Open Access Article
Open Access ArticleSepiolite/CNT/S@PANI composite with stable network structure for high performance lithium sulfur batteries†
Guolong Yuan‡
a,
Junan Pan‡a,
Yaguang Zhanga,
Junxi Yua,
Yanjia Hea,
Yong Sua,
Qi Zhoub,
Hongyun Jin c and
Shuhong Xie
c and
Shuhong Xie *d
*d
aHunan Provincial Key Laboratory of Thin Film Materials and Devices, Xiangtan University, Xiangtan, 411105 Hunan, China
bState Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals, Lanzhou University of Technology, Lanzhou, 730050 Gansu, China
cEngineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan, 430074 Hubei, China
dKey Laboratory of Low Dimensional Materials and Application Technology of Ministry of Education, School of Materials Science and Engineering, Xiangtan University, Xiangtan 411105, Hunan, China. E-mail: shxie@xtu.edu.cn
First published on 16th May 2018
Abstract
Composite materials with a stable network structure consisting of natural sepiolite (Sep) powders, carbon nanotubes (CNTs) and conductive polymer (PANI) have been successfully synthesized using a simple vacuum heat treatment and chemical oxidation method, and they have been used as cathode materials for lithium sulfur batteries. It is found that Sep/CNT/S@PANI composites possess high initial discharge capacity, good cyclic stability and good rate performance. The initial discharge capacity of the Sep/CNT/S@PANI-II composite is about 1100 mA h g−1 at 2C, and remained at 650 mA h g−1 after 300 cycles, and the corresponding coulombic efficiency is above 93%. Such performance is attributed to specific porous structure, outstanding adsorption characteristics, and excellent ion exchange capability of sepiolite, as well as excellent conductivity of CNT. Furthermore, the PANI coating has a pinning effect for sulfur, which enhances the utilization of the active mass and improves the cycling stability and the coulombic efficiency of the composites at high current rates.
1. Introduction
Utilization of sulfur as a cathode material enables the electrochemical system of lithium–sulfur (Li–S) batteries to have a high energy density of 2600 W h kg−1.1–3 Besides the large specific capacity,4,5 sulfur is also inexpensive and environmentally friendly,6,7 stimulating extensive research. However, the commercialization of the Li–S battery is challenged by the severe “shuttle effect” resulting from the dissolution of lithium polysulfide during the charge/discharge process.8,9 The repeated polysulfide shuttling causes the loss of active materials, resulting in low coulombic efficiency and low cycle life.10–12 Furthermore, the low conductivity of sulfur and Li2S leads to poor rate capability.13,14To overcome these drawbacks, significant efforts have been carried out to improve the performance of Li–S battery by incorporating additives into sulfur cathodes, including carbon materials,15–17 conductive polymers18–20 and metal oxides.6,21 It was reported that carbon materials added into sulfur cathode are effective in improving the comprehensive performances of sulfur cathode, including CMK-3,22 carbon nanotubes,23 and carbon nanofibers.24 Meanwhile some conductive polymers were coated onto the sulfur cathode to reduce the dissolution of lithium polysulfides and increase the conductivity of sulfur, including polyaniline25,26 and polypyrrole.27,28 Metal oxides were also applied to improve the performance of Li–S batteries, as they can effectively adsorb lithium polysulfides by chemical adsorption.21,29 Our previous study demonstrated the sepiolite/sulfur (Sep/S) composite as a promising cathode material due to its low cost and simple synthesis method.30 Indeed, the strong adsorption of sepiolite for polysulfide enabled good conversion efficiency of active material. Nevertheless, the poor electrical conductivity and impurity of nature sepiolite result in low coulombic efficiency at high current rate.
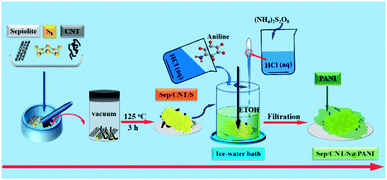
Herein, we design a network structure that can reinforce the stability of the composite by adding both carbon nanotubes (CNTs) and polyaniline (PANI) into the Sep/S matrix, forming a composite cathode denoted as Sep/CNT/S@PANI. As shown in Fig. 1, Sep/CNT/S composite was first synthesized via vacuum heating treatment method, and then followed by the chemical oxidation method to form the Sep/CNT/S@PANI composite. The composite cathode exhibits excellent electrochemical performance, especially at high current rate, since CNTs possess excellent electrical conductivity while PANI coating layer serves as a barrier to restrict the diffusion of polysulfide.
