Open Access Article
Open Access ArticleFluorinated polymer surfactants bearing an alternating peptide skeleton prepared by three-component polycondensation†
Y. Koyama *a,
A. B. Ihsana,
T. Tairab and
T. Imurab
*a,
A. B. Ihsana,
T. Tairab and
T. Imurab
aDepartment of Pharmaceutical Engineering, Faculty of Engineering, Toyama Prefectural University, 5180 Kurokawa, Imizu, Toyama 939-0398, Japan. E-mail: ykoyama@pu-toyama.ac.jp
bResearch Institute for Chemical Process Technology, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba Central 5-2, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan
First published on 16th February 2018
Abstract
A new species of fluorinated polymer surfactant was developed by three component polycondensation analogous to Ugi four-component condensation. The surfactant exhibited unique surface properties, which made cellulose-based materials hydrophobic and decreased the surface tension of CHCl3. It turned out that the polymer forms micelles in CHCl3.
Fluorinated surfactants constitute an intriguing class of surfactants,1 which exhibit high surface activity to not only oil–water interfaces but also gas–liquid and solid–liquid interfaces. The surfactants enable the decrease of surface tension even in organic solvents2 and the fabrication of superhydrophobic surfaces on substrates,3 which are adopted in various fields for wetting and repellency applications including paints, coatings, lubricants, fire-fighting foams, and mist suppression. Perfluorooctane sulfonic acid and perfluorooctanoic acid are the most popular members in this class.4 However, the toxicity and bioaccumulation of the surfactants lead to some inevitable limitations on its use.5 Considering the low biocompatibility of such low-molecular-weight surfactants, our recent efforts have been devoted to the development of new entry for polymeric surfactants. Fluorinated polyethers such as oligo(hexafluoropropylene oxide)6 and poly(fluorooxetane)7 are synthesized via the ring-opening polymerization of the corresponding monomers. The vinyl polymer-based surfactants can also be prepared by the radical polymerization of fluorinated vinyl monomers and subsequent functionalization of the polymer terminus with a hydrophilic group.8 Although these polymer surfactants become promising candidates as the substitute of low-molecular-weight fluorinated surfactants, two problems on the synthetic methods that have to be solved arise for practical use. One is the low skeletal diversity of polymer surfactants, because the diversity is strongly dependent on the availability of fluorinated monomers. The other problem is a low reproducibility to give a uniform polymer surfactant with precisely same amphiphilicity, owing to the synthetic challenges on the control of polymerization and polymer reaction. Thus, we became intrigued by the potential possibility of multi-component polycondensation9 for the creation of fluorinated polymer surfactants bearing well-defined amphiphilicity.
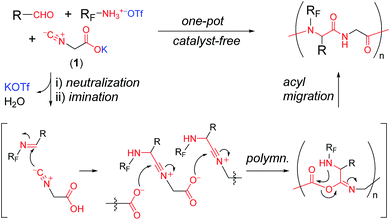
In a recent publication, we reported a one-pot synthetic technique for synthesizing alternating peptides.10 The technique involves catalyst-free polymerization analogous to the Ugi four-component condensation (Ugi 4CC) for the synthesis of dipeptides.11 On the basis of our previous method, we planned three-component polycondensation using an aldehyde, perfluoroammonium triflate, and a potassium salt of glycine-based ambident molecule bearing both isonitrile and carboxylic acid (CN–CH2–COOK, 1) to give alternating peptides with perfluoroalkyl pendant groups (Scheme 1). Neutral perfluoroalkylamine can be generated in situ by the treatment of the ammonium salt with 1, which smoothly react with an aldehyde to give the corresponding imine. Further reaction of imine (RC = NRF) with the ambident molecule would proceed to polymerization, followed by acyl migration to give the alternating peptides without the need of a catalyst. The peptide main chain is expected to serve as the hydrophilic part of surfactant. Because the alternating peptide skeleton consists of the well-defined repeating unit, the amphiphilicity of the polymer would be precisely controlled by the manipulation of R and RF structures, which could be hardly dependent on the polymerization degree.
Building upon this idea, herein, we describe catalyst-free three-component polycondensation to give perfluoroalkyl-containing alternating peptides. The effects of the polymer structure on their surface-active properties were investigated by surface tension and dynamic light scattering (DLS) measurements. We successfully developed a fascinating polymer surfactant, which made cellulose-based materials hydrophobic and decreased the surface tension of CHCl3. It turned out that the polymer forms thermodynamically stable associates, i.e., micelles in CHCl3.
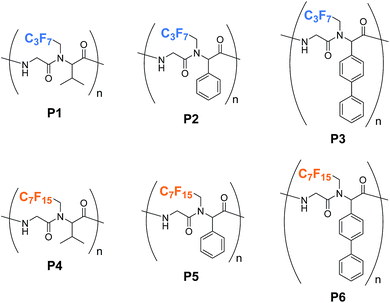
Fig. 1 depicts the structures of alternating peptides with perfluoroalkyl pendant groups (P1–P6). We selected three types of aldehydes, two perfluoroalkylammonium salts, and potassium isocyanoacetate (1) as the precursor of the ambident molecule. The ammonium salts were freshly prepared in i-PrOH in situ before the polymerization. According to the schematic illustration in Scheme 1, the three-component polycondensation began with a combination of isobutyraldehyde, C3F7CH2NH3OTf, and 1. The three components were stirred in i-PrOH at room temperature for 4 d; then, i-PrOH was removed in vacuo. The residue was stirred for an additional 3 d until the magnetic rotator could no longer be stirred, which produced a highly viscous material. After typical workup, a CHCl3 solution of the products was reprecipitated into hexane to give P1 as a hexane-insoluble part.
The polymer structure of P1 was confirmed by 1H NMR, 13C NMR, 19F NMR, and IR spectra.12 The 1H NMR spectrum was found to consist of proton signals from all the three components with an appropriate integral ratio (Fig. S1†). In the 13C NMR spectrum (Fig. S2†), four amide carbon signals were observed at around 170–180 ppm, strongly supporting the formation of amide linkages in the main chain and the presence of cis–trans rotamers of the N,N-disubstituted amide bond. All carbon signals except for the signals attributed to C3F7 are very sharp, indicating the formation of a high-molecular-weight polymer. The broadening of C3F7 carbons in the range of 100–150 ppm would come from the N,N-disubstituted amide rotamers and multiple scalar couplings between 13C and 19F nuclei. We also measured the 19F NMR spectrum (Fig. S3†),12 which clearly provided the direct evidence for the introduction of 19F nuclei to the polymer skeleton. In the IR spectrum, an amide absorption band was observed at around 1660 cm−1 (Fig. S5†).12 These results clearly indicate that the three-component polycondensation formed an alternating peptide polymer, P1. In a similar manner, using other reaction components, we also prepared the other polymers, P2, P3, P4, P5, and P6. The results are summarized in Table 1. The obtained polymers were hardly soluble in water, while the polymers were soluble in various organic solvents. The weight-average molecular weight (Mw) values were estimated by the diffusion coefficients (D) obtained from diffusion-ordered NMR spectroscopy (DOSY) according to the literature.10,13 The polydispersity indices (Mw/Mn) were estimated by a size exclusion column chromatography. Glass transition temperatures (Tg) were measured by differential scanning calorimetry (DSC) analyses.12 Tgs of the alternating peptides were found to be dependent on both the rigidity of the pendant groups (R) and the length of perfluoroalkyl chains, implying that the thermal properties of the polymers would be easily tunable by the selection of appropriate aldehyde and amine components.14
| Polymer | Yielda (%) | Mwb (kDa) | Mw/Mnc | Tgd (°C) |
|---|---|---|---|---|
| a Hexane-insoluble part.b Estimated by DOSY spectra (400 MHz, CDCl3, 298 K).c Estimated by a size exclusion column chromatography on the basis of polystyrene standards (eluent: DMF).d Estimated by a differential scanning calorimetry. | ||||
| P1 | 85 | 7.4 | 1.5 | −23.6 |
| P2 | 82 | 7.8 | 1.8 | −13.3 |
| P3 | 63 | 7.1 | 1.5 | 31.4 |
| P4 | 43 | 7.6 | 1.2 | −1.3 |
| P5 | 67 | 7.4 | 1.3 | 0.7 |
| P6 | 64 | 8.8 | 1.2 | 45.5 |
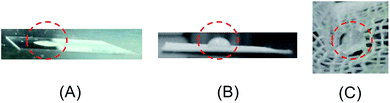
Next, with six types of perfluoroalkyl group-containing peptides, we investigated the surface modification of the polymers on glass plate, filter paper, and cotton gauze (Fig. 2). A water droplet of 6 μL was deposited on each polymer-modified surface to evaluate the water contact angles (WCA). In the cases of glass plates, WCA for all plates modified by the polymers were <10°, similar to that of the original glass plate (Fig. 2A), mainly because of the interaction of the polymers with water. On the other hand, we found that P6 efficiently makes highly hydrophobic filter paper or cotton gauze without dissolution in water (Fig. 2B and C),15 whereas the other polymers hardly change the hydrophilicity of the original materials.
The hydrophobic gauze modified by P6 was characterized by IR spectra before and after the modification (Fig. S38†).12 By a careful comparison, we found that the IR spectrum of the modified gauze includes a small shoulder at around 1660 cm−1, which perfectly matches the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption of P6. The other IR absorption signals of the modified gauze almost match those of the original gauze, suggesting that the high hydrophobicity of the gauze is caused by the trace amount of P6 immobilized on the surface.
O absorption of P6. The other IR absorption signals of the modified gauze almost match those of the original gauze, suggesting that the high hydrophobicity of the gauze is caused by the trace amount of P6 immobilized on the surface.
The solutions of P1, P2, and P3 are transparent, while the solutions of P4 and P5 are slightly opaque (Fig. 3A), because of the low solubility of P4 and P5 in CHCl3. On the other hand, the solution of P6 is transparent. It is noted that the P6 solution easily made bubbles by shaking, also indicating the interfacial activity between CHCl3 phase and atmosphere. For the evaluation of unique surface modification capability of P6, the surface tensions of 1.0 wt% polymer solutions in CHCl3 were measured by the Wilhelmy plate method (Fig. 3B).16 Compared with the surface tension of pure CHCl3 (26.9 mN m−1), those of all polymers clearly decreased (P1: 25.7, P2: 25.5, P3, 26.7, P4: 20.9, P5: 20.0, and P6: 22.0 mN m−1), suggesting that the polymers serve as a surfactant in CHCl3.2 The good contrast between the organophobicity of perfluoroalkyl pendant groups and the organophilicity of the main chain would facilitate the interfacial alignment of the polymers in CHCl3. The C7F15-containing polymers (P4, P5, and P6) exhibit higher surface activity than C3F7-containing polymers (P1, P2, and P3).
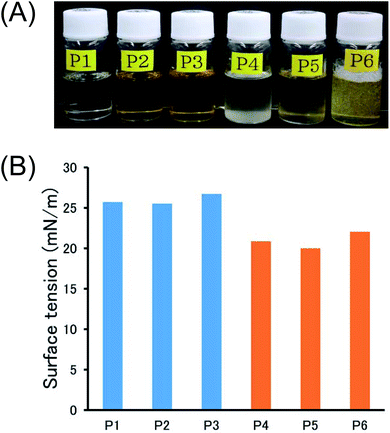 | ||
| Fig. 3 (A) Photographs and (B) surface tension of 1.0 wt% polymer solutions in CHCl3 at room temperature. | ||
The effects of alkyl groups (R) in both C3F7- and C7F15-containing polymer series on the surface activity appear to exhibit a similar trend as follows; phenyl > i-propyl > biphenyl.
Micellization affects interfacial properties such as reduction of surface tension. It is therefore, very important to characterize the surfactants in term of micellization behavior. Quite reasonably, the critical micelle concentration (CMC), i.e., onset concentration to form micelle is an important parameter. Thus, on the assumption, we measured UV-vis spectra of P6 in CHCl3 at various concentrations. The UV-vis spectra include the characteristic absorption to biphenyl structures in the polymer.12 We plotted the relationship between polymer concentration and absorbance at 340 nm normalized by the concentration (Fig. 4). The plots clearly include the intersection point at around 0.6 wt%, indicating the CMC for the surfactant. To our best knowledge, the studies on micelle formation in CHCl3 has not been sufficiently investigated, while there are several reports about surface activities of fluorinated surfactants in organic solvents. The numerical magnitude of CMC of P6 in CHCl3 might be comparable with those of typical hydrocarbon- and fluorocarbon-based low-molecular-weight surfactants in water (e.g. C8H17COONH4: 7.0 wt% and C8F17COONH4: 0.4 wt%),2d suggesting that the perfluoroalkyl pendant groups of P6 would play an important role on the decrease of surface tension even in CHCl3.
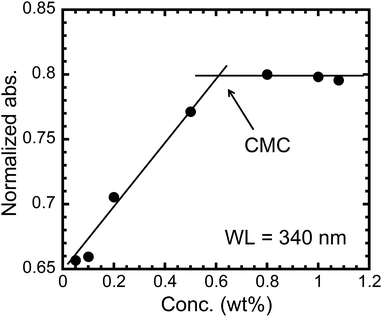 | ||
| Fig. 4 Normalized absorbance of P6 at 340 nm (CHCl3, room temperature) as a function of concentration (wt%). | ||
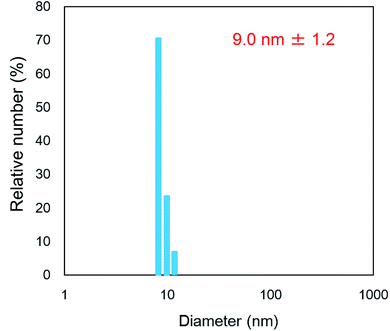
To know the size of micelles, we performed the DLS measurements above the CMC (1.0 wt%) of the polymer solutions in CHCl3. In the profile of P6 solution (Fig. 5), we observed scattered light signals enough for fitting the auto-correlate function, which reveals the existence of associates in the solution. The size of associates was found to be 9.0 ± 1.2 nm. On the other hand, the other polymers didn't exhibit any association, which was confirmed by the DLS analyses. It is interesting that P6 forms micelles in CHCl3, which is a good contrast to the results of P4 and P5 with the same C7F15 groups.
The differences in the micellization result directly from the bulkiness of the substituents in the peptide main chain, since more bulky groups in the surfactants can stabilize the micelle structure. Probably, the organophobic C7F15 groups would self-associate in CHCl3, which could form micelles projecting the organophilic peptide main chain outside. The bulky biphenyl substituents in P6 at the outer face could prevent the aggregation of micelles, probably due to the kinetic suppression of interchain interactions such as hydrogen bondings and fluorous–fluorous interactions. In the cases of P4 and P5, lack of appropriate bulkiness could result in the formation of the unstable aggregated structure, leading to the opaque solution as shown in Fig. 3A. The other factors may also play a role for the formation of superstructure. The micelles in the solution of P6 in CHCl3 would recognize and coat the hydrophilic cellulose surface, which could make the surface hydrophobic. The biphenyl groups in P6 are also expected to suppress the approach of water not only to the cellulose scaffold but also to the polypeptide main chain of P6 on a molecular scale, which could remarkably decrease the dissolution rate of P6 in a water droplet on the P6-modified surface.
Conclusions
In conclusion, we state that we have developed a new three-component polycondensation technique to synthesize well-defined fluorinated polymer surfactants, where aldehyde, ammonium salt, and potassium salt 1 work as the reaction components. The reaction not only enables the one-pot synthesis of alternating peptides but also enables easy introduction of the perfluoroalkyl group to the polypeptide skeleton at a regular interval. Since the synthesis of alternating copolymers is still difficult,17 the present method might provide a new insight into the control of polymer sequence. The rigidity of the side group attributed to the aldehyde strongly influences the Tgs of the polymers. In all polymers, P6 particularly exhibited unique surface activity, which made the cellulose-based materials hydrophobic and decreased the surface tensions of CHCl3. It was indicated that P6 forms micelle even in CHCl3, which was confirmed by both UV-vis spectra and DLS measurements. Our finding about micelle formation in organic solvent might open a new field of surfactant chemistry. The effects of polymer structure, solvent, and temperature on the CMC are currently investigating in detail.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI (Grant Numbers JP17H03070 and JP15H00718), Nagase science technology foundation, and Kanamori foundation.Notes and references
- N. M. Kovalchuk, A. Trybala, V. Starov, O. Matar and N. Ivanova, Adv. Colloid Interface Sci., 2014, 210, 65 CrossRef CAS PubMed.
- (a) G.-L. Li, L.-Q. Zheng and J.-X. Xiao, J. Fluorine Chem., 2009, 130, 674 CrossRef CAS; (b) C. Rodríguez, H. Kunieda, Y. Noguchi and T. Nakaya, J. Colloid Interface Sci., 2001, 242, 255 CrossRef; (c) J. Eastoe, A. Downer, A. Paul, D. C. Steytler, E. Rumsey, J. Penfold and R. K. Heenan, Phys. Chem. Chem. Phys., 2000, 2, 5235 RSC; (d) S. Ohtoshi, Sekiyu Gakkaishi, 1989, 32, 277 CrossRef CAS; (e) S. Ohtoshi, Nippon Setchaku Kyokaishi, 1983, 19, 345 CAS.
- M. Monduzzi, Curr. Opin. Colloid Interface Sci., 1998, 3, 467 CrossRef CAS.
- J. C. Ravey and M. J. Stébé, Colloids Surf., A, 1994, 84, 11 CrossRef CAS.
- (a) Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbühler, Environ. Int., 2013, 60, 242 CrossRef CAS PubMed; (b) A. Zaggia and B. Ameduri, Curr. Opin. Colloid Interface Sci., 2012, 17, 188 CrossRef CAS; (c) H.-J. Lehmler, Chemosphere, 2005, 58, 1471 CrossRef CAS PubMed.
- (a) S. V. Kostjuk, E. Ortega, F. Ganachaud, B. Améduri and B. Boutevin, Macromolecules, 2009, 42, 612 CrossRef CAS; (b) N. Durand, D. Mariot, B. Améduri, B. Boutevin and F. Ganachaud, Langmuir, 2011, 27, 4057 CrossRef CAS PubMed.
- C. M. Kausch, J. E. Leising, R. E. Medsker, V. M. Russell and R. R. Thomas, Langmuir, 2002, 18, 5933 CrossRef CAS.
- (a) V. C. Malshe, S. Elango, S. S. Bhagwat and S. S. Maghrabi, Prog. Org. Chem., 2005, 53, 212 CrossRef CAS; (b) H. Sawada, Polym. J., 2007, 39, 637 CrossRef CAS; (c) F. Boschet, G. K. Kostov, B. Boutevin, A. Jackson and B. Améduri, Polym. Chem., 2012, 3, 217 RSC.
- For related reports, see: (a) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (b) J. G. Rudick, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3985 CrossRef CAS; (c) S. Cheawchan, S. Uchida, H. Sogawa, Y. Koyama and T. Takata, Langmuir, 2016, 32, 309 CrossRef CAS PubMed.
- Y. Koyama and P. G. Gudeangadi, Chem. Commun., 2017, 53, 3846 RSC.
- (a) I. Ugi, R. Meyr, U. Fetzer and C. Steinbrückner, Angew. Chem., 1959, 71, 386 Search PubMed; (b) I. Ugi, B. Werner and A. Dömling, Molecules, 2003, 8, 53 CrossRef; (c) U. K. Sharma, U. K. N. Sharma, D. D. Vachhani and E. V. van der Eycken, Chem. Soc. Rev., 2015, 44, 1836 RSC; (d) A. A. Samad, J. De Winter, P. Gerbaux, C. Jérôme and A. Debuigne, Chem. Commun., 2017, 53, 12240 RSC.
- See ESI.†.
- (a) W. Li, W. H. Chung, C. Daeffler, J. A. Johnson and R. H. Grubbs, Macromolecules, 2012, 45, 9595 CrossRef CAS PubMed; (b) K. F. Morris and C. S. Jr Johnson, J. Am. Chem. Soc., 1992, 114, 3139 CrossRef CAS; (c) C. S. Jr Johnson, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203 CrossRef; (d) A. Chen, D. Wu and C. S. Jr Johnson, J. Am. Chem. Soc., 1995, 117, 7965 CrossRef CAS; (e) A. Jerschow and N. Müller, Macromolecules, 1998, 31, 6573 CrossRef CAS; (f) J. Viéville, M. Tanty and M.-A. Delsuc, J. Magn. Reson., 2011, 212, 169 CrossRef PubMed.
- For a related report, see: T. Modzelewski, E. Wilts and H. R. Allcock, Macromolecules, 2015, 48, 7543 CrossRef CAS.
- G. Li, H. Zheng, Y. Wang, H. Wang, Q. Dong and R. Bai, Polymer, 2010, 51, 1940 CrossRef CAS.
- J. J. Jasper, J. Phys. Chem. Ref. Data, 1972, 1, 841 CrossRef CAS.
- (a) A. Sommazzi and F. Garbassi, Prog. Polym. Sci., 1997, 22, 1547 CrossRef CAS; (b) I. Cho, Prog. Polym. Sci., 2000, 25, 1043 CrossRef CAS; (c) D. Braun and F. Hu, Prog. Polym. Sci., 2006, 31, 239 CrossRef CAS; (d) B. M. Culbertson, Encycl. Polym. Sci. Eng., 1987, 9, 225 CAS; (e) K. A. Parker and N. S. Sampson, Acc. Chem. Res., 2016, 49, 408 CrossRef CAS PubMed; (f) K. Satoh, S. Ozawa, M. Mizutani, K. Nagai and M. Kamigaito, Nat. Commun., 2010, 1, 1–6 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, 1H NMR, 13C NMR, 19F NMR, DOSY, and IR spectra, and DSC profiles. See DOI: 10.1039/c8ra00581h |
| This journal is © The Royal Society of Chemistry 2018 |