Open Access Article
Open Access ArticleSodium borohydride-nickel chloride hexahydrate in EtOH/PEG-400 as an efficient and recyclable catalytic system for the reduction of alkenes†
Kaoxue Li *,
Chuanchao Liu,
Kang Wang,
Yang Ren and
Fahui Li*
*,
Chuanchao Liu,
Kang Wang,
Yang Ren and
Fahui Li*
Chemistry & Chemical and Environmental Engineering College, Weifang University, Weifang 261061, China. E-mail: likaoxue@wfu.edu.cn; fahuili@163.com; Tel: +86-536-8785283
First published on 19th February 2018
Abstract
An efficient, safe and one-pot convenient catalytic system has been developed for the reduction of alkenes using NaBH4–NiCl2·6H2O in EtOH/PEG-400 under mild conditions. In this catalytic system, a variety of alkenes (including trisubstituted alkene α-pinene) were well reduced and the Ni catalyst could be recycled.
The reduction of alkenes is an important transformation in organic synthesis, widely used particularly in petrochemical, pharmaceutical, and fine chemical processes. Traditionally, direct hydrogenation,1–4 catalytic hydrogen transfer 5–7and hydride reduction methods8–10 have been employed for the reduction of alkenes. Among the reported methods, the utility of sodium borohydride for the reduction of simple alkenes first described by Brown in 1962 is well known.11 In recent years, several related catalytic systems on modification of Brown's approach have been developed. These catalytic systems include NaBH4/NiCl2·6H2O/moist alumina in hexane,12 InCl3–NaBH4 reagent system,13 NaBH4/RuCl3 under aqueous conditions,14 NaBH4/CH3COOH in the presence of Pd/C15 and NaBH4-RANEY® nickel system in water.16 Nevertheless, most of these systems require costly transition metal catalyst, long reaction time and a large excess of NaBH4. In addition to this, little has been done to recycle the catalyst for the reduction of alkenes using NaBH4. Thus, a very simple, efficient and recyclable system for the reduction of alkenes by NaBH4 would be highly desirable.
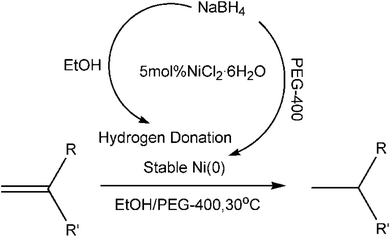
In the reduction of alkenes by NaBH4, the in situ generated metal nanoparticles (NPs) from the combination of appropriate metal salts and NaBH4 catalyze the reduction of alkene.15 In general, metal NPs tend to agglomerate during the catalytic processes and therefore need to be protected by stabilizers.17 Castro et al. ever noticed the aggregation of NPs after just one time in the reduction of alkenes by NaBH4 without use of a stabilizer.18 Immobilized nanoparticles (NPs) on insoluble solid supports were generally used for this process in the past literature.12,15,16 One significant example was that Takashi Morimoto and coworkers reached 90% yield of ethylbenzene within 3 h using NiCl2·6H2O on moist alumina reducted by NaBH4 in hexane at 30 °C.12 Unfortunately, heterogeneous catalysts of NPS on solid supports are often more inert than corresponding soluble NPs catalysts.19 In view of the above, we wanted to explore the use of soluble NPs generated in situ for this process. PEG-400 is known to be an excellent dispersion agent and stabilizer for soluble metal NPs.20,21 Abdul Rahman Mohamed et al. found that iron metal NPs formed in an ethanol-PEG-400 solution displayed a more uniform distribution.22 In this work, we introduce NaBH4/NiCl2·6H2O in EtOH/PEG-400 to the reduction of alkenes, to the best of our knowledge, the system is novel for the reaction. The novel system was expected to show the following advantages (Scheme 1): (a) NaBH4 not only reduces Ni2+ to soluble Ni (0) NPs in situ, but also serves as hydrogen donation for the reduction of alkenes with ethanol; (b) the in situ generated soluble Ni (0) NPs catalyst stabilized by PEG-400 is stable, efficient and recyclable for the reduction of alkenes.
We first chose the reduction of styrene as model reaction (Table 1). Initially, the effect of VEtOH/VPEG-400 (volume ratio of ethanol to PEG-400) on the reaction was investigated. Curiously, using pure ethanol and PEG-400 as the solvent gave lower yields (entries 1, 2), while adding certain amounts of PEG-400 to the ethanol led to an enhancement in activity first (entries 3, 4), then further addition of PEG-400 retarded the reaction (entries 5, 6), and a best yield was obtained when using 3/2 ratio of ethanol and PEG-400 (entry 4), which might be due to the good dispersion and stabilization of the Ni (0) NPs in the mixture. Subsequently, the influence of PEG-400 in different solvent on the reduction of styrene was also investigated. It was found that the combination of PEG-400 with MeOH also gave excellent yield (entry 7), whereas PEG-400 in 1-propanol, 1-butanol, 2-propanol, H2O, ethyl acetate, toluene, cyclohexane afforded the lower yields of product (entries 8–14). The very low yields were obtained in HCOOH-PEG-400 and CH3COOH-PEG-400 system (entries 15, 16). These results indicated that EtOH and MeOH were the suitable solvents for the reduction of styrene in the presence of PEG-400. Studies on the effects of the amounts of NaBH4 in EtOH/PEG-400 (3/2 ratio) showed that the yield of ethylbenzene decreased with the increase of the molar equivalents of NaBH4 (entries 4, 17–19). It is possible that excess amounts of boron products strongly bound the nanocatalyst surface that passivates the active sites of the Ni catalyst. Importantly, the appropriate amounts of the NaBH4 were only 0.5 molar equivalents (entry 4), which were lower than those in the earlier reports.12,15,23,24 This is possibly because the hydrogen source for the reduction can be sufficiently derived from the B–H of NaBH4 and the O–H of ethanol in our catalytic system (ideally 1 molar of NaBH4 can reduce 4 molar of alkenes with ethanol), as recently reported by Bai et al. for the semihydrogenation of alkynes with NaBH4 in methanol.25 The activity of the in situ generated Ni (0) NPs catalyst in EtOH/PEG-400 (3/2 ratio) had also been compared with the commercial RANEY® nickel catalyst. The results revealed that the in situ generated Ni (0) NPs exhibited a higher activity for the reduction of styrene (entries 4, 20). Thus, we can conclude that the EtOH/PEG-400 using NiCl2·6H2O–NaBH4 is a very simple and efficient system for the reduction of styrene.
| Entry | Solvent (v/v) | NaBH4 (equiv.) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: N2 atmosphere, 30 °C, NiCl2·6H2O 0.25 mmol, solvent (5 mL), styrene 5 mmol.b GC yield.c Catalyzed by RANEY® nickel. | |||
| 1 | EtOH | 0.5 | 76 |
| 2 | PEG-400 | 0.5 | 41 |
| 3 | EtOH-PEG400(4/1) | 0.5 | 84 |
| 4 | EtOH-PEG400(3/2) | 0.5 | 99 |
| 5 | EtOH-PEG400(2/3) | 0.5 | 92 |
| 6 | EtOH-PEG400(1/4) | 0.5 | 79 |
| 7 | MeOH-PEG400(3/2) | 0.5 | 98 |
| 8 | 1-Propanol-PEG400(3/2) | 0.5 | 80 |
| 9 | 1-Butanol-PEG400(3/2) | 0.5 | 60 |
| 10 | 2-Propanol-PEG400(3/2) | 0.5 | 18 |
| 11 | H2O-PEG400(3/2) | 0.5 | 26 |
| 12 | Ethyl acetate-PEG400(3/2) | 0.5 | 23 |
| 13 | Toluene-PEG400(3/2) | 0.5 | 21 |
| 14 | Cyclohexane-PEG400(3/2) | 0.5 | 26 |
| 15 | HCOOH-PEG400(3/2) | 0.5 | <1 |
| 16 | CH3COOH-PEG400(3/2) | 0.5 | <1 |
| 17 | EtOH-PEG400(3/2) | 0.75 | 89 |
| 18 | EtOH-PEG400(3/2) | 1.0 | 79 |
| 19 | EtOH-PEG400(3/2) | 1.25 | 68 |
| 20 | EtOH-PEG400(3/2) | 0.5 | 54c |
The in situ generated Ni (0) NPs in EtOH/PEG-400 were characterized by UV-vis, XPS after model reaction. Fig. 1a showed the UV-vis spectrum of nickel chloride hexahydrate in EtOH/PEG-400 before and after reaction. Apparently, a broad band at 250–270 nm appeared after reaction, which indicated the formation of Ni (0) NPs.26 XPS spectra (Fig. 1b) showed that Ni 2p3/2 peak at approximately 852.8 eV and Ni 2p1/2 peak at 870.9 eV, respectively, indicating the generation of Ni (0) NPs.27
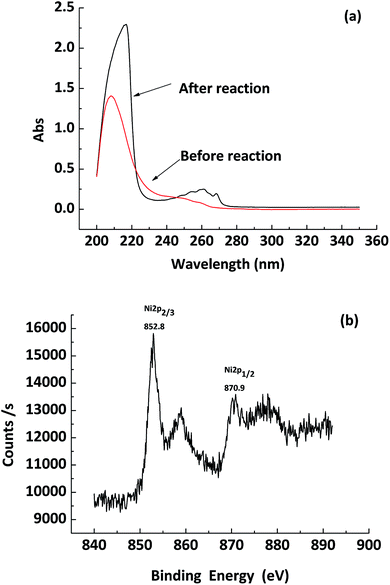 | ||
| Fig. 1 (a) UV-vis spectra of the solution of NiCl2·6H2O in EtOH/PEG-400 before and after the reduction of styrene using NaBH4. (b) XPS of in situ generated Ni NPs. | ||
The motive to use PEG-400 as a stabilizer in ethanol was the possibility to protect and recycle the Ni (0) NPs catalyst. After model reaction, the catalyst could be separated by simple extracting with n-heptane followed by decantation and reused directly without further purification. The results for the reuse of the Ni catalyst were shown in Table 2. As can be seen from Table 2, in the first run, the yield of ethylbenzene reached 99% for 15 min. In subsequent consecutive runs the catalytic activity decreased, similar observations have also been made about the decrease for in situ generated Nps catalyst during recycling.28,29 However, prolonging the reaction time from 15 min to 120 min could still keep a high yield of ethylbenzene. Notably, the activities of Ni (0) NPs from second run still higher than the corresponding heterogeneous Ni NPS on moist alumina reported by Takashi Morimoto et al.12
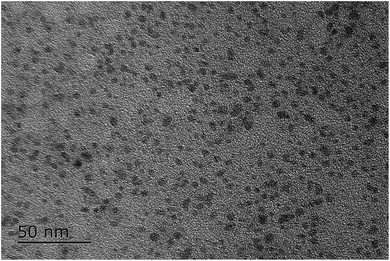
To explore the reason why the catalytic activity decreased after first run, the leaching, size and distribution of Ni NPs were characterized after reaction. The leached Ni species were checked by ICP-AES and found to be only 0.3%. HRTEM image showed that Ni (0) NPs were well-dispersed with an average diameter of 3–5 nm (Fig. 2), indicating the good dispersion and stabilization of the Ni (0) NPs in EtOH/PEG-400 system. Therefore, the reason for the decrease of catalyst could hardly be explained by Ni leaching or the aggregate of the Ni (0) NPs.
Meanwhile, control experiments were performed between in situ generated Ni (0) NPs in the second run and preformed Ni (0) NPs catalyst (see the ESI† for details)for the reduction of styrene (Fig. 3). Obviously, the activities of the two kinds of Ni (0) NPs catalysts for the model reaction were almost equivalent. This promoted us to infer Ni (0) NPs had been changed from in situ generated to preformed catalyst, which may be the main reason for the reduced activity of the catalyst in subsequent runs during recycling. The details are under investigation.
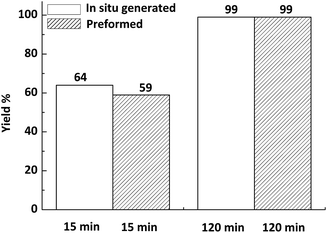 | ||
| Fig. 3 Control experiments between in situ generated Ni (0) NPs in the second run and preformed Ni (0) NPs catalyst for the model reaction. | ||
The scope of this catalytic system was also examined for the reduction of various olefins (Table 3). Both aliphatic and acyclic olefins were reactive in the catalytic system, affording their corresponding alkanes with excellent yields. For example, monosubstituted terminal alkenes were efficiently reduced to their corresponding alkanes within 15 min (entries 1–7). Disubstituted olefins were also reduced efficiently (entries 8–13), but with longer reaction times compared to monosubstituted terminal alkenes except for norbornene (entry 10). Reduction of 1,5-cyclooctadiene afforded cyclooctene and cyclooctane with 94% yield within 300 min (entry 13). Importantly, trisubstituted alkene α-pinene (in contrast with previous studies of NaBH4/CoCl2 (ref. 30) and NaBH4/RuCl3 system,14,31 which were inert for the reduction of α-pinene) was also reduced without any difficulty (entry 14).
| Entry | Substrate | Time (min) | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: N2 atmosphere, 30 °C, NiCl2·6H2O 0.25 mmol, VEtOH/VPEG-400 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (5 mL), alkenes 5 mmol, NaBH4 2.5 mmol.b GC yield.c Ratio of cyclooctene/cyclooctane. 2 (5 mL), alkenes 5 mmol, NaBH4 2.5 mmol.b GC yield.c Ratio of cyclooctene/cyclooctane. |
|||
| 1 | 1-Hexene | 15 | 98 |
| 2 | 1-Octene | 15 | 96 |
| 3 | 1-Decene | 15 | 95 |
| 4 | 1-Dodecene | 15 | 94 |
| 5 | Styrene | 15 | 99 |
| 6 | 4-Methylstyrene | 15 | 100 |
| 7 | Allyl phenyl ether | 15 | 100 |
| 8 | trans-Anethole | 120 | 94 |
| 9 | β-Pinene | 240 | 96 |
| 10 | Norbornene | 15 | 100 |
| 11 | Cyclopentene | 30 | 96 |
| 12 | Cyclohexene | 120 | 99 |
| 13 | 1,5-Cyclooctadiene | 300 | 94(72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28)c 28)c |
| 14 | α-Pinene | 300 | 91 |
Conclusions
In summary, we have developed a novel system for the reduction of alkenes in one pot. The catalytic system involving NaBH4/NiCl2·6H2O/EtOH/PEG-400 exhibited good activity, recyclability and general applicability. Further the protocol is convenient, cheap and safe to carry out on a large scale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2014BL016) and Weifang Science & Technology Development Plan (2016GX004, 2014GX024).Notes and references
- K. Li, Y. Wang, J. Jiang and Z. Jin, Catal. Commun., 2010, 11, 542 CrossRef CAS.
- D. A. struc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef PubMed.
- D. J. Frank, L. Guiet, A. Käslin, E. Murphy and S. P. Thomas, RSC Adv., 2013, 3, 25698 RSC.
- P. Upadhyay and V. Srivastava, RSC Adv., 2015, 5, 740 RSC.
- D. Wang and D. Astruc, Chem. Rev., 2015, 115, 6621 CrossRef CAS PubMed.
- G. Zhang, Z. Yin and J. Tan, RSC Adv., 2016, 6, 22419 RSC.
- K. Imamura, Y. Okubo, T. Ito, A. Tanaka and K. Hashimoto, RSC Adv., 2014, 4, 19883 RSC.
- R. A. W. Johnstone, A. H. Wilby and I. D. Entwistle, Chem. Rev., 1985, 85, 129 CrossRef CAS.
- J. Mondal, K. T. Nguyen, A. Jana and K. Kurniawan, Chem. Commun., 2014, 50, 12095 RSC.
- A. Dhakshinamoorthy and K. Pitchumani, Tetrahedron Lett., 2008, 49, 1818 CrossRef CAS.
- H. C. Brown and C. A. Brown, J. Am. Chem. Soc., 1962, 84, 1494 CrossRef CAS.
- S. Yakabe, M. Hirano and T. Morimoto, Tetrahedron Lett., 2000, 41, 6795 CrossRef CAS.
- B. C. Ranu and S. Samanta, Tetrahedron, 2003, 59, 7901 CrossRef CAS.
- P. K. Sharma, S. Kumar, P. Kumar and P. Nielsen, Tetrahedron Lett., 2007, 48, 8704 CrossRef CAS.
- A. T. Tran, V. A. Huynh, E. M. Friz, S. K. Whitney and D. B. Cordes, Tetrahedron Lett., 2009, 50, 1817 CrossRef CAS.
- G. K. Rao, N. B. Gowda and R. A. Ramakrishna, Synth. Commun., 2012, 42, 893 CrossRef CAS.
- A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757 CrossRef CAS PubMed.
- K. A. De Castro, S. Oh, J. Yun, J. K. Lim, G. An, D. K. Kim and H. Rhee, Synth. Commun., 2009, 39, 3509 CrossRef CAS.
- J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 191, 187 CrossRef CAS.
- F. A. Harraz, S. E. El-Hout, H. M. Killa and I. A. Ibrahim, J. Catal., 2012, 286, 184 CrossRef CAS.
- Y. Zhong, X. Gong, X. Zhu, Z. Ni, H. Wang, J. Fu and W. Han, RSC Adv., 2014, 4, 63216 RSC.
- C. M. Seah, S. P. Chai, S. Ichikawa and A. R. Mohamed, Carbon, 2012, 50, 960 CrossRef CAS.
- B. C. Ranu and S. Samanta, J. Org. Chem., 2003, 68, 7130 CrossRef CAS PubMed.
- V. V. Kalashnikov and L. G. Tomilova, Mendeleev Commun., 2007, 17, 343 CrossRef CAS.
- X. Wen, X. Shi, X. Qiao, Z. Wu and G. Bai, Chem. Commun., 2017, 53, 5372 RSC.
- P. Calandra, Mater. Lett., 2009, 63, 2416 CrossRef CAS.
- Y. Richardson, J. Blin, G. Volle and J. Motuzas, Appl. Catal., A, 2010, 382, 220 CrossRef CAS.
- Z. Du, W. Zhou, F. Wang and J. X. Wang, Tetrahedron, 2011, 67, 4914 CrossRef CAS.
- L. Ackermann and R. Vicente, Org. Lett., 2009, 11, 4922 CrossRef CAS PubMed.
- N. Satyanarayana and M. Periasamy, Tetrahedron Lett., 1984, 25, 2501 CrossRef CAS.
- J. H. Babler and N. A. White, Tetrahedron Lett., 2010, 51, 439 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00905h |
| This journal is © The Royal Society of Chemistry 2018 |