Open Access Article
Open Access ArticleRetracted Article: Resveratrol attenuates inflammation and reduces matrix-metalloprotease expression by inducing autophagy via suppressing the Wnt/β-catenin signaling pathway in IL-1β-induced osteoarthritis chondrocytes
Ci Li,
Wenliang Wu,
Guangjun Jiao,
Yunzhen Chen and
Haichun Liu *
*
Department of Orthopedics, Qilu Hospital of Shandong University, 107# Wenhua West Road, Jinan 250012, China. E-mail: liuhaichunsd@163.com; Tel: +86-531-82166531
First published on 4th June 2018
Abstract
Resveratrol (Res), a naturally occurring polyphenolic compound, has been reported to exert many biological effects like anti-inflammatory and anti-oxidant effects. In this study, we investigated the role of Res on IL-1β-induced osteoarthritis (OA) chondrocytes and its possible mechanism. Results demonstrated that Res suppressed IL-1β-induced IL-1, IL-6 and TNF-α production in a dose-dependent manner. Res also decreased MMP-1, MMP-3 and MMP-13 production in IL-1β-induced OA chondrocytes. These results suggested that Res suppressed IL-1β-induced inflammation and matrix-metalloproteases (MMP) expression in OA chondrocytes. In addition, Res was found to reverse the decreased autophagy level through increasing the expression of Beclin1, LC3 II/I ratio and LC3+ puncta in IL-1β-induced OA chondrocytes. Inhibition of autophagy by 3-methyladenine (3-MA) abolished the inhibitory effect of Res on inflammation and MMP expression in IL-1β-induced OA chondrocytes. Moreover, the Wnt/β-catenin signaling pathway was activated in IL-1β-induced OA chondrocytes. However, Res was found to suppress this activated Wnt/β-catenin signaling pathway. Activation of the Wnt/β-catenin signaling pathway counteracted the promoted effect on autophagy and inhibitory effect on inflammation and MMP expression of Res in IL-1β-induced OA chondrocytes. Taken together, our data demonstrated that Res attenuated inflammation and reduced MMP expression through inducing autophagy via inhibiting the Wnt/β-catenin signaling pathway in IL-1β-induced OA chondrocytes. Res may be used as a potential therapeutic agent for OA treatment.
Introduction
Osteoarthritis (OA) is a complex progressively degenerative joint disorder characterized by degradation and destruction of cartilage matrix and inflammatory responses in chondrocytes.1,2 Although a great number of risk factors of OA including obesity, bone mass, joint injury and deformity have been identified, the pathophysiology of OA has still remained largely unknown.3 Accumulating evidence indicated that inflammation played a vital role in the development of OA.4,5 IL-1β is an important inflammatory cytokine which is involved in the pathologic process of OA.6 IL-1β could induce the production of matrix metalloproteinases (MMPs) and inflammatory mediator PGE2 and NO production in chondrocytes which lead to the clinical manifestations of OA.7 Csaki's study reported that IL-1β could induce the activation of the NF-κB signaling pathway, thus leading to the release of inflammatory mediators and apoptosis in human articular chondrocytes.8 These inflammatory mediator, including MMP and nitric oxide (NO), lead to articular cartilage damage.9 Recently, non-steroidal anti-inflammatory drugs (NSAIDs) are often used for the treatment of OA. However, these medications often relieve OA symptoms but also possess serious side effects.10 Therefore, the development of effective and safe drugs to treat OA is urgently needed.Resveratrol (3,4′,5-trihydroxystilbene (Res)) is a polyphenolic compound commonly identified in grape skin, berries and peanuts and exerts a variety of biological effects such as anti-oxidation, anti-proliferation and anti-inflammation effects.11 Res is often used for the treatment of skin inflammations, cardiovascular and liver diseases.12,13 Res has also been reported to have a positive effect against OA. Wei demonstrated that Res ameliorated inflammatory damage and protects against OA in a rat model of OA via NF-κB and HO-1/Nrf-2 signaling.14 However, to date, the effect and mechanism of Res on the IL-1β-induced OA remains unclear.
Autophagy is a conserved catabolic process which mediates the degradation of damaged proteins and dysfunctional organelles for energy recycling to maintain homeostasis under stress.15 Autophagy is closely associated with apoptosis in the pathogenesis of many degenerative diseases. Decreased autophagy is often observed in OA and activation of autophagy may reduce the severity of OA.16,17
In our present study, we investigated the role of Res and its underlying mechanism on the treatment of IL-1β-induced OA. We observed that Res induced autophagy via suppressing Wnt/β-catenin signaling pathway to ameliorate inflammation and reduce MMP expression, thus leading to the attenuation of OA.
Materials and methods
Statement
The animal experiments in this study were approved by the Animal Care and Research Committee of Qilu Hospital of Shandong University. All experiments were performed in compliance with relevant laws and guidelines. Besides, all experiments were conducted following institutional guidelines of Qilu Hospital of Shandong University.Chondrocyte isolation, culture and treatment
Chondrocytes were isolated from the knee joints of 5-day-old Wistar rats by enzymatic digestion as described before.18 Briefly, the cartilages were removed, minced and then digested with 0.2% type II collagenase (Invitrogen) in DMEM with high glucose medium (Gibco, Rockville, MD) for 5 h at 37 °C with shaking to harvest individual cells. Primary chondrocytes were added into T75 flasks (Greiner Bio-One) that contained DMEM/F-12 with 10% fetal bovine serum (Hyclone) at 37 °C with 5% CO2. Chondrocytes were treated with 10 ng mL−1 IL-1β (Sigma Chemical Co. MO, USA) for 24 h to mimic OA in vitro and used between passage 0–2. Chondrocytes were then treated with different concentrations of Res (Sigma Chemical Co. MO, USA).Cell viability assay
MTT assay was used to detect cell viability. The cells were seeded at a density of 5 × 103 cells per mL into 96-well plates. After treated with different concentrations of Res (0, 5, 10, 20, 30 μM), 10 μL MTT (Beyotime Institute of Biotechnology, Haimen, China) was added and incubated in 37 °C for 4 h. The solution containing MTT was removed and cells were mixed with 150 μL dimethyl sulfoxide for 10 min. The plate was read spectrophotometrically at a wavelength of 560 nm by a Bio-Rad model 550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to detect cell viability.Enzyme-linked immunosorbent assay (ELISA)
The levels of MMP1, MMP3, and MMP13 in cell culture supernatants were monitored by ELISA kits (R&D systems, Minneapolis, MN, USA) according to the manufacturer's instructions.Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's protocol. Same amount of proteins of each sample were separated by 10% SDS-PAGE gel and then transferred into PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk for 1 h at room temperature, the membrane was respectively incubated with the primary antibodies against MMP-1 (Abcam), MMP-3, MMP-13, Beclin1, LC3 I/II, β-catenin, T-cell factor 4 (TCF4), c-Myc, cyclin D1 and GAPDH (Cell Signaling Technology) overnight at 4 °C at a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000. After incubation with HRP-conjugated secondary antibodies for 1 h at room temperature, the signals were detected by ECL.
