Open Access Article
Open Access ArticleSynthesis of a MnS/NixSy composite with nanoparticles coated on hexagonal sheet structures as an advanced electrode material for asymmetric supercapacitors†
Qing Pan‡
a,
Xijia Yang‡b,
Xiaohong Yanga,
Lianfeng Duanb and
Lijun Zhao *a
*a
aKey Laboratory of Automobile Materials (Jilin University), Ministry of Education, College of Materials Science and Engineering, Nanling Campus, Changchun, 130025, P. R. China. E-mail: lijunzhao@jlu.edu.cn; Fax: +86-431-85095876
bKey Laboratory of Advanced Structural Materials, Ministry of Education, Department of Materials Science and Engineering, Changchun University of Technology, Changchun 130012, China
First published on 15th May 2018
Abstract
Herein, a facile hydrothermal method was designed to synthesize a novel structure of micro-flowers decorated with nanoparticles. The micro-flower structure consists of enormous cross-linked flat hexagonal nanosheets with sufficient internal space, providing fluent ionic channels and enduring volume change in the electrochemical storage process. As expected, the MnS/NixSy (NMS) electrode exhibits a relatively high specific capacitance of 1073.81 F g−1 (at 1 A g−1) and a good cycling stability with 82.14% retention after 2500 cycles (at 10 A g−1). Furthermore, the assembled asymmetric supercapacitor achieves a high energy density of 46.04 W h kg−1 (at a power density of 850 W kg−1) and exhibits excellent cycling stability with 89.47% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The remarkable electrochemical behavior corroborates that NMS can serve as an advanced electrode material.
000 cycles. The remarkable electrochemical behavior corroborates that NMS can serve as an advanced electrode material.
1. Introduction
Driven by the growing demand for clean, renewable and uninterruptible energy supply as well as mobile power sources, the development of highly efficient energy conversion and storage devices has become a key challenge.1 Supercapacitors (SCs), as newly emerging energy-storage devices, have attracted significant scientific interest due to their sustainable cycle life, high power density, and easy preparation process. These supercapacitors have been applied in various fields such as in hybrid electric vehicles, military devices, high-power electric devices and portable electronics.2–8 However, the poor energy density (∼5–20 W h kg−1) and low potential window of electric double-layer capacitors (EDLCs) inhibit their large-scale practical application to a great degree.9–11 An efficacious way to improve this situation is to utilize battery-type electrode materials, such as Ni(OH)2, cobalt oxides, and sulfides, as the energy source.11–13At present, metal oxides have drawn significant attention because the reversible faradaic reaction and multiple valence state changes endow them with higher theoretical capacity.14–18 For example, Xia et al. successfully synthesized KxMnO2 and Na0.5MnO2 electrodes for high-performance supercapacitors.19,20 However, the surface redox reactions of the metal oxides are always retarded; this is because of the intrinsic poor electrical conductivity or the slow ion transport kinetics.21 Contrary to transition metal oxides (such as NiO, Co3O4, MnO2),18,22–24 their sulfides present an enhanced electronic conductivity owing to their lower energy gap values.25,26 Moreover, sulfur has a low electron negativity that endows it with more feasibilities of creating more flexible structures and thus prevents structure disintegration and ensures easier charge transportation.27 Amongst all sulfur compounds, MnS is a promising candidate for potential application in energy storage devices due to the rich variable valence states of the Mn element as well as its low cost. To date, a number of studies have reported that binary Ni/Mn nanocomposites have achieved more superior capacitive performance than the monometallic sulfides due to the beneficial synergistic effect between Ni and Mn-based components. Zhao et al. successfully synthesized a NiMn-LDH/CNT architecture as an electrode with good electrochemical performance.28 Wan et al. synthesized hedgehog-like hollow Ni–Mn oxides and sulfides by a one-step directional Mn-induced approach, and the capacitance improved about 26.35% after sulfuring.29 Chen et al. reported a microflower-like Ni–Mn composite with nanosheet petals. After the sulfurization process, not only the capacitance improved, but also the cycling retention extended about 12% after 1500 cycles as compared to the case of the pristine NiMn-layered double hydroxides.4
Moreover, the design of functionalized microstructures plays a crucial role in improving the capacitance performance of nanohybrids. Extensive endeavors have been made to explore the flower-like structures owing to their versatile merits of large specific surface area and abundant available internal space. In addition, previous studies have stated that ultrafine nanoparticles in situ grown on the nanosheets will not only significantly promote the specific surface area but also efficiently stimulate the synergistic effect owing to the robust incorporation of heterostructures. Pang et al. synthesized a heterogeneous Co3O4-nanocube/Co(OH)2-nanosheet hybrid via a facial hydrothermal reaction.30 Guan et al. designed ZnMnO4 nanoparticles encapsulated in carbon sheets and employed them for supercapacitors.31 Therefore, a distinct micro-flower structure decorated with nanoparticles has been successfully synthesized in our research.
Herein, the MnS/NixSy (NMS) composite with nanoparticles coated on hexagonal sheet structures has been successfully synthesized via a facial hydrothermal method. The suitable hexagonal sheet morphology with plenty of nanoparticles accelerated the electron transfer rate and ensured a fast and reversible faradaic reaction. When utilized for a supercapacitor electrode, NMS exhibited remarkable electric properties of a high specific capacitance (1073.81 F g−1 at 1 A g−1) and elevated cycling retention (82.14% retention after 2500 cycles). Furthermore, an asymmetric supercapacitor (based on positive NMS and negative AC electrodes) was fabricated, which also displayed an excellent performance with long-term cycling duration and a high energy density (46.04 W h kg−1 at a power density of 0.85 kW kg−1). All these properties corroborate the high feasibility of NMS for application in the energy-storage field.