2. Experimental section
2.1 Fabrication of Sep/CNT/S composites
Natural sepiolite powders (Sep) were bought from a mineral processing factory in Hunan Province, China. Carbon nanotubes (CNT), sublimed sulfur (S), aniline monomers (An), ammonium persulfate ((NH4)2S2O8), hydrochloric acid (HCl), polytetrafluoroethylene (PTFE) and acetylene black were purchased from Sinopharm Chemical Reagent Co. Ltd. (China). All chemicals of analytical grade were used without further purification.The Sep/CNT/S composite was prepared via a universal vacuum heat treatment method. Typically, 0.3 g sepiolite powders, 0.6 g sulfur and 0.1 g carbon nanotubes with mass ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed by the gravimetric method. The mixture was then heated under vacuum at 125 °C for 3 h, forming the Sep/CNT/S composite.
1 were mixed by the gravimetric method. The mixture was then heated under vacuum at 125 °C for 3 h, forming the Sep/CNT/S composite.
2.2 Fabrication of Sep/CNT/S@PANI composites
Three Sep/CNT/S samples (each of 0.2 g) were used, and each sample was mixed with 30 mL ethanol by ultrasonication to make them disperse uniformly. Three 15 mL 2 M HCl solutions and anilines with different weights of 0.05 g, 0.1 g and 0.2 g were mixed respectively, which were then added into the three Sep/CNT/S samples, followed by adding the mixture solution of 15 mL 2 M HCl with (NH4)2S2O8 (0.1 g, 0.2 g and 0.4 g). After 12 hours stirring at ice-water bath, the dark green products were obtained through centrifugation and dried for 12 h at 60 °C in vacuum. Finally, the polyaniline (PANI) layer with different amount of PANI to Sep/CNT/S composites were obtained and denoted as Sep/CNT/S@PANI-I, II, and III respectively.2.3 Material characterization
The structure and composition of the composites were examined using X-ray diffraction (XRD, Rigaku D/max-2400) with Cu Kα radiation (λ = 1.5406 Å). The morphology of the synthesized composites were observed by scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, FEI Tecnai G2 F20) with energy dispersive X-ray spectroscopy (EDS). The Brunauer–Emmett–Teller (BET) surface area and pore size distributions were measured using a JW-BK122W system. The infrared spectroscopy (IR, Perkin-Elmer Spectrum One spectrophotometer) was applied to confirm the structures of composites. Thermogravimetric (TG) analysis was carried out by SDT-Q600 thermal analyzer in N2 atmosphere.2.4 Electrode fabrication and electrochemical test
For positive electrode fabrications, 70 wt% Sep/CNT/S composite or Sep/CNT/S@PANI-I, II and III composites, 20 wt% acetylene black and 10 wt% polytetrafluoroethylene (PTFE) binders were homogeneously mixed in an ethanol solvent under continuous magnetic stirring for 3 h, and then the slurry was coated uniformly on an aluminum foil. The electrode was dried under vacuum at 60 °C for 12 h. After that 2016 type coin cells were assembled in an argon-filled glove box. Lithium metal was used as a counter electrode and polypropylene membrane was used as the separator. The electrolyte was 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio mixture of 1,3-dioxolane and dimethoxy ethane.
1 volume ratio mixture of 1,3-dioxolane and dimethoxy ethane.
Electrochemical charge–discharge and cyclic performances were measured at room temperature using a BTS-5V3A battery test system (Neware, Shenzhen, China), with the voltage range from 3.0 to 1.0 V (vs. Li/Li+). Cyclic voltammetry (CV) and electro-chemical impedance spectroscopy (EIS) were tested using a CHI660D electrochemical workstation (Chenhua, Shanghai, China) over a frequency range of 1 MHz to 0.1 Hz, with an open-circuit AC voltage amplitude of 5 mV.
3. Results and discussion
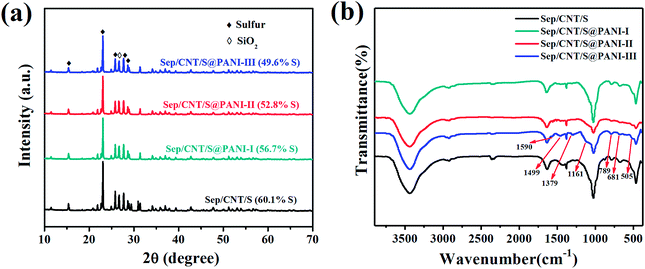
The X-ray diffraction patterns of Sep/CNT/S, Sep/CNT/S@PANI-I, Sep/CNT/S@PANI-II and Sep/CNT/S@PANI-III are shown in Fig. 2(a), and the sharp diffraction peaks of sulfur illustrate its good crystallinity in the four composites. The diffraction peak near 27° can be assigned to SiO2, which indicates the existence of sepiolite. In addition, with the increased PANI fraction (from Sep/CNT/S@PANI-I to Sep/CNT/S@PANI-III), the intensity of characteristic diffraction peaks decreases due to the relative low crystallinity of PANI.31,32The FTIR spectrums of Sep/CNT/S composites with and without PANI coating are shown in Fig. 2(b). The absorption peaks at 1030 cm−1 and 469 cm−1 correspond to the Si–O–Si stretching.33 After coating PANI the absorption peaks corresponding to Si–O stretching and bending of sepiolite gradually weaken, and the peaks at 789 cm−1 and 681 cm−1 and peaks at 1590 cm−1 and 1499 cm−1 are observed, which are consistent with those of the quinone ring and benzene ring of polyaniline.34 Furthermore, the absorption peaks at 1379 cm−1 and 1161 cm−1 correspond to the stretching vibration of C–N bond in aromatic amine and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond in polyaniline, respectively.35 The characteristic peak at 505 cm−1 refers to the bending vibration of aromatic ring. Thus FTIR spectra confirms the existence of PANI in Sep/CNT/S@PANI composites.25,36 Meanwhile, the intensity of characteristic peaks of polyaniline increases with increased aniline content.37 Furthermore, thermogravimetry (TG) is used to analyze the sulfur content of the Sep/CNT/S and Sep/CNT/S@PANI composites as shown in Fig. S1,† suggesting that Sep/CNT/S composite and Sep/CNT/S@PANI-I, II and III composites contain 60.1 wt%, 56.7 wt%, 52.8 wt% and 49.6 wt% of S, respectively. The differences between Sep/CNT/S composite before and after coating PANI indicate the corresponding mass percent of PANI in the Sep/CNT/S@PANI-I, II and III composites are 3.4 wt%, 7.3 wt% and 10.5 wt%, respectively.
N– bond in polyaniline, respectively.35 The characteristic peak at 505 cm−1 refers to the bending vibration of aromatic ring. Thus FTIR spectra confirms the existence of PANI in Sep/CNT/S@PANI composites.25,36 Meanwhile, the intensity of characteristic peaks of polyaniline increases with increased aniline content.37 Furthermore, thermogravimetry (TG) is used to analyze the sulfur content of the Sep/CNT/S and Sep/CNT/S@PANI composites as shown in Fig. S1,† suggesting that Sep/CNT/S composite and Sep/CNT/S@PANI-I, II and III composites contain 60.1 wt%, 56.7 wt%, 52.8 wt% and 49.6 wt% of S, respectively. The differences between Sep/CNT/S composite before and after coating PANI indicate the corresponding mass percent of PANI in the Sep/CNT/S@PANI-I, II and III composites are 3.4 wt%, 7.3 wt% and 10.5 wt%, respectively.
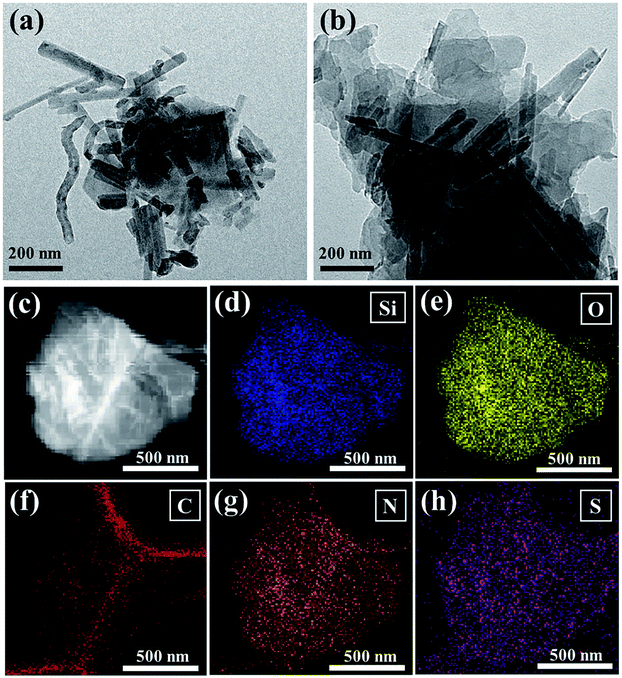
The morphology of Sep/CNT/S and Sep/CNT/S@PANI-II composites are characterized by TEM with EDS, as shown in Fig. 3(a) and (b). It is observed that the network structure of Sep/CNT/S composite are composed of short sepiolite fibrous bundles with length in the range of 200 nm to 1 μm, as well as bended carbon nanotube with length around 1 μm, and the surfaces of sepiolite and CNTs are covered with sulfur. After PANI is coated on the surface of Sep/CNT/S composite, the network structure remains and aggregation appears due to the viscosity of PANI as seen in Fig. 3(b). Comparing the images of the three kinds of Sep/CNT/S@PANI composites in Fig. 3, S2 and S3† carefully, it is observed that the thickness of PANI coating is inhomogeneous, and the three dimensional network structure stability increase with increasing PANI content moderately, and excessive PANI coating reduce the surface area and the effective pore numbers due to aggregation. Moreover, the element distributions of Si, O, C, N, and S in Fig. 3(c)–(h) are uniform in the Sep/CNT/S@PANI-II composite. The Si element is ascribed to sepiolite, while the O element is from both polyaniline (PANI) and sepiolite, whose area is slightly larger than that of Si element. The C element arises from CNT and TEM sample substrate. Concentration of N element is relatively high at the edge of Si element, suggesting that PANI layer covers the Sep/CNT/S composite. The elemental mapping of S confirms that the sulfur is dispersed uniformly. These results demonstrate that PANI has been successfully deposited on Sep/CNT/S composite.38 Since the oxidation reactions can only occur at the Sep/CNT/S interface during charging/discharging process, the highly uniform distribution of PANI coating can increase the contact area between PANI and the electrolyte, and hence increase the number of active sites for electrochemical reaction in Li–S batteries. The TEM images of Sep/CNT/S@PANI-I and III composites are shown in Fig. S2 and S3† is the SEM images of Sep/CNT/S and Sep/CNT/S@PANI composites.
N2 adsorption and desorption isotherm was used to measure the specific surface area of the Sep/CNT/S composites without and with different content of PANI coating, as shown in Fig. 4(a). The IV type isotherm of mesoporous materials is observed,39 and the specific surface area of Sep/CNT/S increases after being coated with moderate PANI, and then decreases with further coating. Fig. 4(b) reveals the change of pore diameter before and after PANI coating, showing a homogeneous distribution of pore size. Further, Table 1 lists that the specific surface area of the Sep/CNT/S composite and Sep/CNT/S@PANI-I, II and III composites are 9.297 m2 g−1, 17.515 m2 g−1, 18.731 m2 g−1 and 6.320 m2 g−1, respectively, and the corresponding pore volume are 0.027 cm3 g−1, 0.044 cm3 g−1, 0.045 cm3 g−1 and 0.021 cm3 g−1, which is consistent with the results of TEM and SEM images.
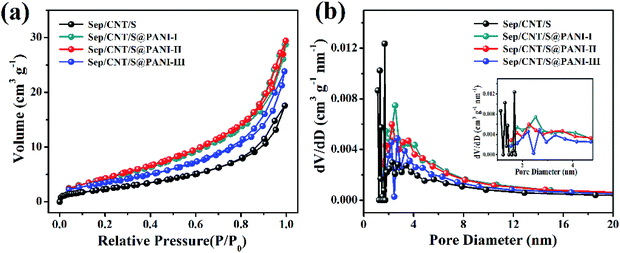 | ||
| Fig. 4 (a) BET surface area and (b) pore size distribution of Sep/CNT/S and Sep/CNT/S@PANI-I, II and III composites. | ||
| Sample | Specific surface area (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|
| Sep/CNT/S | 9.297 | 0.027 |
Sep/CNT/S@PANI (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
17.515 | 0.044 |
Sep/CNT/S@PANI (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
18.731 | 0.045 |
Sep/CNT/S@PANI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6.320 | 0.021 |
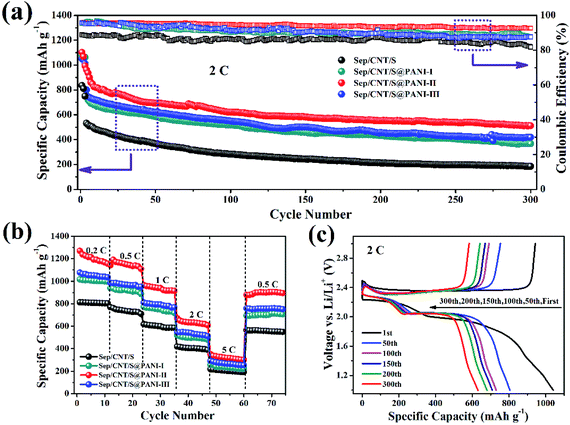
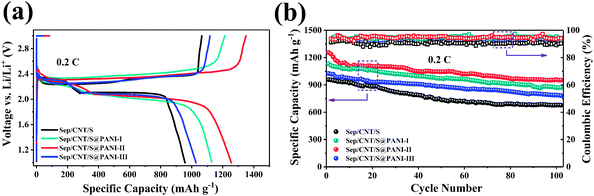
The cycling performance and coulombic efficiency of the Sep/CNT/S and Sep/CNT/S@PANI-I, II and III cathodes over 300 cycles at 2C rate (1C = 1675 mA g−1) are shown in Fig. 5(a). It is observed that the first specific capacity of Sep/CNT/S composite cathode is about 800 mA h g−1, smaller than 1050 mA h g−1 for Sep/CNT/S@PANI-I, III and 1100 mA h g−1 for the Sep/CNT/S@PANI-II cathode. Both Sep/CNT/S and Sep/CNT/S@PANI composites show larger capacity at 2C rate, compared to our previous study of Sep/S composite with capacity of 750 mA h g−1.30 It is also obvious that the discharge capacity of Sep/CNT/S@PANI after 300 cycles is much better than that of Sep/CNT/S. The discharge capacity of Sep/CNT/S only remains about 200 mA h g−1 after 300 cycles, while corresponding capacity of Sep/CNT/S@PANI-I, III are about 400 mA h g−1 and 450 mA h g−1 respectively. In particular, the discharge capacity of Sep/CNT/S@PANI-II is the best among the four composites, keeping about 650 mA h g−1 after 300 cycles. Meanwhile, the coulombic efficiency of Sep/CNT/S cathode is around 85%, and corresponding value of Sep/CNT/S@PANI-I, II and III cathodes are around 89%, 93% and 90%. The rate capability of Sep/CNT/S composite and Sep/CNT/S@PANI-I, II and III composites under different discharge rate is shown in Fig. 5(b), increasing from 0.2C to 5C by five steps and then decreasing to 0.5C finally. It is obvious that the rate capacities of Sep/CNT/S composite with PANI coating is much better than that of Sep/CNT/S. In particular, Sep/CNT/S@PANI-II composite possesses discharge capacity around 1270 mA h g−1 under 0.2C, and maintains a high value of 1000 mA h g−1 at 1C, 700 mA h g−1 at 2C, and keeps 400 mA h g−1 at 5C. When the current density returns to 0.5C, the discharge capacity recovers the high value around 1100 mA h g−1. These indicate that the Sep/CNT/S@PANI cathode has an excellent high rate discharge capacity. Fig. 5(c) shows the charge–discharge curves at different cycles for Sep/CNT/S@PANI-II cathode under 2C rate. Combining Fig. 5(c) with the corresponding first charge–discharge curves of the four kinds of samples at 0.2C rate in Fig. 6(a), it is obvious that there are two clear discharge plateaus in the first charge–discharge curves, where the high voltage platform is around 2.30 V and the low voltage platform is around 2.10 V, which represents the S8 transformed into lithium polysulfides and the polysulfides transformed into the Li2S2 and Li2S, respectively.40,41 As seen in Fig. 5(c), the discharge capacities are 1085, 810, 760, 720, 685, and 650 mA h g−1 at cycle number 1, 50, 100, 150, 200, 300, respectively.
The cycling performance of the four kinds of composites under 0.2C rate is shown in Fig. 6(a). The first discharge capacity of Sep/CNT/S composite at 0.2C rate is 958.2 mA h g−1 and remains 678.6 mA h g−1 after 100 cycles, the corresponding coulombic efficiency is around 84.5%. Meanwhile, the first discharge capacity of Sep/CNT/S@PANI-I, II and III composites at 0.2C rate are 1026.2, 1132.6 and 1256.3 mA h g−1, remains 787.8, 869.9 and 958.5 mA h g−1 after 100 cycles, the corresponding coulombic efficiency are around 88%, 92.5% and 90% respectively. The results show that the Sep/CNT/S composite with the PANI coating has good cycling performance at low current density 0.2C, especially the Sep/CNT/S@PANI-II composite.
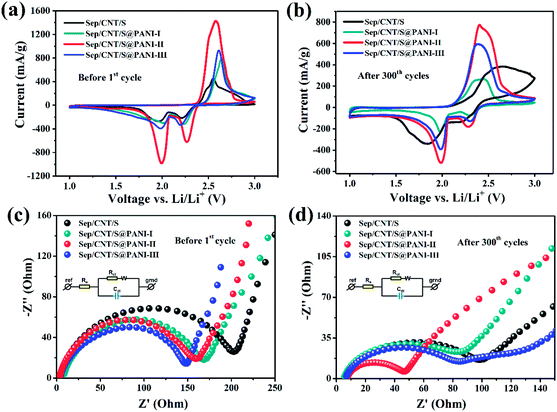
Fig. 7(a) and (b) shows the cyclic voltammograms of Sep/CNT/S cathode and Sep/CNT/S@PANI-I, II and III cathodes in a voltage range between 1.0 and 3.0 V before the first cycle and after 300 cycles at 2C with a scanning speed of 0.1 mV s−1. The peak potential of CV curves for the four composites before 1st cycle is similar, and both the Sep/CNT/S composite and Sep/CNT/S@PANI-I, II and III composites have two cathodic reduction peaks around 2.25 V and 2.0 V and an anodic oxidation peak around 2.60 V. The first peak around 2.25 V is attributed to the elemental sulfur converted into long chain lithium polysulfides (Li2Sn, 4 ≤ n ≤ 8), while the peak around 2.0 V corresponds to the process of the long chain lithium polysulfides deoxidized into short chain lithium polysulfides (Li2Sn, n < 4) and solid lithium sulfide (Li2S), respectively. The corresponding oxidation peak around 2.6 V represents the process that the lithium polysulfides and Li2S are oxidized into elemental sulfur.42,43 It is obvious that the instantaneous current of cathodic reduction and anodic oxidation peaks for Sep/CNT/S@PANI-I, II and III cathodes are higher than that of Sep/CNT/S, which indicates that the active masses of Sep/CNT/S@PANI-I, II and III cathodes are larger than that of Sep/CNT/S, especially the Sep/CNT/S@PANI-II cathode. It is also observed that after 300th cycles the cathodic reduction and anodic oxidation peaks with PANI coating are still obvious, which indicates that the Sep/CNT/S@PANI-I, II, and III cathodes are more stable than that of Sep/CNT/S after 300 cycles.44
Fig. 7(c) and (d) represent the EIS behaviors of Sep/CNT/S cathode and Sep/CNT/S@PANI-I, II and III cathodes before cycle and after 300 cycles, respectively. As seen in Fig. 7(c), the charge transfer resistances of Sep/CNT/S@PANI-I, II and III cathodes are smaller than that of Sep/CNT/S cathode owing to the good interface contact and conductivity of PANI, the corresponding value of Sep/CNT/S and Sep/CNT/S@PANI-I, II and III composites are 204.2, 166.2 and 148.7 Ω respectively. After 300 cycles as shown in Fig. 7(d), the four electrodes exhibit obviously decreased resistances compared with the corresponding fresh cells, especially in the Sep/CNT/S@PANI-II cathode, the corresponding resistance (Rct) of Sep/CNT/S and Sep/CNT/S@PANI-I, II and III composites are 89.8, 76.3, 39.4 and 72.8 Ω respectively. The decrease of impedance may be due to the formation of some conducting interfacial layer, favoring the electrochemical stability of the system and resulting in the reduction of charge transfer resistance as the number of cycles increases.45–47 The Sep/CNT/S@PANI-II cathode shows the smallest charge transfer resistance after 300 cycles, which is consistent with the results in Fig. 5. However, the Sep/CNT/S@PANI-II and III composites appear another semicircle in medium frequency region, that is similar with the electrochemical impedance spectra (EIS) in the reported literature,48 which is probably owing to the formation of a passive layer on the PANI interface.49 Based on the equivalent circuit models in the insets of Fig. 7(c) and (d), the electrode resistance data is shown in Table S2 as the ESI.†
By synthetically analyzing the results of Fig. 5, 6 and 7, it is obtained that appropriate PANI coating has pinning effect for sulfur, which can enhance the utilization of the active mass and improve the cycling stability and the coulombic efficiency of the composites at different current rates.
4. Conclusions
In this work, the Sep/CNT/S@PANI-I, II and III composites material were obtained by using mechanical mixing, vacuum heat treatment and chemical oxidation methods in turn. Owing to the stable network structure of Sep/CNT/S composites with PANI coating, the cycling performance of the Sep/CNT/S composites with PANI coating are better than that of Sep/CNT/S composite at 2C rate, and Sep/CNT/S@PANI-II cathode shows the best capacity, cyclic and rate performance, with first specific capacity about 1100 mA h g−1 at 2C current density, and keeping about 650 mA h g−1 after 300 cycles. With optimized surface modification of PANI coating, the initial capacity increased 37.5% and the corresponding coulombic efficiency at 2C discharge rate increased from 85% to 93% compared with the composite without PANI coating. The sepiolite and sulfur raw materials are abundant and inexpensive, and the manufacturing process is simple, making the system promising for the commercialization of lithium–sulfur batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the support from the National Key Research and Development Program of China (2016YFA0201001), National Natural Science Foundation of China (11627801 and 11472236), the National Training Program of Innovation and Entrepreneurship for Undergraduates (201510530004) and Engineering Research Center of Nano-Geo Materials of Ministry of Education of China University of Geosciences (NGM2018KF008).References
- Y. Fu, A. Manthiram and Y. S. Su, Acc. Chem. Res., 2013, 46, 1125 CrossRef PubMed.
- J. Kim, D. J. Lee, H. G. Jung, Y. K. Sun, J. Hassoun and B. Scrosati, Adv. Funct. Mater., 2013, 23, 1076 CrossRef.
- M. A. Pope and I. A. Aksay, Adv. Energy Mater., 2015, 5, 1500124 CrossRef.
- X. Fang and H. Peng, Small, 2015, 11, 1488 CrossRef PubMed.
- Y. Sun, Z. W. Seh, Q. Zhang and Y. Cui, Chem. Soc. Rev., 2016, 45, 5605 RSC.
- S. Zheng, Y. Wen, Y. Zhu, Z. Han, J. Wang, J. Yang and C. Wang, Adv. Energy Mater., 2014, 4, 1400482 CrossRef.
- C. Zu, L. Li, J. Guo, S. Wang, D. Fan and A. Manthiram, J. Phys. Chem. Lett., 2016, 7, 1392 CrossRef PubMed.
- W. Hua, Z. Yang, H. Nie, Z. Li, J. Yang, Z. Guo, C. Ruan, X. Chen and S. Huang, ACS Nano, 2017, 11, 2209 CrossRef PubMed.
- N. Xu, T. Qian, X. Liu, J. Liu, Y. Chen and C. Yan, Nano Lett., 2017, 17, 538 CrossRef PubMed.
- A. F. Hofmann, D. N. Fronczek and W. G. Bessler, J. Power Sources, 2014, 259, 300 CrossRef.
- H. Al Salem, G. Babu, C. V. Rao and L. M. Arava, J. Am. Chem. Soc., 2015, 137, 11542 CrossRef PubMed.
- J. Bruckner, S. Thieme, A. Meier, I. Bauer, K. Gruber, J. Kaspar, A. Helmer, H. Althues, M. Schmuck and S. Kaskel, J. Mater. Chem. A, 2015, 3, 3808 Search PubMed.
- Y. Qu, Z. Zhang, X. Zhang, G. Ren, X. Wang, Y. Lai, Y. Liu and J. Li, Electrochim. Acta, 2014, 137, 439 CrossRef.
- F. Wu, A. Magasinski and G. Yushin, J. Mater. Chem. A, 2014, 2, 6064 Search PubMed.
- S. Jiang, Z. Zhang, X. Wang, Y. Qu, Y. Lai and J. Li, RSC Adv., 2013, 3, 16318 RSC.
- S. Lu, Y. Chen, X. Wu, Z. Wang, L. Lv, W. Qin and L. Jiang, RSC Adv., 2014, 4, 18052 RSC.
- J. Liu, B. Liu, C. Wang, Z. Huang, L. Hu, X. Ke, L. Liu, Z. Shi and Z. Guo, J. Alloys Compd., 2017, 718, 373 CrossRef.
- M. Wang, W. Wang, A. Wang, K. Yuan, L. Miao, X. Zhang, Y. Huang, Z. Yu and J. Qiu, Chem. Commun., 2013, 49, 10263 Search PubMed.
- Y. Li, L. Yuan, Z. Li, Y. Qi, C. Wu, J. Liu and Y. Huang, RSC Adv., 2015, 5, 44160 RSC.
- J. Rao, R. Xu, T. Zhou, D. Zhang and C. Zhang, J. Alloys Compd., 2017, 728, 376 CrossRef.
- X. Tao, J. Wang, C. Liu, H. Wang, H. Yao, G. Zheng, Z. W. Seh, Q. Cai, W. Li, G. Zhou, C. Zu and Y. Cui, Nat. Commun., 2016, 7, 11203 CrossRef PubMed.
- Q. Li, C. Zhou, Z. Ji, B. Han, L. Feng and J. Wu, J. Mater. Chem. A, 2015, 3, 7241 Search PubMed.
- Y. Zhang, K. Li, H. Li, Y. Wang, Y. Peng, S. Lin, B. J. Hwang and J. Zhao, J. Alloys Compd., 2017, 729, 331 CrossRef.
- N. W. Li, Y. X. Yin and Y. G. Guo, RSC Adv., 2016, 6, 617 Search PubMed.
- J. Wang, K. Yue, X. Zhu, K. L. Wang and L. Duan, PCCP Phys. Chem. Chem. Phys., 2016, 18, 261 RSC.
- J. H. Kim, K. Fu, J. Choi, K. Kil, J. Kim, X. Han, L. Hu and U. Paik, Sci. Rep., 2015, 5, 8946 CrossRef PubMed.
- G. Ma, Z. Wen, J. Jin, Y. Lu, K. Rui, X. Wu, M. Wu and J. Zhang, J. Power Sources, 2014, 254, 353 CrossRef.
- N. Nakamura, T. Yokoshima, H. Nara, T. Momma and T. Osaka, J. Power Sources, 2015, 274, 1263 CrossRef.
- Y. Su, F. Li and J. Zhao, PCCP Phys. Chem. Chem. Phys., 2016, 18, 25241 RSC.
- J. Pan, C. Wu, J. Cheng, Y. Pan, Z. Ma, S. Xie and J. Li, J. Power Sources, 2015, 293, 527 CrossRef.
- X. H. Huang, J. P. Tu, X. H. Xia, X. L. Wang and J. Y. Xiang, Electrochem. Commun., 2008, 10, 1288 CrossRef.
- Y. An, P. Wei, M. Fan, D. Chen, H. Chen, Q. Ju, G. Tian and K. Shu, Appl. Surf. Sci., 2016, 375, 215 CrossRef.
- X. Liang, Y. Xu, G. Sun, L. Wang, Y. Sun, S. Yang and Q. Xu, Chem. Eng. J., 2011, 174, 436 CrossRef.
- L. Xiao, Y. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176 CrossRef PubMed.
- D. F. Acevedo, C. R. Rivarola, M. C. Miras and C. A. Barbero, Electrochim. Acta, 2011, 56, 3468 CrossRef.
- M. Trchová, I. Šeděnková, E. Tobolková and J. Stejskal, Polym. Degrad. Stab., 2004, 86, 179 CrossRef.
- S. Sathiyanarayanan, S. S. Azim and G. Venkatachari, Electrochim. Acta, 2007, 52, 2068 CrossRef.
- L. Jin, X. Huang, G. Zeng, H. Wu and M. Morbidelli, Sci. Rep., 2016, 6, 32800 CrossRef PubMed.
- L. G. J. E. P. Barrett and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef.
- S. E. Cheon, K. S. Ko, J. H. Cho, S. W. Kim, E. Y. Chin and H. T. Kim, J. Electrochem. Soc., 2003, 150, A796 CrossRef.
- X. Ji and L. F. Nazar, J. Mater. Chem., 2010, 20, 9821 RSC.
- Z. Ma, S. Dou, A. Shen, L. Tao, L. Dai and S. Wang, Angew. Chem., Int. Ed. Engl., 2015, 54, 1888 CrossRef PubMed.
- A. G. H. Yamin, J. Penciner, Y. Sternberg and E. Peled, J. Electrochem. Soc., 1988, 135, 1045 CrossRef.
- W. L. Y. Mi, Q. Wang, J. Jiang, G. W. Brudvig, H. Zhou and H. Wang, J. Mater. Chem. A, 2017, 5, 23 Search PubMed.
- G. C. Li, G. R. Li, S. H. Ye and X. P. Gao, Adv. Energy Mater., 2012, 2, 1238 CrossRef.
- Z. Deng, Z. Zhang, Y. Lai, J. Liu, J. Li and Y. Liu, J. Electrochem. Soc., 2013, 160, A553 CrossRef.
- K. Xie, W. Wei, H. Yu, M. Deng, S. Ke, X. Zeng, Z. Li, C. Shen, J. g. Wang and B. Wei, RSC Adv., 2016, 6, 35746 RSC.
- Z. Zhang, L. L. Kong, S. Liu, G. R. Li and X. P. Gao, Adv. Eng. Mater., 2017, 7, 1602543 Search PubMed.
- X. Zhao, H. J. Ahn, K. W. Kim, K. K. Cho and J. H. Ahn, J. Phys. Chem. C, 2015, 119, 7996 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01925h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |