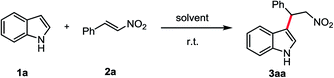Open Access Article
Open Access ArticleFriedel–Crafts alkylation reaction with fluorinated alcohols as hydrogen-bond donors and solvents†
Ren-Jin Tang,
Thierry Milcent and
Benoit Crousse *
*
Faculty of Pharmacy, UMR 8076, BioCIS, Univ. Paris-Sud, CNRS, Université Paris-Saclay, 92290, Châtenay-Malabry, France. E-mail: benoit.crousse@u-psud.fr
First published on 13th March 2018
Abstract
An effective and clean FC alkylation of indoles and electron-rich arenes with β-nitroalkenes in HFIP was reported. The desired products are formed rapidly in excellent yields under mild conditions without the need for any additional catalysts or reagents. Further, this methodology can be applied to one-pot synthesis of biologically active tryptamine derivatives.
Friedel–Crafts (FC) alkylation is one of the most important methods for C–C bond formation in organic chemistry.1 The FC alkylation between indoles and β-nitroalkenes is of particular interest due to the versatility by which the nitro group can be transformed into other functional groups.2 Numerous researchers have developed new conditions and effective catalysts in the past decades.3 Among them, H-bond donor (HBD) catalysts have become a focus of an increasing amount of research.4 Significant advances have been made in the area of homogeneous HBD catalysts by using urea or thiourea,4a 2-aminopyridinium ions4b boronate ureas,4e and silanediol.4d,4f However, the strong hydrogen-bonding nature of the HBD catalysts drives a detrimental self-association or aggregation, thus reducing or enhancing catalytic activity.5
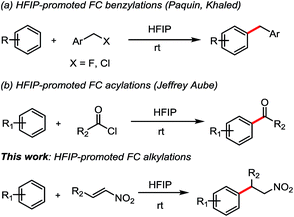
Trifluoroethanol (TFE) and hexafluoro-2-propanol (HFIP), exhibit unique features in organic synthesis. Indeed, the presence of the strong electron-withdrawing trifluoromethyl group influences several key parameters: ionizing power, Brønsted acidity, and hydrogen-bond donation HBD (or H-bond acidity) are increased, whereas nucleophilicity and hydrogen-bond acceptance HBA (or H-bond basicity) are significantly depleted.6 Furthermore, the HBD was strongly connected to the conformation along the C–O bond. Moreover, it was suggested that aggregation of HFIP as dimers or trimers could also enhance this property.7 Few FC alkylations have been studied solely in HFIP in last years. In 2010, Qu and co-workers described the intra- and intermolecular FC alkylations of electron-rich arenes with epoxides promoted by fluorinated alcohols.8 Recently, FC alkylations of arenes with benzyl halides in HFIP have been developed by Paquin et al.9 and Khaled et al. (Scheme 1a). More recently, HFIP promoting intramolecular and intermolecular Friedel–Crafts acylation have been reported by Jeffrey Aube and co-workers (Scheme 1b).10
In our interest for the use of fluorous medium in organic synthesis,11 and in the pursuit of more efficient catalytic systems, we found that fluorinated alcohols promoted the FC alkylation between indoles and β-nitroalkenes.
The FC alkylation between indole (1a) and β-nitroalkene (2a) was used as model system to optimize the reaction conditions (Table 1). We first examined the reaction in pure HFIP solvent and the reaction mixture was stirred at r.t. for 2 h. The product 3aa was isolated in 96% yield (entry 1). When HFIP was used as additive with other solvents (entries 2–7), good to moderate yields of 3aa were obtained. In DCM, the addition of HFIP from 2.0 to 9.5 equiv. (entries 4–6) decreased product yield and reaction rate. Good results were obtained when added 9.5 equiv. HFIP in water and toluene (entries 5 and 6). With THF the reaction was completely unfavourable (entry 7), probably because of the hydrogen bond between HFIP and THF which inhibits the reaction6b With other fluorinated alcohols, perfluoro-2-methylpropan-2-ol (PFTB) was more efficient than the trifluoroethanol (TFE) (entries 10 and 11). In contrast, in EtOH, only traces of product were observed (entry 12). When performed the reaction in the dark had no effect on the yield (entry 13), which suggested HFIP promoted FC alkylation is not a photochemical pathway.12
| Entry | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Conditions: 1a (0.6 mmol, 1.2 equiv.) and 2a (0.5 mmol) was dissolved in solvent (0.25 M) and stirred at r.t. for a specified period unless otherwise noted.b NMR yields using mesitylene as internal standard.c Isolated yield in parentheses.d The reaction was performed in the dark. | |||
| 1 | HFIP | 2 | 99 (96)c |
| 2 | DCM/HFIP (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4 | 97 |
| 3 | DCM/HFIP (6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
16 | 89 |
| 4 | DCM/HFIP (17.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 45 |
| 5 | H2O/HFIP (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 93 |
| 6 | Toluene/HFIP (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 85 |
| 7 | THF/HFIP (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 24 |
| 8 | (CF3)3COH (PFTB) | 0.75 | 99 |
| 9 | CF3CH2OH (TFE) | 8 | 90 |
| 10 | EtOH | 16 | Trace |
| 11d | HFIP | 2 | 99 |
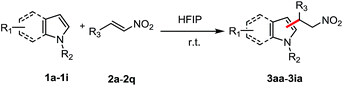
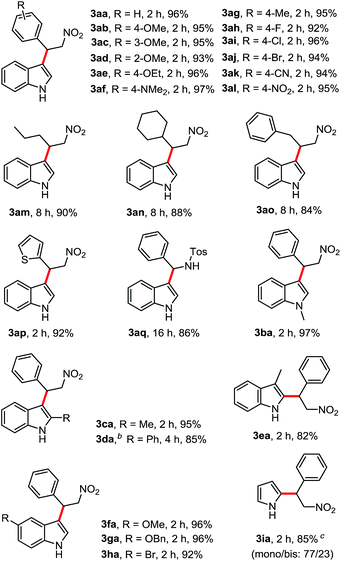
Under the optimized conditions (Table 1, entry 1), the substrate scope and limitation for this FC alkylation between various indoles and β-nitroalkenes were explored. As shown by the results in Table 2, all the aryl-β-nitroalkene analogs tested (2a–2q) reacted with indoles (1a–1h) to produce the corresponding products in excellent yields in HFIP, no matter the presence of substituents with electron withdrawing or electron donating groups in various positions. A variety of functional groups such as methoxy (3ab–3ad), ethoxy (3ae), N,N-dimethylamino (3af), halides (3ah–3aj), nitrile (3ak) and nitro (3al) were tolerated. The aliphatic nitroalkenes were successfully transformed into the desired products (3am–3ao) in good yields after 8 h under the mild conditions. Trans-2-(2-nitrovinyl)-thiophene was also applicable in this reaction providing the product 3ap in 92% yield. Less reactive substrate N-tosyl imine showed good reactivity, giving 3aq in 86% yield after 16 h. This study expands upon our scope of imines with similarly satisfying results. Then various indoles and pyrrole with aryl β-nitroalkene were tested. Reactions of functionalized indoles (1b–h) reacted with β-nitroalkene smoothly in HFIP to lead to the corresponding products in excellent yields. Treatment of the pyrrole (1i) with β-nitroalkene afforded a mixture of mono- and bis-substituted products in 77/23 ratio in 85% yield.
After development of FC alkylation of indoles and pyrrole, we focused on the FC alkylation of arene derivatives. To the best of our knowledge, there are few reports on the FC alkylation of arenes with β-nitroalkene in the literature.3f,13
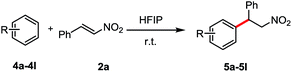
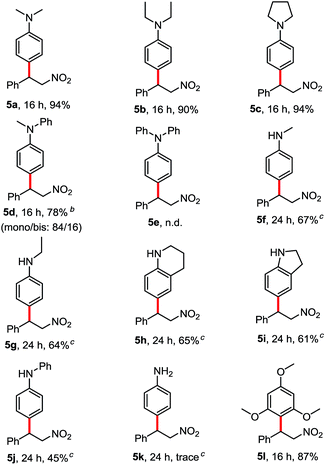
We tested N,N-dimethyl aniline with β-nitroalkene 2a in HFIP at room temperature. To our delight, the corresponding product 5a was obtained after 16 h in 94% yield. Other tertiary anilines such as N,N-diethyl aniline (4b), N-phenylpyrrolidine (4c) and N-methyl-N-phenylaniline (4d) were also efficient in these conditions to afford regioselectively only the para-alkylated products. Triphenylamine (4e) showed no reactivity under the same conditions, probably because of the lower electron density at the phenyl ring. Interestingly, the reaction also worked with secondary anilines (4f–4j) in a moderate yield without the presence of the aza Michael product. In contrast to the primary aniline (4k), the major product was the aza-Michael adduct. Besides, the electron-rich arene, 1,3,5-trimethoxybenzene (4l) reacted with β-nitroalkene to afford the corresponding alkylated product in good yield (87%) (Table 3).
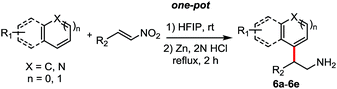
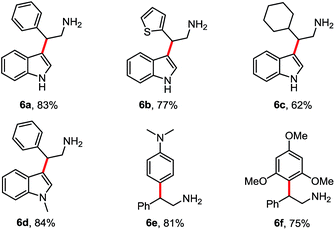
Then following to these effective and mild conditions, we investigated the one-pot combination FC alkylation/NO2 reduction. Interestingly, the one-pot synthesis of tryptamines has not been reported previously. In preliminary experiments after completion of the first step, stoichiometric amounts of zinc powder or granular were added. Unfortunately, despite the slight acidity of HFIP, the reduction of the nitro group was not observed at room temperature and at reflux. On the other hand, when HCl (2 N) was added, the corresponding tryptamine was formed successfully after 2 h in reflux. Thus, different reactions have been performed and reported in Table 4. Indoles and benzene, thiophene and cyclohexane β-nitroalkenes were tolerated (6a–d). Additionally, reaction with electron-rich arenes such as N,N-dimethylaniline, 1,3,5-trimethoxybenzene succeed to lead to the corresponding products 6e–f in good yields.
| a Conditions: 0.6 mmol of indoles or arenes (1.2 equiv.), 0.5 mmol of β-nitroalkenes in 2 mL HFIP for the first step 2.0 mmol of Zn (4.0 equiv.) and 2.5 mL 2 N HCl (10 equiv.) were added for the second step. Isolated yields. |
|---|
 |
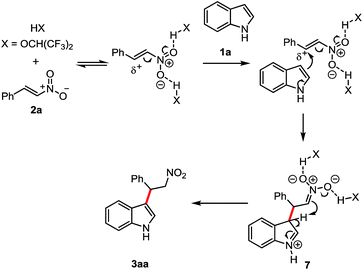
Based on the literature6e,10,14 and our studies,11a,15 a plausible H-bond activation mechanism was proposed in Scheme 2. First HFIP activated the nitro group through hydrogen bond and following by the nucleophilic attack of the indole. The generated intermediate 7 underwent to the subsequent hydrogen transfer to afford the expected product 3aa.
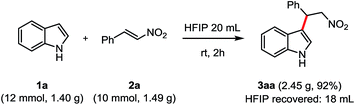
To further explore the synthetic utility of this system, a gram-scale reaction was also evaluated (Scheme 3). The FC alkylation of 1a (12 mmol, 1.40 g) with 2a (10.0 mmol, 1.49 g) was performed in 20 mL HFIP for 2 h. The desired product 3aa was obtained in 92% yield (2.45 g) and 18 mL of HFIP was recovered after distillation directly from the reaction.
Conclusions
In conclusion, we have developed an effective and clean FC alkylation between β-nitroalkenes and arenes in HFIP. The desired products formed smoothly in short times at ambient temperature without any additional catalysts or reagents. Further, this methodology can be applied to one-pot synthesis of biologically active tryptamine derivatives. Besides, because of low boiling point (b.p. = 59 °C) and low viscosity, HFIP can be easy recovered and reused.Experimental section
A general experimental procedure is described as follows:To a stirred solution of indole 1a (70.2 mg, 0.6 mmol, 1.2 equiv.) in HFIP (2 mL) was added β-nitroalkene 2a (74.5 mg, 0.5 mmol, 1.0 equiv.) under air. The reaction mixture was stirred at room temperature for 2 h. After, the reaction mixture was evaporated under reduced pressure and the crude product was purified by column chromatography on silica gel using cyclohexane/ethyl acetate (10/1) as the eluent afforded a white solid 3aa (128 mg, 96% yield).
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Central Glass Co. Ltd. is gratefully acknowledged for kindly providing HFIP. R.-J. T. thanks the China Scholarship Council for a doctoral fellowship.References
- (a) C. Friedel and J. M. Crafts, J. Chem. Soc., 1877, 32, 725 RSC; (b) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS PubMed; (c) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (d) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (e) V. Terrasson, R. Marcia de Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635 CrossRef CAS; (f) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 6 Search PubMed; (g) R. R. Naredla and D. A. Klumpp, Chem. Rev., 2013, 113, 6905 CrossRef CAS PubMed.
- S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108 CrossRef CAS PubMed.
- (a) I. Komoto and S. Kobayashi, Org. Lett., 2002, 4, 1115 CrossRef CAS PubMed; (b) Z.-P. Zhan, R.-F. Yang and K. Lang, Tetrahedron Lett., 2005, 46, 3859 CrossRef CAS; (c) H. Firouzabadi, N. Iranpoor and F. Nowrouzi, Chem. Commun., 2005, 789 RSC; (d) R. Ballini, R. R. Clemente, A. Palmieri and M. Petrini, Adv. Synth. Catal., 2006, 348, 191 CrossRef CAS; (e) L.-T. An, J.-P. Zou, L.-L. Zhang and Y. Zhang, Tetrahedron Lett., 2007, 48, 4297 CrossRef CAS; (f) N. T. Tran, S. O. Wilson and A. K. Franz, org. lett., 2012, 14, 186 CrossRef CAS PubMed; (g) P. M. Habib, V. Kavala, C.-W. Kuo and C.-F. Yao, Tetrahedron Lett., 2008, 49, 7005 CrossRef CAS; (h) L. Liang, Q. Liu, J. Zhang, F. Wang and Y. Yuan, Res. Chem. Intermed., 2012, 39, 1957 CrossRef; (i) M. De Rosa and A. Soriente, Tetrahedron, 2010, 66, 2981 CrossRef CAS.
- (a) A. Ricci, R. P. Herrera and G. Dessole, Synlett, 2004, 2004, 2374 Search PubMed; (b) N. Takenaka, R. S. Sarangthem and S. K. Seerla, org. Lett., 2007, 9, 2819 CrossRef CAS PubMed; (c) J. Cai, P. Wu and Y. Wan, Synlett, 2008, 2008, 1193 CrossRef; (d) A. G. Shafer, J. M. Wieting and A. E. Mattson, Org. Lett., 2011, 13, 5228 CrossRef PubMed; (e) S. S. So, J. A. Burkett and A. E. Mattson, Org. Lett., 2011, 13, 716 CrossRef CAS PubMed; (f) N. T. Tran, S. O. Wilson and A. K. Franz, org. Lett., 2012, 14, 186 CrossRef CAS PubMed.
- (a) M. C. Etter, Z. Urbakzyk-Lipkowska, S. M. Zia-Ebrahimi and T. W. Panunto, J. Am. Chem. Soc., 1990, 112, 8415 CrossRef CAS; (b) C. M. McGuirk, C. L. Stern and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 4689 CrossRef CAS PubMed.
- (a) C. Reichardt, Chem. Rev., 1994, 94, 231 CrossRef; (b) A. Berkessel, J. A. Adrio, D. H. ttenhain and J. M. Neudo, J. Am. Chem. Soc., 2006, 128, 8421 CrossRef CAS PubMed; (c) A. Börner, I. Shuklov and N. Dubrovina, Synthesis, 2007, 2007, 2925 CrossRef; (d) C. Laurence, J. Legros, P. Nicolet, D. Vuluga, A. Chantzis and D. Jacquemin, J. Phys. Chem. B, 2014, 118, 7594 CrossRef CAS PubMed; (e) S. Gennen, M. Alves, R. Mereau, T. Tassaing, B. Gilbert, C. Detrembleur, C. Jerome and B. Grignard, ChemSusChem, 2015, 8, 1845 CrossRef CAS PubMed; (f) S. Khaksar, J. Fluorine Chem., 2015, 172, 51 CrossRef CAS; (g) C. Laurence, J. Legros, A. Chantzis, A. Planchat and D. Jacquemin, J. Phys. Chem. B, 2015, 119, 3174 CrossRef CAS PubMed.
- (a) A. Berkessel, J. A. Adrio, D. Huttenhain and J. M. Neudorfl, J. Am. Chem. Soc., 2006, 128, 8421 CrossRef CAS PubMed; (b) J. Mars, M. Mezger, A. Wiebe, S. R. Waldvogel and B. Kirchner, ACS Catal., 2017, 7, 1846 CrossRef.
- G. X. Li and J. Qu, Chem. Commun., 2010, 46, 2653 RSC.
- (a) P. A. Champagne, Y. Benhassine, J. Desroches and J. F. Paquin, Angew Chem Int Ed Engl, 2014, 53, 13835 CrossRef CAS PubMed; (b) N. Weisner and M. G. Khaledi, Green Chem., 2016, 18, 681 RSC.
- (a) H. F. Motiwala, R. H. Vekariya and J. Aube, Org. Lett., 2015, 17, 5484 CrossRef CAS PubMed; (b) R. H. Vekariya and J. Aube, Org. Lett., 2016, 18, 3534 CrossRef CAS PubMed.
- (a) K. De, J. Legros, B. Crousse and D. Bonnet-Delpon, J. Org. Chem., 2009, 74, 6260 CrossRef CAS PubMed; (b) C. Venkateswarlu, P. V. Balaji, K. De, B. Crousse, B. Figadère and J. Legros, J. Fluorine Chem., 2013, 152, 94 CrossRef CAS; (c) A. Fedotova, B. Crousse, I. Chataigner, J. Maddaluno, A. Y. Rulev and J. Legros, J. Org. Chem., 2015, 80, 10375 CrossRef CAS PubMed.
- S. Yadav, M. Srivastava, P. Rai, B. P. Tripathi, A. Mishra, J. Singh and J. Singh, New J. Chem., 2016, 40, 9694 RSC.
- (a) N. Takenaka, R. S. Sarangthem and S. K. Seerla, org. lett., 2007, 9, 2819 CrossRef CAS PubMed; (b) A. Z. Halimehjani, M. V. Farvardin, H. P. Zanussi, M. A. Ranjbari and M. Fattahi, J. Mol. Catal. A: Chem., 2014, 381, 21 CrossRef CAS; (c) X.-W. Dong, T. Liu, Y.-Z. Hu, X.-Y. Liu and C.-M. Che, Chem. Commun., 2013, 49, 7681 RSC.
- M. O. Ratnikov, V. V. Tumanov and W. A. Smit, Angew. Chem., Int. Ed., 2008, 47, 9739 CrossRef CAS PubMed.
- (a) K. De, J. Legros, B. Crousse and D. Bonnet-Delpon, Tetrahedron, 2008, 64, 10497 CrossRef CAS; (b) A. Di Salvo, M. David, B. Crousse and D. Bonnet-Delpon, Adv. Synth. Catal., 2006, 348, 118 CrossRef CAS; (c) R.-J. Tang, T. Milcent and B. Crousse, Eur. J. Org. Chem., 2017, 4753 CrossRef CAS; (d) R.-J. Tang, T. Milcent and B. Crousse, J. Org. Chem., 2018, 83, 930 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01397g |
| This journal is © The Royal Society of Chemistry 2018 |