2. Experimental
2.1 Preparation of the Ni–Mn precursor
The Ni–Mn precursor was synthesized by a facile hydrothermal method. In a typical process, 1.5 mmol of manganese chloride tetrahydrate (MnCl2·4H2O), 3 mmol of nickel chloride hexahydrate (NiCl2·6H2O), and 12 mmol of urea were dissolved in 75 mL of deionized water under magnetic stirring for 30 minutes. Subsequently, the as-prepared solution was transferred to a 100 mL Teflon-lined autoclave, which was heated at an oven temperature of 120 °C for 6 h to obtain the Ni–Mn precursor. The synthesized precursor powder was centrifuged at 8500 rpm, rinsed with deionized water and absolute ethanol, and vacuum dried at 60 °C overnight.2.2 Preparation of the NMS composite
Typically, 20 mL of Na2S·9H2O (360 mg) aqueous solution was poured into 20 mL of Ni–Mn precursor (100 mg) aqueous solution under magnetic stirring until the solution became black. The black suspension was transferred into a 50 mL Teflon-lined autoclave, which was kept at 100 °C for 6 h. In the end, the resultant powder was thoroughly centrifuged, washed with deionized water and absolute ethanol, and freeze-dried for further use. The synthesized sample was named as NMS. Similarly, we also adjusted the concentration of the precursor solution (1.5 mmol of NiCl2·6H2O and 3 mmol MnCl2·4H2O, as well as 3 mmol of NiCl2·6H2O and 3 mmol MnCl2·4H2O), and the other conditions were kept unchanged; the as-synthesized powders were named as NMS-1 and NMS-2. For comparison, MS and NS were also synthesized via the same process without the introduction of Ni2+ or Mn2+.2.3 Material characterization
A field emission scanning electron microscope (FE-SEM, JSM-6700F) and high-resolution TEM (HRTEM) were applied to study the morphologies and size of the as-prepared samples. The XRD spectra of the sample were characterized by powder X-ray diffraction (XRD, Rigaku D/MAX 2500PC). X-ray photoelectron spectroscopy (XPS) was conducted to investigate the electronic states via an ESCALAB MkII (Vacuum generators) spectrometer with Al Kα X-ray (240 W).2.4 Electrode preparation and electrochemical measurements
The NMS composite, acetylene black, and polyvinylidene difluoride (PVDF) were mixed in a ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 by weight with N-methyl-2-pyrrolidone (NMP) to form the coating paste. The paste was then applied to nickel foam (the spread area was about 10 mm × 10 mm), which was then dried at 80 °C for 8 h in vacuum. The loading density of the active materials was about 1.5–2.0 mg cm−2. To fabricate the asymmetric supercapacitor, a negative electrode was also prepared, for which AC and polytetrafluoroethylene (as a binder) were mixed in a weight ratio of 9
5 by weight with N-methyl-2-pyrrolidone (NMP) to form the coating paste. The paste was then applied to nickel foam (the spread area was about 10 mm × 10 mm), which was then dried at 80 °C for 8 h in vacuum. The loading density of the active materials was about 1.5–2.0 mg cm−2. To fabricate the asymmetric supercapacitor, a negative electrode was also prepared, for which AC and polytetrafluoroethylene (as a binder) were mixed in a weight ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then, the mixture was applied to a 10 mm × 10 mm Ni foam under a pressure of 10 MPa for 30 s.
1, and then, the mixture was applied to a 10 mm × 10 mm Ni foam under a pressure of 10 MPa for 30 s.
An electrochemical working station (CHI660e, Shanghai, China) was used to perform the electrochemical experiments. The NMS composite electrode was utilized as a working electrode; the platinum counter electrode and the saturated calomel electrode (SCE) reference electrodes were placed in a 3 M KOH aqueous electrolyte.
2.5 Calculations
The specific capacitance of the single electrode and assembly device can be easily calculated by the following equation:| Cs = IΔt/(mΔV) | (1) |
The most optimal mass ratio of the NMS positive electrode and AC negative electrode can be easily calculated referring to eqn (2) as follows:
| m+/m− = Cs− ΔV−/(Cs+ΔV+) | (2) |
The energy density (E) and power density (P) for the two-electrode devices can be calculated using the following formulae:
| E = 0.5Cs V2 | (3) |
| P = E/Δt | (4) |
3. Result and discussion
3.1 Structure and morphology of the NMS composite
The morphological details of the NMS composite were examined using FESEM. Fig. 1a shows the novel NMS morphology of the nanoparticles coated on the 3D micro-flower structure; these micro-flowers display a diameter of ca. 2–3 μm and thickness of ca. 70 nm. Moreover, the flat internal microsheets and open porous characteristics provide more active sites for the diffusion reaction between the NMS composite and KOH electrolyte. Conversely, the absence of either Ni or Mn will cause agglomeration of the system as well as lead to different structures (Fig. S1a and b†). Therefore, we adjusted the molar ratio of Ni/Mn salts to further control the morphology and highlight the superiority of the NMS composite. As displayed in Fig. S1c,† when the molar ratio of Ni/Mn was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the sheet-like structure became smaller, whereas the number of nanoparticles increased. In Fig. S1d,† these samples display thinner and smaller sheets with the absence of nanoparticles. Hence, we expected that NMS with heterogeneous structures would show higher electrochemical performance.
2, the sheet-like structure became smaller, whereas the number of nanoparticles increased. In Fig. S1d,† these samples display thinner and smaller sheets with the absence of nanoparticles. Hence, we expected that NMS with heterogeneous structures would show higher electrochemical performance.