1000. After incubation with HRP-conjugated secondary antibodies for 1 h at room temperature, the signals were detected by ECL.
Fluorescence microscopy
Chondrocytes were transfected with GFP-LC3 plasmid by Lipofectamine 3000™ (Invitrogen) according to the manufacturer's protocol. After chondrocytes were treated IL-1β and Res, the number of puncta formation of GFP-LC3 was determined under fluorescent microscopy. Cells with more than 5 puncta counted were considered to have accumulated autophagosomes.Statistical analysis
Experiments were performed in triplicate on three independent occasions. All data are represented as the mean ± standard deviation (SD). The Student's t test was used to compare means of two groups and One-Way ANOVA was used for comparing means of multiple samples using SPSS version 17 (SPSS, Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.Results
Res suppresses inflammation and reduces MMP expression in IL-1β-induced OA chondrocytes
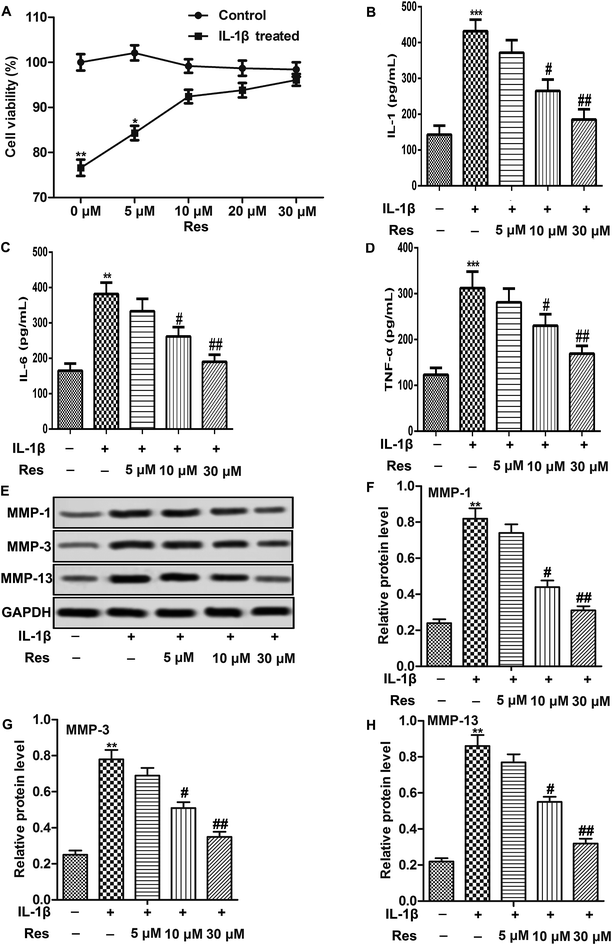
IL-1β was used to induce OA in chondrocytes in our study. We first explore the effect of IL-1β on the cell viability of chondrocytes. As shown in Fig. 1A, Res treatment didn't influence cell viability of IL-1β untreated cells significantly while increased cell viability of IL-1β treated chondrocytes in a dose-dependent manner (Fig. 1A, *P < 0.05, **P < 0.01). Results from ELISA showed that IL-1β increased the release of IL-1, IL-6 and TNF-α significantly in chondrocytes. However, Res treatment decreased IL-1β-induced IL-1, IL-6 and TNF-α production dose-dependently (Fig. 1B–D, ***P < 0.001, #P < 0.05, ##P < 0.01). In addition, results from western blot showed that Res could also suppressed IL-1β-induced MMP-1, MMP-3 and MMP-13 production remarkably in a dose-dependent manner (Fig. 1E–H, **P < 0.01, #P < 0.05, ##P < 0.01). These results suggested that Res suppressed IL-1β-induced inflammation and MMP expression in OA chondrocytes.Res promotes autophagy in IL-1β-induced OA chondrocytes
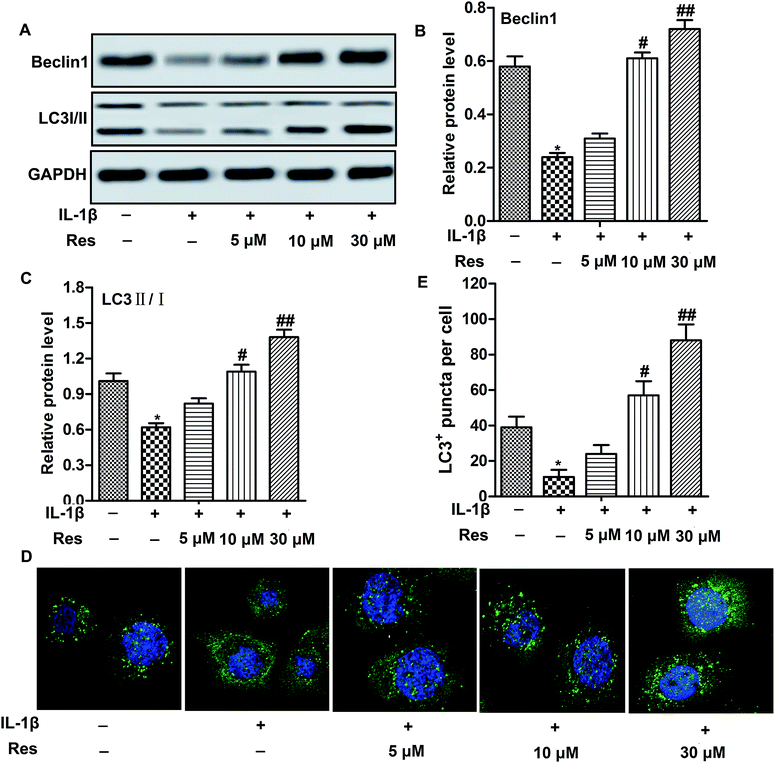
Decreased autophagy is often observed in OA.16 Similarly, we also observed that IL-1β decreased relative expression of autophagy-related proteins Beclin1 and the ratio of LC3 II/I. Moreover, GFP-LC3 puncta formation observed as punctate dots of green fluorescence was also significantly decreased in chondrocytes treated with IL-1β, indicating that autophagy was suppressed in IL-1β-induced OA chondrocytes. However, Res up-regulated the expression of Beclin1 and the ratio of LC3 II/I, as well as LC3+ puncta in cells compared with IL-1β group in a dose-dependent manner (Fig. 2A–E, *P < 0.05, #P < 0.05, ##P < 0.01). These results indicated that Res promoted autophagy in IL-1β-induced OA chondrocytes.Autophagy inhibitor abolishes the inhibitory effect of Res on inflammation and MMP expression in IL-1β-induced OA chondrocytes
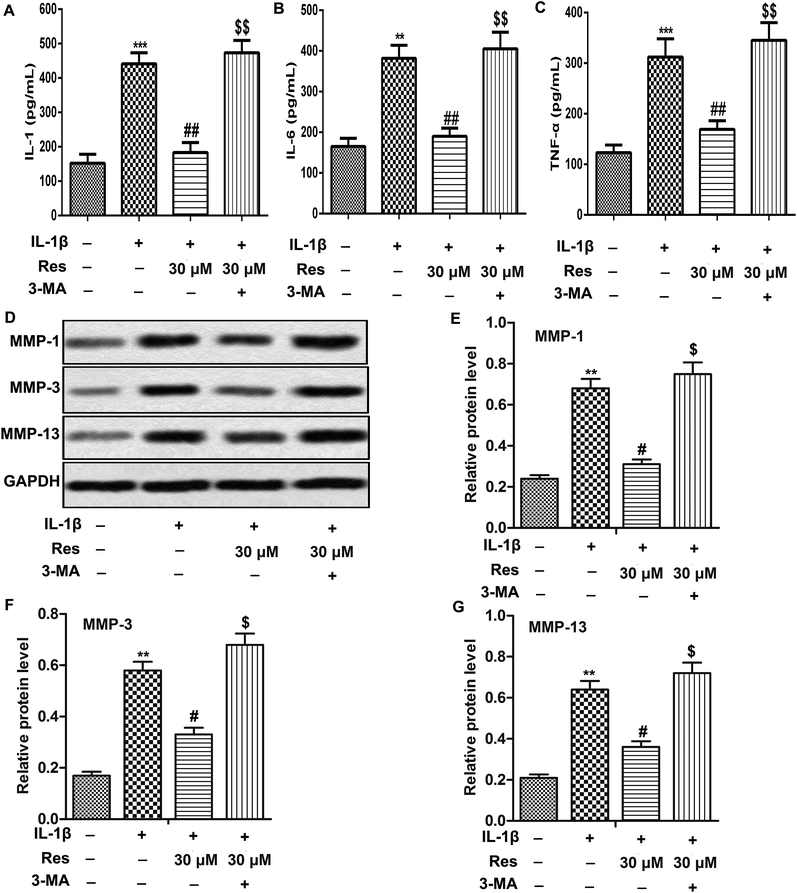
Our results above demonstrated that Res might inhibit inflammation and MMP expression through inducing autophagy in IL-1β-induced OA chondrocytes. To verify our hypothesis, 3-methyladenine (3-MA, a small molecule inhibitor of autophagy) was used to treat chondrocytes at the concentration of 10 mmol L−1 in our study. Our data showed that 3-MA treatment reversed the decreased release of IL-1, IL-6 and TNF-α in IL-1β + Res 30 μM group. Moreover, results from western blot also indicated that inhibition of autophagy by 3-MA down-regulated relative protein level of MMP-1, MMP-3 and MMP-13 compared with IL-1β + Res 30 μM group (Fig. 3A–G, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, $P < 0.05, $$P < 0.01). Our results demonstrated that inhibition of autophagy abolished the inhibitory effect of Res on inflammation and MMP expression in IL-1β-induced OA chondrocytes.Res induces autophagy through suppressing Wnt/β-catenin signaling pathway in IL-1β-induced OA chondrocytes
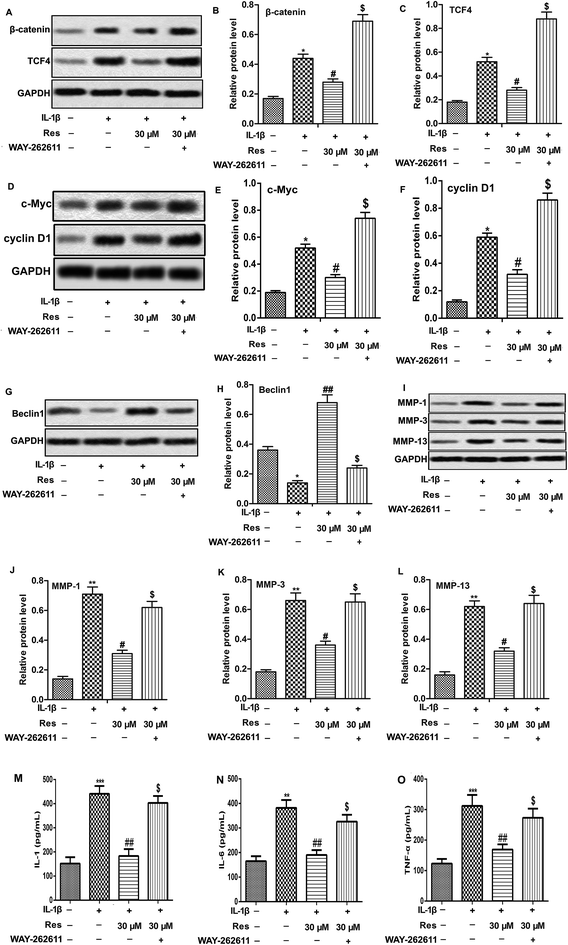
Activation of Wnt/β-catenin has been reported to be involved in OA aggravation.19 Here, we investigated the effect of Res in Wnt/β-catenin signaling pathway. The results showed that IL-1β treatment increased the expression of β-catenin and TCF4, as well as Wnt target genes c-Myc and cyclin D1. However, Res down-regulated IL-1β-induced β-catenin, TCF4, c-Myc and cyclin D1 production significantly, indicating that Res suppressed Wnt/β-catenin signaling pathway in IL-1β-induced OA chondrocytes. Wnt activator WAY-262611 (1 μM) was used to treat chondrocytes and it could increase the expression of β-catenin, TCF4, c-Myc and cyclin D1 markedly (Fig. 4A–F, *P < 0.05, #P < 0.05, $P < 0.05). Activation of Wnt/β-catenin signaling pathway by WAY-262611 decreased relative protein level of Beclin1 while increased the release of IL-1, IL-6, TNF-α and MMP-1, MMP-3 and MMP-13 expression compared with IL-1β + Res 30 μM group, suggesting that activation of Wnt/β-catenin signaling pathway counteracted the promoted effect on autophagy and inhibitory effect on inflammation and MMP expression of Res in IL-1β-induced OA chondrocytes (Fig. 4G–L and O, *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, $P < 0.05). Taken together, results above elucidated that Res suppressed inflammation and MMP expression through inducing autophagy via inhibiting of Wnt/β-catenin signaling pathway in IL-1β-induced OA chondrocytes.Discussion
Res is a natural phytoalexin and has been demonstrated to play an important role in OA chondrocyte metabolism, apoptosis and proliferation.20,21 In our present study, we investigated the role and the underlying mechanism of Res in IL-1β-induced OA chondrocytes. Our data revealed that Res suppressed inflammation and MMP expression to protect chondrocytes from IL-1β-induced OA in a dose-dependent manner through inducing autophagy via suppressing Wnt/β-catenin signaling pathway.A great number of studies demonstrated that inflammation played a vital role in the development of OA through releasing various inflammation cytokines.22 IL-6 and TNF-α were reported to activate macrophages, which subsequently synthesize pro-inflammatory chemokines to maintain inflammation in the development of OA.23 IL-1β is often used to induce OA in chondrocytes in many researches.24,25 Previous studies suggested that IL-1β contributed to cartilage matrix degradation through inducing the expression of MMPs and inflammatory mediators.26 MMPs are a family of 23 enzymes with a specific function to inhibit synthesis of type II collagen which is one of the primary extracellular matrix (ECM) macromolecules in cartilage.27 Abnormal expression of MMP members could lead to proteolysis and pathological cartilage breakdown in OA.28 Among these MMP members, MMP-1, MMP-3 and MMP-13 have been demonstrated to induce degradation of ECM in OA articular cartilage.29 Here, we also used IL-1β to induce OA in chondrocytes and observed that IL-1β inhibited cell viability and induced release of inflammation cytokines (IL-1, IL-6, TNF-α) and MMPs expression (MMP-1, MMP-3, MMP-13). However, Res treatment suppressed IL-1β-induced inflammation and MMP expression in OA chondrocytes in a dose-dependent manner, indicating that Res could attenuate development of OA.
The role of autophagy has gained much attention of researches in the past few years. At the early stage of OA, activation of autophagy can protect chondrocytes from limiting catabolic degradation.17 While in the late stage of OA, decreased autophagy is observed in chondrocytes, combined with the increase of apoptosis in chondrocytes.30 Tang's study observed that autophagy was blocked while apoptosis was increased in human OA.31 Autophagy is regulated by multiple genes. Among these genes, Beclin1 and LC3 are important regulator and markers of autophagy.32 Beclin1 can form a complex with type III phosphatidylinositol which allows nucleation of the autophagic vesicles. LC3-II which is converted from LC3-I can attach to the membrane of the autophagosome during the process of autophagy activation.33 Similarly with previous studies, we found decreased expression of Beclin1, LC3 II/I ratio and LC3+ puncta in IL-1β treated chondrocytes, suggesting that autophagy was blocked in IL-1β-induced OA chondrocytes. Res treatment reversed the blocked autophagy induced by IL-1β in OA chondrocytes in a dose-dependent manner. Our results suggested that Res might inhibit inflammation and MMP expression through activation of autophagy in IL-1β-induced OA chondrocytes. In order to verify our hypothesis, 3-MA, an inhibitor of autophagy was used in our present study. We observed that inhibition of autophagy counteracted the inhibitory effect of Res on inflammation and MMP expression. Results above demonstrated that Res attenuated inflammation and reduced MMP expression by inducing autophagy in IL-1β-induced OA chondrocytes.
Wnt signaling pathway is a conserved pathway which constitutes one of the most critical biological processes during cell fate assignment and homeostasis. Aberration in Wnt signaling pathway may be involved in the development of OA.34 Yuasa reported that Wnt/β-catenin was a powerful stimulator of chondrocyte matrix catabolic action and activation of Wnt/β-catenin signaling pathway might be involved in OA aggravation.19 Wnt/β-catenin signaling pathway is responsible for the activation of collagen X (COLX) which leads to the degradation of cartilage-specific ECM and chondrocyte hypertrophy. Wnt/β-catenin signaling pathway is also a powerful stimulator of chondrocyte matrix degradation.35 TCF4 is an important transcription factor involved in Wnt/β-catenin signaling pathway.36 Activation of Wnt signaling pathway is dependent on the abnormal accumulation of β-catenin in cytoplasm, which then binds with TCF in nucleus to form transcription factor complexes. These complexes are important to regulate the expression of various downstream genes such as c-Myc and cyclin D1.37 In our study, we found increased β-catenin, TCF4, c-Myc and cyclin D1 expression in IL-1β group, indicating that Wnt/β-catenin signaling pathway was activated in IL-1β-induced OA chondrocytes. In addition, Res was found to suppress activated Wnt/β-catenin signaling pathway in OA chondrocytes. Activation of Wnt/β-catenin signaling pathway counteracted the promoted effect on autophagy and inhibitory effect on inflammation and MMP expression of Res in IL-1β-induced OA chondrocytes.
Taken together, our data demonstrated that Res attenuated inflammation and reduced MMP expression through inducing autophagy via inhibiting of Wnt/β-catenin signaling pathway in IL-1β-induced OA chondrocytes. Res may be a potential therapeutic agent for OA treatment.
Conflicts of interest
None.Abbreviations
| Res | Resveratrol |
| OA | Osteoarthritis |
| MMP | Matrix-metalloproteases |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SD | Standard deviation |
| 3-MA | 3-Methyladenine |
| TCF4 | T-cell factor 4 |
| ECM | Extracellular matrix |
References
- C. Sanchez, M. A. Deberg, N. Piccardi, P. Msika, J. Y. Reginster and Y. E. Henrotin, Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes, Osteoarthr. Cartil, 2005, 13(11), 988–997 CrossRef PubMed.
- C. Sanchez, M. A. Deberg, A. Bellahcene, V. Castronovo, P. Msika, J. P. Delcour, J. M. Crielaard and Y. E. Henrotin, Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone, Arthritis Rheumatol., 2008, 58(2), 442–455 CrossRef PubMed.
- X. Zhou, W. Li, L. Jiang, J. Bao, L. Tao, J. Li and L. Wu, Tetrandrine Inhibits the Wnt/beta-Catenin Signalling Pathway and Alleviates Osteoarthritis: An In Vitro and In Vivo Study, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 809579 Search PubMed.
- M. Kapoor, J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier and H. Fahmi, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis, Nat. Rev. Rheumatol., 2011, 7(1), 33–42 CrossRef PubMed.
- J. P. Pelletier, J. Martel-Pelletier and S. B. Abramson, Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets, Arthritis Rheumatol., 2001, 44(6), 1237–1247 CrossRef.
- P. Richette, M. Francois, E. Vicaut, C. Fitting, T. Bardin, M. Corvol, J. F. Savouret and F. Rannou, A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis, J. Rheumatol., 2008, 35(8), 1650–1654 Search PubMed.
- M. Daheshia and J. Q. Yao, The interleukin 1beta pathway in the pathogenesis of osteoarthritis, J. Rheumatol., 2008, 35(12), 2306–2312 CrossRef PubMed.
- C. Csaki, A. Mobasheri and M. Shakibaei, Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis, Arthritis Res. Ther., 2009, 11(6), R165 Search PubMed.
- S. B. Abramson, M. Attur, A. R. Amin and R. Clancy, Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis, Curr. Rheumatol. Rep., 2001, 3(6), 535–541 CrossRef PubMed.
- S. Rashad, F. Low, P. Revell, A. Hemingway, K. Rainsford and F. Walker, Effect of non-steroidal anti-inflammatory drugs on course of osteoarthritis, Lancet, 1989, 2(8672), 1149 CrossRef.
- M. Yadav, S. Jain, A. Bhardwaj, R. Nagpal, M. Puniya, R. Tomar, V. Singh, O. Parkash, G. B. Prasad and F. Marotta, et al., Biological and medicinal properties of grapes and their bioactive constituents: an update, J. Med. Food, 2009, 12(3), 473–484 CrossRef PubMed.
- W. D. Johnson, R. L. Morrissey, A. L. Usborne, I. Kapetanovic, J. A. Crowell, M. Muzzio and D. L. McCormick, Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity, Food Chem. Toxicol., 2011, 49(12), 3319–3327 CrossRef PubMed.
- T. Szekeres, P. Saiko, M. Fritzer-Szekeres, B. Djavan and W. Jager, Chemopreventive effects of resveratrol and resveratrol derivatives, Ann. N. Y. Acad. Sci., 2011, 1215, 89–95 CrossRef PubMed.
- Y. Wei, J. Jia, X. Jin, W. Tong and H. Tian, Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis, Mol. Med. Rep., 2018, 17(1), 1493–1498 Search PubMed.
- N. Mizushima and M. Komatsu, Autophagy: renovation of cells and tissues, Cell, 2011, 147(4), 728–741 CrossRef PubMed.
- B. Carames, N. Taniguchi, S. Otsuki, F. J. Blanco and M. Lotz, Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis, Arthritis Rheumatol., 2010, 62(3), 791–801 CrossRef PubMed.
- H. Sasaki, K. Takayama, T. Matsushita, K. Ishida, S. Kubo, T. Matsumoto, N. Fujita, S. Oka, M. Kurosaka and R. Kuroda, Autophagy modulates osteoarthritis-related gene expression in human chondrocytes, Arthritis Rheumatol., 2012, 64(6), 1920–1928 CrossRef PubMed.
- M. Lei, J. G. Wang, D. M. Xiao, M. Fan, D. P. Wang, J. Y. Xiong, Y. Chen, Y. Ding and S. L. Liu, Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-kappaB activity, Eur. J. Pharmacol., 2012, 674(2–3), 73–79 CrossRef PubMed.
- T. Yuasa, T. Otani, T. Koike, M. Iwamoto and M. Enomoto-Iwamoto, Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration, Lab. Invest., 2008, 88(3), 264–274 CrossRef PubMed.
- J. Wang, J. S. Gao, J. W. Chen, F. Li and J. Tian, Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit, Rheumatol. Int., 2012, 32(6), 1541–1548 CrossRef PubMed.
- H. J. Kim, H. J. Braun and J. L. Dragoo, The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism, Bone Jt. Res., 2014, 3(3), 51–59 CrossRef PubMed.
- J. Sokolove and C. M. Lepus, Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations, Ther. Adv. Musculoskeletal Dis., 2013, 5(2), 77–94 CrossRef PubMed.
- W. Zheng, Z. Feng, Y. Lou, C. Chen, C. Zhang, Z. Tao, H. Li, L. Cheng and X. Ying, Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo, Oncotarget, 2017, 8(59), 99649–99665 Search PubMed.
- Y. X. Liu, G. D. Wang, X. Wang, Y. L. Zhang and T. L. Zhang, Effects of TLR-2/NF-kappaB signaling pathway on the occurrence of degenerative knee osteoarthritis: an in vivo and in vitro study, Oncotarget, 2017, 8(24), 38602–38617 Search PubMed.
- P. Kong, G. Chen, A. Jiang, Y. Wang, C. Song, J. Zhuang, C. Xi, G. Wang, Y. Ji and J. Yan, Sesamin inhibits IL-1beta-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway, Oncotarget, 2016, 7(50), 83720–83726 CrossRef PubMed.
- S. Ahmed, N. Wang, B. B. Hafeez, V. K. Cheruvu and T. M. Haqqi, Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro, J. Nutr., 2005, 135(9), 2096–2102 CrossRef PubMed.
- L. Dahlberg, R. C. Billinghurst, P. Manner, F. Nelson, G. Webb, M. Ionescu, A. Reiner, M. Tanzer, D. Zukor and J. Chen, et al., Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1), Arthritis Rheumatol., 2000, 43(3), 673–682 CrossRef.
- G. Murphy, V. Knauper, S. Atkinson, G. Butler, W. English, M. Hutton, J. Stracke and I. Clark, Matrix metalloproteinases in arthritic disease, Arthritis Res., 2002, 4(Suppl 3), S39–S49 CrossRef PubMed.
- Y. Yoshihara, H. Nakamura, K. Obata, H. Yamada, T. Hayakawa, K. Fujikawa and Y. Okada, Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis, Ann. Rheum. Dis., 2000, 59(6), 455–461 CrossRef PubMed.
- Y. Zhang, F. Vasheghani, Y. H. Li, M. Blati, K. Simeone, H. Fahmi, B. Lussier, P. Roughley, D. Lagares and J. P. Pelletier, et al., Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis, Ann. Rheum. Dis., 2015, 74(7), 1432–1440 CrossRef PubMed.
- Q. Tang, G. Zheng, Z. Feng, Y. Chen, Y. Lou, C. Wang, X. Zhang, Y. Zhang, H. Xu and P. Shang, et al., Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development, Cell Death Dis., 2017, 8(10), e3081 CrossRef PubMed.
- C. He and D. J. Klionsky, Regulation mechanisms and signaling pathways of autophagy, Annu. Rev. Genet., 2009, 43, 67–93 CrossRef PubMed.
- Z. Li, Y. Li, Y. Li, K. Ren, X. Li, X. Han and J. Wang, Long non-coding RNA H19 promotes the proliferation and invasion of breast cancer through upregulating DNMT1 expression by sponging miR-152, J. Biochem. Mol. Toxicol., 2017, 31(9) CrossRef PubMed.
- N. Sassi, L. Laadhar, M. Allouche, A. Achek, M. Kallel-Sellami, S. Makni and S. Sellami, WNT signaling and chondrocytes: from cell fate determination to osteoarthritis physiopathology, J. Recept. Signal Transduction Res., 2014, 34(2), 73–80 CrossRef PubMed.
- L. Chen, Y. Wu, Y. Wu, Y. Wang, L. Sun and F. Li, The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/beta-catenin pathway, Sci. Rep., 2016, 6, 29176 CrossRef PubMed.
- M. Yan, G. Li and J. An, Discovery of small molecule inhibitors of the Wnt/beta-catenin signaling pathway by targeting beta-catenin/Tcf4 interactions, Exp. Biol. Med., 2017, 242(11), 1185–1197 CrossRef PubMed.
- H. Shang, Z. Q. Hao, X. B. Fu, X. D. Hua, Z. H. Ma, F. L. Ai, Z. Q. Feng, K. Wang, W. X. Li and B. Li, Intermedin promotes hepatocellular carcinoma cell proliferation through the classical Wnt signaling pathway, Oncol. Lett., 2018, 15(4), 5966–5970 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |