6240 CrossRef PubMed
; (d) M. Hayakawa, H. Kaizawa and K. Kawaguchi, Bioorg. Med. Chem., 2007, 15, 403 CrossRef PubMed
; (e) C. W. Lisa, K. Jacobien and M. K. Thea, J. Med. Chem., 2007, 50, 828 CrossRef PubMed
; (f) T. Palani, K. Park, M. R. Kumar, H. M. Jung and S. Lee, Eur. J. Org. Chem., 2012, 26, 5038 CrossRef
; (g) G. C. Moraski, L. D. Markley, P. A. Hipskind, H. Boshoff, S. Cho, S. G. Franzblau and M. J. Miller, ACS Med. Chem. Lett., 2011, 2, 466 CrossRef PubMed
; (h) G. Chen, Z. Liu, Y. Zhang, X. Shan, L. Jiang, Y. Zhao, W. He, Z. Feng, S. Yang and G. Liang, ACS Med. Chem. Lett., 2013, 4, 69 CrossRef PubMed
.
-
(a) J.-T. Zhang, Z.-H. Zhang, Y. Wang, X.-Q. Zheng and Z.-Y. Wang, Eur. J. Org. Chem., 2008, 30, 5112 CrossRef
; (b) L. Tang, X.-F. Guo, Y. Yang, Z.-G. Zha and Z.-Y. Wang, Chem. Commun., 2014, 50, 6145 RSC
; (c) L. Tang, Y. Yang, L.-X. Wen, S. Zhang, Z.-G. Zha and Z.-Y. Wang, Org. Chem. Front., 2015, 2, 114 RSC
; (d) P. L. Reddy, R. Arundhathi, M. Tripathia and D. S. Rawat, RSC Adv., 2016, 6, 53596 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra. CCDC 1818722. XRD spectra and SEM image of CuI NPs. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra02552e |
| This journal is © The Royal Society of Chemistry 2018 |