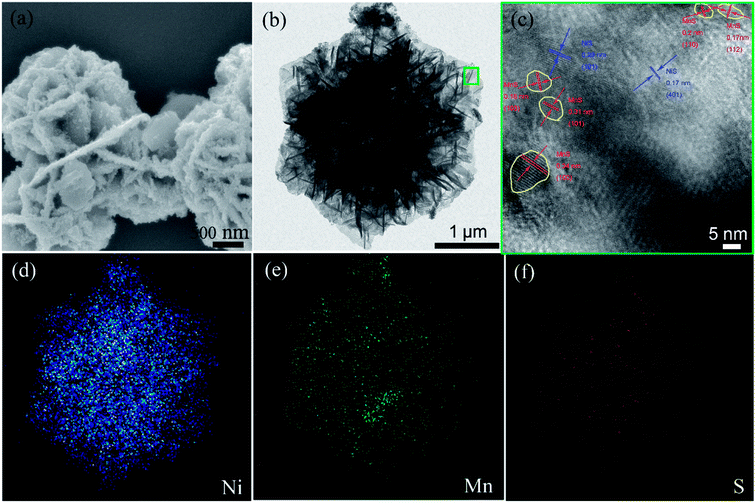 | ||
| Fig. 1 (a) FE-SEM; (b) TEM image of the as-prepared NMS; (c) HRTEM image of the green square in “(b)”; the elemental mapping of the NMS composite in (b): (d) Ni, (e) Mn and (f) S element. | ||
To further investigate the morphology of NMS, TEM and HRTEM measurements were performed. Fig. 1b revealed that the hierarchical nanosheets vertically expanded outward along the normal direction. The dark area in the TEM image results from the overlap of the multi-sheet structure. In the HRTEM image shown in Fig. 1c, the lattice fringe spacings of 0.17 and 0.29 nm separately correspond to the (401) and (101) planes of NiS, whereas the distances 0.34, 0.31, 0.18, 0.20, and 0.17 nm conform to the (100), (101), (103), (110), and (112) planes of MnS, respectively. In addition, the distribution of MnS fringes is close to a circle (as marked by the yellow circle in Fig. 1c), whereas NiS is distributed widely and continuously; thus, there is a great possibility that the particles are MnS, and the hexagonal sheets are NiS. In addition, the chemical content of NMS is accurately evaluated via EDX measurement, as depicted in Fig. S2,† powerfully demonstrating the existence of Ni, Mn and S elements. In the EDX measurement, we used the copper network as a substrate. Thus, apart from the three elements Ni, Mn, and S, the Cu peak can also be detected. Moreover, the elemental distribution of NMS in Fig. 1d–f reveals uniform distribution of these elements.
The as-prepared NMS composite was further tested through XRD patterns, as provided in Fig. 2a. The peaks at 25.8°, 27.6°, 29.3°, 38.2°, 45.6°, 49.9°, 54.1° and 55.1° separately correspond to the (100), (002), (101), (102), (110), (103), (201) and (112) planes of the MnS phase (JCPDS card no. 65-3413).32 The peaks at 18.7°, 30.4°, 37.9° and 40.8° are attributed to the (110), (101), (220) and (211) planes of the NiS phase (JCPDS card no.02-1208),33,34 respectively. Some other diffraction peaks, as shown in Fig. 2a, may result from nickel sulfides (NiS2 phase (JCPDS card no. 65-3325)35 and Ni7S6 phase (JCPDS card no. 54-0931)).36 The XRD patterns of the Ni–Mn precursor and other sulfides are also shown. As shown in Fig. S3b,† the diffraction peaks of the Ni–Mn precursor can be reasonably indexed to a series of crystal planes, demonstrating successful preparation of NiMn hydroxide,4,28 whereas the MS samples are MnS/MnS2 composites, and NS is mainly attributed to the Ni7S6 phase (Fig. S3a†).
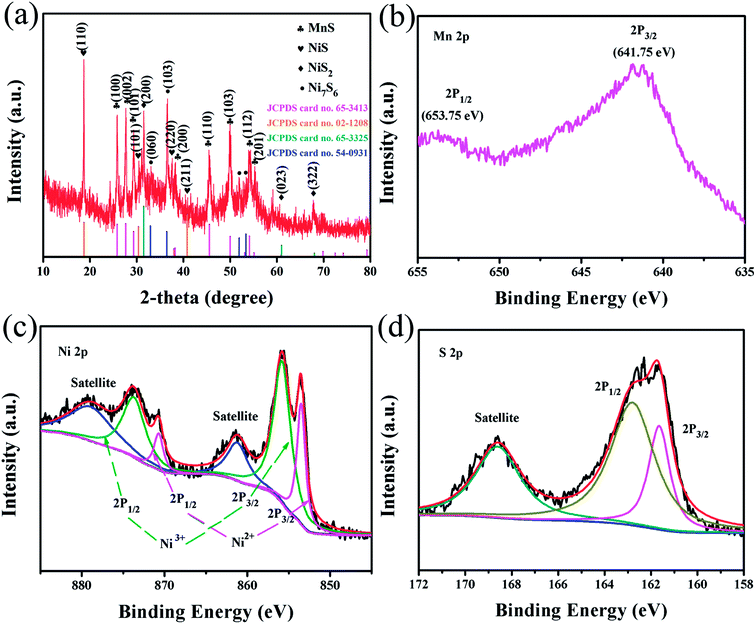 | ||
| Fig. 2 (a) XRD pattern of the as-prepared NMS; XPS spectra of the NMS: (b) Mn 2p peaks, (c) Ni 2p peaks, and (d) S 2p peaks. | ||
Furthermore, the chemical composition and metal states of the NMS composite were detected by XPS measurement. In the Mn 2p spectrum shown in Fig. 2b, two major peaks at 653.75 eV and 641.75 eV are separately attributed to the Mn 2p1/2 and 2p3/2 levels, wherein the Mn 2p3/2 characteristic peak reveals the presence of Mn ions in the divalent state.37 The Ni 2p spectrum (Fig. 2c) can be best fitted with two spin–orbit doublets and two shakeup satellites via the Gaussian fitting method. The doublets at 853.5 and 870.7 eV separately correspond to the binding energies of Ni 2p3/2 and 2p1/2 levels, representing the typical binding energies of Ni2+.16 However, the peaks at 855.8 and 873.7 eV are respectively attributed to the 2p3/2 and 2p1/2 levels of Ni3+.38,39 As clearly shown in Fig. 2d, the detailed spectrum of S 2p shows two peaks of S 2p1/2 and 2p3/2 at 162.8 and 161.65 eV, respectively, as well as a concomitant shake-up satellite peak.39,40 Therefore, the XPS spectra further coincide well with the multi-phase composition of NMS, composed of various nickel sulfides and MnS phases. Interestingly, the pronounced divergence about the valence state of the Ni element is displayed in the XRD and XPS pattern. Thus, we generally denoted these various nickel sulfides as NixSy.
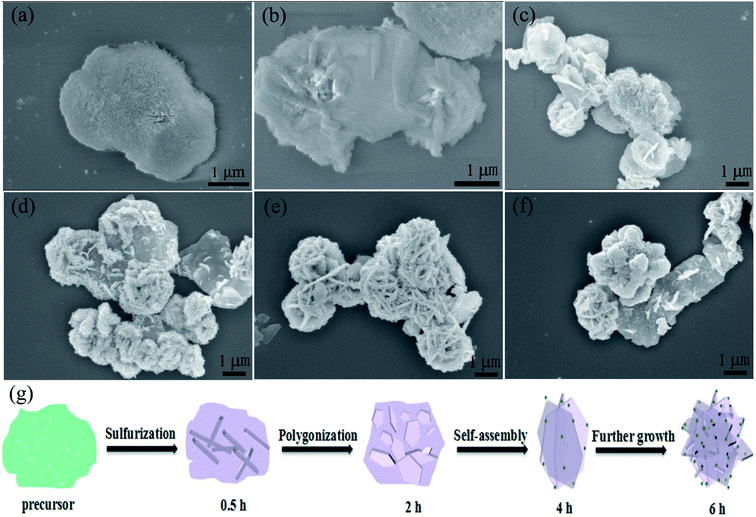
To shed light on the growth progress of this novel NMS structure, we further explored the samples (obtained at different sulfurization stages) via the FE-SEM techniques (Fig. 3), and the corresponding XRD patterns are shown in Fig. S3b.† Fig. 3a shows the FE-SEM image of blurry flowery Ni–Mn precursor. After sulfurization for 0.5 h (Fig. 3b), the blurry Ni–Mn precursor becomes smooth, and a large thick piece begins to regenerate. As shown in Fig. S3b,† some NixSy diffraction peaks can be observed. After sulfurization treatment for 2 h, hexagonal sheets gradually regenerate with a thicker size, as depicted in Fig. 3c. In the stage of 4 h (Fig. 3d), the hexagonal sheets gather and self-assemble to form a micro-flower structure, which exhibit an obvious decrease in thickness. After 6 h (Fig. 3e), NMS was obtained, possessing uniform morphology with a covering of nanoparticles. Compared to the case of the samples obtained after sulfurization for 2 h or 4 h, stronger MnS peaks can be detected in NMS. However, as shown in Fig. 3f, excess sulfurization time (8 h) would result in obvious enrichment of nanoparticles and increase the thickness of microsheets.
On the basis of the abovementioned FE-SEM description and the corresponding XRD results, the growth progress of the NMS structures is probably as follows (Fig. 3g): (1) in the beginning, S ion formed by Na2S·9H2O reacts with the Ni–Mn precursor; then, the product aggregates to cause nucleation; (2) after nucleation, the nucleation center grows into hexagonal sheets; (3) then, the hexagonal sheets self-assemble with the nanoparticles coated on them; and (4) after further growth, the NMS novel structure is finally built.
3.2 Electrochemical performance of the NMS electrodes
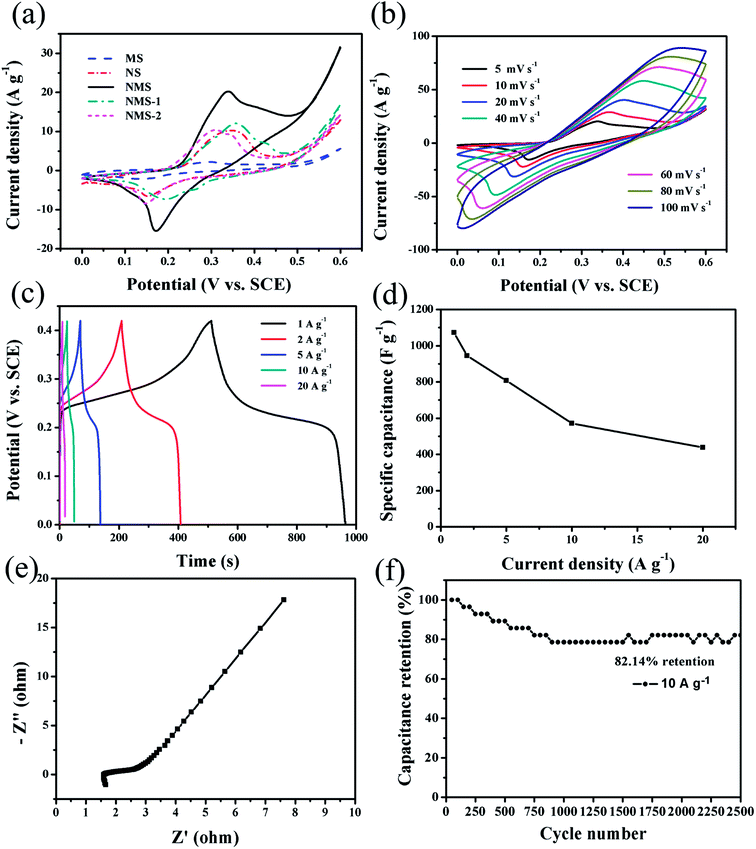
A three-electrode test system was employed to further evaluate the energy-storage capacitance of MS, NS, NMS, NMS-1 and NMS-2 electrodes. Fig. 4a shows comparison of the different CV curves of these electrodes at a scan rate of 5 mV s−1. Amongst them, NMS showed larger integrated area with stronger redox peaks, suggesting its superior capacitance behavior. A series of typical anodic and cathodic peaks are visible in these CV curves, which show significant difference in comparison with the ideal rectangular CV curves of EDLCs.5,10 Then, the detailed CV curves of the NMS electrode are shown in Fig. 4b at different scan rates ranging from 5 to 100 mV s−1. With the increasing scan rates, the initial shapes could be generally maintained, but the anodic peak shifted positively and the cathodic peak shifted negatively; this was due to the increased diffusion resistance and electrode polarization.41,42 The faradaic reactions corresponding to the redox peaks are as follows:| NiS + OH− ↔ NiSOH + e− |
| NiSOH + e− ↔ NiS + OH− |
| NiS2 + OH− ↔ NiS2OH + e− |
| NiS2OH + e− ↔ NiS2 + OH− |
| Ni7S6 + OH− ↔ Ni7S6OH + e− |
| Ni7S6OH + e− ↔ Ni7S6 + OH− |
| MnS + OH− ↔ MnSOH + e− |
| MnSOH + e− ↔ MnS + OH− |
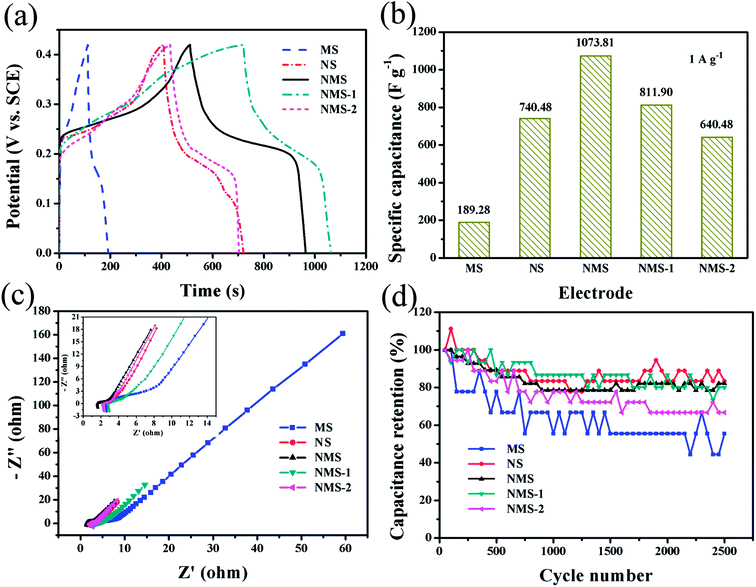
Fig. 4c shows the GCD characteristics of the as-prepared NMS electrode in the potential range of 0–0.42 V. The specific capacitance at various discharge current densities can be calculated via eqn (1) and are displayed in Fig. 4d. The obtained specific capacitance values of the NMS electrode are 1073.81, 944.76, 807.14, 571.43 and 428.57 F g−1 at the current densities of 1, 2, 5, 10, and 20 A g−1, respectively. For comparison, the electrochemical performances of MS, NS, NMS-1, and NMS-2 were also evaluated at a current density of 1 A g−1 (Fig. 5a and b). Compared with MS, NS, NMS-1, and NMS-2 electrodes, NMS presented an improved specific capacitance. The good electrochemical properties of NMS can be attributed to the synergistic effect of MnS and NixSy: (1) the hybrid manganese sulfide and nickel sulfide undergo redox reactions, which result in enhanced specific capacitances; (2) the stable 3D micro-flowers are constructed by the 2D hexagonal sheets, increasing the specific surface area and shortening the diffusion route to a great extent, benefiting both ion and electron transfer during the charge–discharge process; (3) the micro-flower structure can endure volume change in the electrochemical storage process; and (4) nanoparticles directly growing on the 3D networked micro-flowers not only provide more electrochemical active sites for redox reactions, but are also beneficial for the contact with the electrolyte. Hence, the NMS electrode performed best amongst all our materials.
The EIS measurements of NMS were carried out to further analyze the resistances of these electrodes. In the Nyquist plots of Fig. 4e and 5c, Z′ and Z′′ represent the real and imaginary parts of the impedance, respectively.43,44 The intersection of x-axis at a high frequency demonstrates the equivalent series resistance (ESR), which reveals multiple resistances including the intrinsic resistance of the active materials, electrolytic resistance, and the contact resistance between the electrolyte and active material. The ESR values of the MS, NS, NMS, NMS-1 and NMS-2 electrodes are 2.667, 1.668 1.66, 2.956 and 2.365 Ω, respectively. Moreover, contrary to other electrodes, NMS exhibited a smaller semicircle diameter and steeper straight line in high-frequency area; this verified low charge transfer resistance (RCT) and diffusion resistance (Zw). Therefore, the formation of binary metal sulfurization compounds and the optimization of mole ratio could serve as an efficient strategy to significantly reduce the resistances, then cause a synergistic effect among different components, and finally promote the electrochemical performance.
The long-term cycling stability is always considered as a critical factor for extensive applications in energy-storage devices. In Fig. 4f, the capacitance retention of NMS dramatically attenuated during the initial 800 cycles and tended to stably decrease in the latter cycling measurement. Moreover, 82.14% retention was observed after 2500 cycles at 10 A g−1, which was superior than those reported in previous studies and that of our other electrodes.4,45 The improved supercapacitor property of the NMS electrode benefited from the distinct 3D cross-linked flowery structure with ultrafine nanoparticles on a great scale.46 During the GCD processes, this 3D architecture has the self-adaptive strain-relaxation capability for more stable structures, thus leading to an improved cycle life.43,47 Furthermore, the MnS nanoparticles directly coated onto the NixSy nanosheets construct a heterostructure to shorten the diffusion length, which are greatly beneficial for the high-rate charge/discharge process, thus leading to the good electrochemical performance.48 In addition, as shown in Fig. 5d, great discreteness from other comparisons was presented in long-term cycling curves, which originated from the lower discharge time causing extremely fluctuant capacitance retention.
3.3 Asymmetric supercapacitor characterization
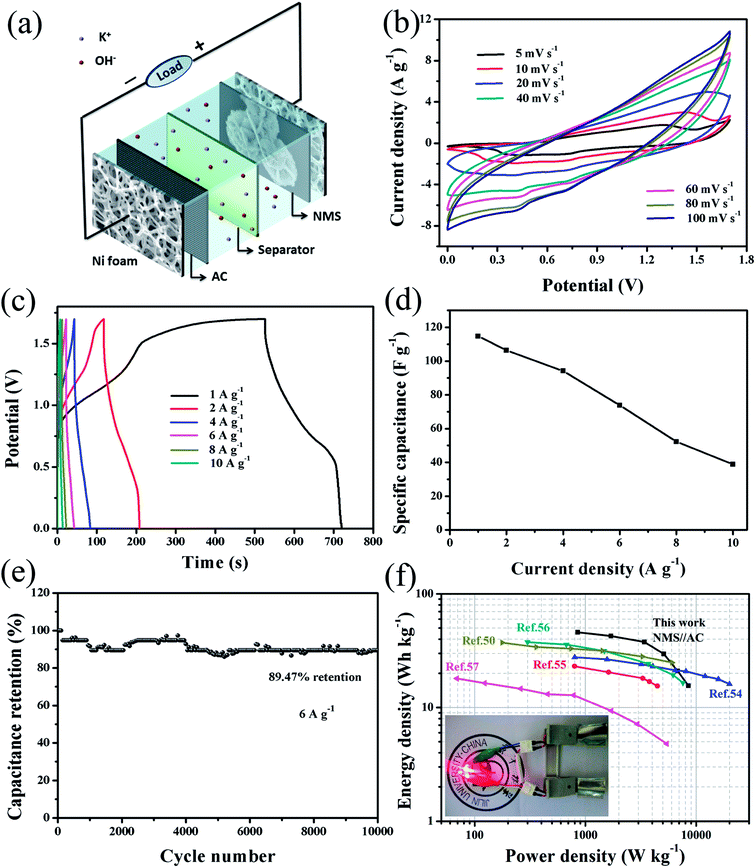
To further study the electrochemical property of the NMS electrode, we have further fabricated an asymmetric supercapacitor (NMS//AC, composed of positive NMS and negative AC electrodes), as exhibited in Fig. 6a. We fabricated the two-electrode asymmetric supercapacitor following the eqn (2), and the mass ratio of NMS to AC was about 1.73. Furthermore, the corresponding electrochemical test results are shown in Fig. S4.† The CV curves of the NMS//AC device (in Fig. 6b) exhibit a nearly rectangular shape, implying that there is a good charge matching between the positive and negative electrodes. The corresponding GCD curves were obtained (Fig. 6c) at various current densities in a wide potential window between 0 and 1.7 V. At a current density of 1 A g−1, the capacitance for the device can reach as high as 114.71 F g−1. On the other hand, the capacitance diminished with an increase in the current densities due to the incomplete charge–discharge process and electrode polarization at high current densities. However, the device still kept a value of 38.83 F g−1 at a high current density of 10 A g−1 (as shown in Fig. 6d).The cycling duration of NMS//AC was also investigated. As presented in Fig. 6e, the specific capacitance decreased in the initial 2050 cycles and then generated a slight rebound (2100–3950 cycles); this might be attributed to electrode activation. After this, the capacitance remained relatively stable and achieved a higher retention of 89.47%, enduring 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Furthermore, the Ragone plots for NMS//AC and other references are shown in Fig. 6f. According to the eqn (3) and (4), our NMS//AC asymmetric supercapacitor revealed a high energy density of 46.04 W h kg−1 at a power density of 0.85 kW kg−1, which was superior to that of Mn- and Ni/Mn-based asymmetric supercapacitors such as γ-MnS/rGO-60//rGO,54 α-MnS/N-rGO hybrid//N-rGO,55 AC//LNC,56 MnS//EDAC50 and M-MCNF-based full cell supercapacitor.57 The high specific capacitance (114.71 F g–1) and large cell voltage (1.7 V) lead to the high energy density. In addition, as presented in Table 1, we also compared other important parameters with those of other related asymmetric supercapacitors to highlight the advantages of our device. Finally, two NMS//AC asymmetric supercapacitors were further assembled in series to intuitively display their practical application, which could effortlessly light up four red light-emitting diodes (LED) (1.8 V, 20 mA) (as shown in the inset of Fig. 6f). These impressive results powerfully testify the enormous potential of NMS for applications in energy storage.
000 cycles. Furthermore, the Ragone plots for NMS//AC and other references are shown in Fig. 6f. According to the eqn (3) and (4), our NMS//AC asymmetric supercapacitor revealed a high energy density of 46.04 W h kg−1 at a power density of 0.85 kW kg−1, which was superior to that of Mn- and Ni/Mn-based asymmetric supercapacitors such as γ-MnS/rGO-60//rGO,54 α-MnS/N-rGO hybrid//N-rGO,55 AC//LNC,56 MnS//EDAC50 and M-MCNF-based full cell supercapacitor.57 The high specific capacitance (114.71 F g–1) and large cell voltage (1.7 V) lead to the high energy density. In addition, as presented in Table 1, we also compared other important parameters with those of other related asymmetric supercapacitors to highlight the advantages of our device. Finally, two NMS//AC asymmetric supercapacitors were further assembled in series to intuitively display their practical application, which could effortlessly light up four red light-emitting diodes (LED) (1.8 V, 20 mA) (as shown in the inset of Fig. 6f). These impressive results powerfully testify the enormous potential of NMS for applications in energy storage.
| Electrode material | Specific capacity/capacitance | Energy density | Cycling stability | Reference |
|---|---|---|---|---|
| NMS | 1073.81 F g−1 at 1 A g−1 | 46.04 W h kg−1 at 850 W kg−1 | 89.47% 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 6 A g−1 000 cycles 6 A g−1 |
This work |
| Ni–Mn LDH nanosheets | 881 F g−1 at 0.5 A g−1 | 46.3 W h kg−1 at 112.6 W kg−1 | 89% 6000 cycles 5 A g−1 | 49 |
| γ-MnS | 573.9 F g−1 at 0.5 A g−1 | 37.6 W h kg−1 at 181.2 W kg−1 | 89.87% 5000 cycles 1 A g−1 | 50 |
| (Mn–Co) oxysulfide on Ni foam | 490C g−1 (capacity) at 2 A g−1 | — | 86.5% 3000 cycles 20 A g−1 (three-electrode system) | 10 |
| Co2.5Mn0.5 sulfide | 289C g−1 at 1 A g−1 | 22.3 W h kg−1 at 750 W kg−1 | 85% 5000 cycles 3 A g−1 | 11 |
| Mn/Zn-BMO | 251 F g−1 at 0.5 A g−1 | 33.1 W h kg−1 at 4416 W kg−1 | 99.6% 5000 cycles/— | 51 |
| Zn0.25Mn0.75S hollow spheres | 664 F g−1 at 1 A g−1 | — | — | 52 |
| MnS/GO-NH3 | 390.8 F g−1 at 0.25 A g−1 | 14.9 W h kg−1 at 66.5 W kg−1 | 81% 2000 cycles/— | 53 |
4. Conclusion
In our study, we have successfully fabricated an NMS electrode through a facile hydrothermal approach and subsequent sulfurization treatment. A novel flowery structure composed of flat hexagonal nanosheets and ultrafine nanoparticles endowed MNS with sufficient active areas for the diffusion reaction. The synergistic effect was due to the heterojunction between NixSy and MnS at a bigger scale, promoting the diffusion of ions on the surface of the electrode; thus, NMS endured the change in volume in the long-term cycling process. Therefore, the as-synthesized NMS electrode can reach a high specific capacitance with favorable cycling stability. Moreover, we have further fabricated an NMS//AC asymmetric supercapacitor, which delivers a high specific energy density as well as an excellent cycling stability. In addition, two NMS//AC device in series could easily trigger the LED indicator, indicating potential for practical application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51501068), the Science and Technology Key Project of Jilin Province (20160204017GX), and the National Natural Science Foundation of China (Grant No. 61774022).References
- E. Mourad, L. Coustan, P. Lannelongue, D. Zigah, A. Mehdi, A. Vioux, S. A. Freunberger, F. Favier and O. Fontaine, Nat. Mater., 2017, 16, 446–453 CrossRef PubMed.
- X. Liu, Z. Wu and Y. Yin, Chem. Eng. J., 2017, 323, 330–339 CrossRef.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- J. Chen, X. Wang, J. Wang and P. S. Lee, Adv. Energy Mater., 2016, 6, 1501745 CrossRef.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- L. Wu, L. Hao, B. Pang, G. Wang, Y. Zhang and X. Li, J. Mater. Chem. A, 2017, 5, 4629–4637 Search PubMed.
- Y. Zhao, L. Hu, S. Zhao and L. Wu, Adv. Funct. Mater., 2016, 26, 4085–4093 CrossRef.
- R. R. Salunkhe, J. Lin, V. Malgras, S. X. Dou, J. H. Kim and Y. Yamauchi, Nano Energy, 2015, 11, 211–218 CrossRef.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166–5180 CrossRef PubMed.
- M. Liu, Y. Fu, H. Ma, T. Wang, C. Guan and K. Hu, Electrochim. Acta, 2016, 191, 916–922 CrossRef.
- S. Chen, H. Chen, C. Li, M. Fan, C. Lv, G. Tian and K. Shu, J. Mater. Sci., 2017, 52, 6687–6696 CrossRef.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef PubMed.
- T. Brousse, D. Belanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117–1123 CrossRef PubMed.
- G. He, J. Li, W. Li, B. Li, N. Noor, K. Xu, J. Hu and I. P. Parkin, J. Mater. Chem. A, 2015, 3, 14272–14278 Search PubMed.
- J. Yang, C. Yu, X. Fan, S. Liang, S. Li, H. Huang, Z. Ling, C. Hao and J. Qiu, Energy Environ. Sci., 2016, 9, 1299–1307 Search PubMed.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597 Search PubMed.
- S. Zhu, L. Li, J. Liu, H. Wang, T. Wang, Y. Zhang, L. Zhang, R. S. Ruoff and F. Dong, ACS Nano, 2018, 12, 1033–1042 CrossRef PubMed.
- N. Jabeen, A. Hussain, Q. Xia, S. Sun, J. Zhu and H. Xia, Adv. Mater., 2017, 29, 1700804 CrossRef PubMed.
- N. Jabeen, Q. Xia, S. V. Savilov, S. M. Aldoshin, Y. Yu and H. Xia, ACS Appl. Mater. Interfaces, 2016, 8, 33732–33740 Search PubMed.
- L. Liu, Nanoscale, 2013, 5, 11615–11619 RSC.
- Y. Lu, L. Yu, M. Wu, Y. Wang and X. W. D. Lou, Adv. Mater., 2018, 30, 12464–12472 Search PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef PubMed.
- Y. Ouyang, X. Xia, H. Ye, L. Wang, X. Jiao, W. Lei and Q. Hao, ACS Appl. Mater. Interfaces, 2018, 10, 3549–3561 Search PubMed.
- M. S. Javed, X. Han, C. Hu, M. Zhou, Z. Huang, X. Tang and X. Gu, ACS Appl. Mater. Interfaces, 2016, 8, 24621–24628 Search PubMed.
- Y. Tang, S. Chen, S. Mu, T. Chen, Y. Qiao, S. Yu and F. Gao, ACS Appl. Mater. Interfaces, 2016, 8, 9721–9732 Search PubMed.
- Z. Zeng, D. Wang, J. Zhu, F. Xiao, Y. Li and X. Zhu, CrystEngComm, 2016, 18, 2363–2374 RSC.
- J. Zhao, J. Chen, S. Xu, M. Shao, Q. Zhang, F. Wei, J. Ma, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 2938–2946 CrossRef.
- H. Wan, J. Jiang, Y. Ruan, J. Yu, L. Zhang, H. Chen, L. Miao and S. Bie, Part. Part. Syst. Charact., 2014, 31, 857–862 CrossRef.
- H. Pang, X. Li, Q. Zhao, H. Xue, W.-Y. Lai, Z. Hu and W. Huang, Nano Energy, 2017, 35, 138–145 CrossRef.
- Y. Guan, Y. Feng, Y. Mu, L. Fang, H. Zhang and Y. Wang, Nanotechnology, 2016, 27, 475402 CrossRef PubMed.
- X. Li, J. Shen, N. Li and M. Ye, J. Power Sources, 2015, 282, 194–201 CrossRef.
- B. Guan, Y. Li, B. Yin, K. Liu, D. Wang, H. Zhang and C. Cheng, Chem. Eng. J., 2017, 308, 1165–1173 CrossRef.
- X. Yang, L. Zhao and J. Lian, J. Power Sources, 2017, 343, 373–382 CrossRef.
- Z. Dai, X. Zang, J. Yang, C. Sun, W. Si, W. Huang and X. Dong, ACS Appl. Mater. Interfaces, 2015, 7, 25396–25401 Search PubMed.
- Z. Li, J. Han, L. Fan and R. Guo, CrystEngComm, 2015, 17, 1952–1958 RSC.
- J. Tang, J. Shen, N. Li and M. Ye, J. Alloys Compd., 2016, 666, 15–22 CrossRef.
- C. Lamiel, V. H. Nguyen, D. R. Kumar and J.-J. Shim, Chem. Eng. J., 2017, 316, 1091–1102 CrossRef.
- Z. Zhang, Q. Wang, C. Zhao, S. Min and X. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 4861–4868 Search PubMed.
- Y. Ruan, J. Jiang, H. Wan, X. Ji, L. Miao, L. Peng, B. Zhang, L. Lv and J. Liu, J. Power Sources, 2016, 301, 122–130 CrossRef.
- J. Ji, L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef PubMed.
- J. Liu, J. Wang, Z. Ku, H. Wang, S. Chen, L. Zhang, J. Lin and Z. X. Shen, ACS Nano, 2016, 10, 1007–1016 CrossRef PubMed.
- X. Wang, J. Hao, Y. Su, F. Liu, J. An and J. Lian, J. Mater. Chem. A, 2016, 4, 12929–12939 Search PubMed.
- X. Zhang, Y. Zhao and C. Xu, Nanoscale, 2014, 6, 3638–3646 RSC.
- S. Sahoo, R. Mondal, D. J. Late and C. S. Rout, Microporous Mesoporous Mater., 2017, 244, 101–108 CrossRef.
- T. Zhu, H. B. Wu, Y. Wang, R. Xu and X. W. D. Lou, Adv. Energy Mater., 2012, 2, 1497–1502 CrossRef.
- L. Qu, Y. Zhao, A. M. Khan, C. Han, K. M. Hercule, M. Yan, X. Liu, W. Chen, D. Wang, Z. Cai, W. Xu, K. Zhao, X. Zheng and L. Mai, Nano Lett., 2015, 15, 2037–2044 CrossRef PubMed.
- C. Jiang, E. Hosono and H. Zhou, Nano Today, 2006, 1, 28–33 CrossRef.
- H. Sim, C. Jo, T. Yu, E. Lim, S. Yoon, J. H. Lee, J. Yoo, J. Lee and B. Lim, Chemistry, 2014, 20, 14880–14884 CrossRef PubMed.
- T. Chen, Y. Tang, Y. Qiao, Z. Liu, W. Guo, J. Song, S. Mu, S. Yu, Y. Zhao and F. Gao, Sci. Rep., 2016, 6, 23289 CrossRef PubMed.
- T. Prasankumar, V. S. Irthaza Aazem, P. Raghavan, K. Prem Ananth, S. Biradar, R. Ilangovan and S. Jose, J. Alloys Compd., 2017, 695, 2835–2843 CrossRef.
- Y. Jin, J. Xu, L. Wang, Q. Lu and F. Gao, Chemistry, 2016, 22, 18859–18864 CrossRef PubMed.
- Y. Tang, T. Chen, S. Yu, Y. Qiao, S. Mu, J. Hu and F. Gao, J. Mater. Chem. A, 2015, 3, 12913–12919 Search PubMed.
- G. Zhang, M. Kong, Y. Yao, L. Long, M. Yan, X. Liao, G. Yin, Z. Huang, A. M. Asiri and X. Sun, Nanotechnology, 2017, 28, 065402 CrossRef PubMed.
- H. Quan, B. Cheng, D. Chen, X. Su, Y. Xiao and S. Lei, Electrochim. Acta, 2016, 210, 557–566 CrossRef.
- H. Chen, Y. Ai, F. Liu, X. Chang, Y. Xue, Q. Huang, C. Wang, H. Lin and S. Han, Electrochim. Acta, 2016, 213, 55–65 CrossRef.
- H. Wang, J. Deng, Y. Chen, F. Xu, Z. Wei and Y. Wang, Nano Res., 2016, 9, 2672–2680 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02063a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |