Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Theoretical study of the oxidation reactions of sulfurous acid/sulfite with ozone to produce sulfuric acid/sulfate with atmospheric implications†
Fang Shenga,
Liu Jingjing*b,
Chen Yua,
Tao Fu-Mingc,
Duan Xuemei*a and
Liu Jing-yao a
a
aInstitute of Theoretical Chemistry, Laboratory of Theoretical and Computational Chemistry, Jilin University, Changchun 130023, China. E-mail: duanxm@jlu.edu.cn
bDepartment of Chemistry, Key Laboratory of Organic Optoelectronics & Molecular Engineering of Ministry of Education, Tsinghua University, Beijing 100084, China. E-mail: enouragement@mail.tsinghua.edu.cn
cDepartment of Chemistry and Biochemistry, California State University, Fullerton, California 92834, USA
First published on 20th February 2018
Abstract
Herein, theoretical studies were performed on the atmospheric oxidation of sulfurous acid (H2SO3) and sulfite ions (HSO3−) by ozone (O3) to produce sulfuric acid and hydrosulfate ions. The most favorable path for the H2SO3 + O3 reaction has been found to be initiated from concerted H-abstraction and oxygen addition, with an overall energy barrier of 18.3 kcal mol−1. On the other hand, the most favorable path for the HSO3− + O3 reaction is initiated from oxygen addition, with an overall energy barrier of only 0.3 kcal mol−1. Kinetic simulations were performed to estimate the significance of these reactions in the formation of atmospheric sulfate and destruction of the ozone layer. The results provide new insight into the missing source of atmospheric sulfate and particulate matter.
1. Introduction
Oxidation reactions are the most important reactions in the atmosphere, which connect anthropogenic and natural species.1,2 The oxidation of sulfur species is considered to be the main channel for the production of atmospheric sulfuric acid (H2SO4) or sulfate species.3,4 Gaseous sulfuric acid and sulfate have been identified as the major drivers to generate atmospheric aerosols.3,5–7 As is well known, aerosols have a significant impact on the global environment such as climate change, reduction in visibility, and public health effects.8 In addition, hexavalent sulfur species are confirmed as the major fraction of PM2.5 (aerosol particles with an aerodynamic diameter less than 2.5 μm), which are associated with certain sicknesses and other acute or chronic health effects.7 Air pollution caused by sulfur emissions will remain for a long time in China due to its coal-based energy structure.9 Haze days with high concentrations of PM2.5 appear frequently in the northern cities of China during the cold winter and spring seasons10 due to the increase in emissions from heating.11,12 Atmospheric aerosols are hazardous to both human health and the environment.3,7,8 Therefore, understanding the formation process of sulfuric acid or sulfate species is a critical step in developing the atmospheric sulfur cycle.The oxidation of sulfur dioxide (SO2) is known as the main source of atmospheric sulfate.4,13–15 The oxidation reactions of SO2 have been extensively studied in a variety of experimental approaches and theoretical calculations.1,12–14,16–27 Several reaction mechanisms have been proposed, ranging from gas-phase oxidation by atmospheric radicals (Criegee intermediate16,17,25–27 and hydroxyl4,13,14 and hydroperoxy radicals18) to aqueous-phase reaction by O3,20,24,28 hydrogen peroxide,29 and others.30 To date, a consensus has emerged that the production of gaseous sulfate is determined by the SO2 + OH reaction.30 However, recent studies have shown that the formation of sulfate from traditional air quality models does not account for the high sulfate levels observed; this suggests the existence of missing pathways for sulfate production.31,32 Thus, new reaction pathways should be explored for the formation of atmospheric sulfate.
New models have been recently proposed for the formation of sulfate in the troposphere, clouds, aerosols, acid rain, and fog to bridge the gap between the modeled and observed sulfate.20,32–35 SO2 can be quickly taken into fog and rain droplets,36 which is followed by its liquid phase oxidation by O3 and H2O2.20,24,28,29,37,38 Model studies suggest that the oxidation of SO2 in the aqueous phase results in more than 80% of the global sulfate production.39 In some of these models, the hydrolysis reaction of SO2 plays an important role, and bisulfite anion (HSO3−) serves as a product of hydrolysis, which is one of the dominant sulfur(IV) species.40 The extensive vibrational spectroscopy studies conducted by Simon and Waldman revealed the presence of bisulfite anion (HSO3−), which resulted from the dissolution of SO2 in water.41–45 In addition, it has been proven that the existence of NH3 in hydrated SO2 clusters accelerates the production of HSO3−;33 moreover, the rate of oxidation of dissolved SO2 by O3 to form sulfate is enhanced.46 As another product of hydrolysis, sulfurous acid (H2SO3) was first generated in an experiment conducted by Schwarz et al. in 1988 and was considered stable in the gas phase.47 Recent studies have shown that SO2 can hydrolyze to produce sulfurous acid (H2SO3) or HSO3− in the gas phase in the presence of acid molecules or clusters such as water, hydrated ammonia, and hydrated sulfuric acid clusters.33,34 In addition, sulfurous acid and bisulfite were thought to be potential precursors for the formation of atmospheric aerosols. However, to the best of our knowledge, no studies have been reported on the atmospheric oxidation of H2SO3/HSO3− to produce H2SO4/HSO4−.
O3 is a reactive oxidant in natural and polluted tropospheres.48 It also acts as an oxidant in the aqueous oxidation of SO2 (ref. 24) together with transition metal ion catalysts (Co2+, Fe3+, and Mn2+).20,40 Furthermore, the gas-phase oxidation of SO2 by O3 is known to have minor contributions to atmospheric sulfate formation due to its relatively high energy barrier and small rate constant.49 However, Cheng et al. have reported that the concentrations of O3 drop dramatically during the haze period together with an increase in sulfate production; this suggests new possible pathways for the formation of sulfate in the presence of O3.32 Consequently, we carried out a detailed theoretical investigation on the reaction mechanism of H2SO3/HSO3− + O3. To further evaluate the atmospheric implications of the title reactions, the rate constants were calculated utilizing the transition state theory. Our results will provide potential insights into the new mechanisms for atmospheric sulfate generation.
2. Computational methods
The structures of the reactants, intermediates, transition states, and products were optimized by the M06-2X functional (density functional theory)50 combined with the 6-311++G(3df,3pd) basis set using the Gaussian 09 program.51 The M06-2X method has been widely used in the computation of atmospheric reactions and proven to provide reliable results.52–56 Harmonic vibrational analyses were conducted to provide the zero-point energy (ZPE) corrections as well as to confirm the minima character of the obtained geometries at the same level of theory (i.e. the local minimal with positive frequencies and saddle points with only one imaginary frequency). The ZPE corrections were included in the determination of relative energy for each stationary point. Intrinsic reaction coordinate (IRC) calculations were performed to confirm that the transition states corresponded to the designated reactants and products. To obtain more accurate energetic information, single point energy calculations were carried out at the CCSD(T)/aug-cc-pVTZ level of theory.Due to the partial biradical character of O3,57 the reliability of the results obtained had to be determined from the single-reference-based coupled-cluster wave function (CCSD(T)); one of the best methods to determine this is through the T1 diagnostic.58
| T1 = ‖t1‖/(N)elec1/2 | (1) |
3. Results and discussion
3.1 Mechanism of the H2SO3 + O3 reaction
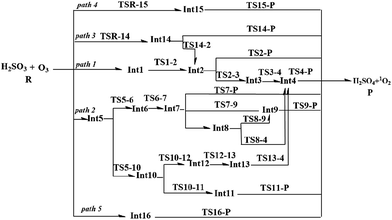
Due to the multiple reaction sites in H2SO3 and O3, there are five reaction pathways (paths 1–5) according to the different entrance channels. As shown in Fig. 1, in path 1, path 2, and path 5, the reactions start with the collision of the reactants; this leads to the formation of the hydrogen-bond intermediates Int1, Int5, and Int16, respectively. In paths 3 and 4, the reactions initiate from the cycloaddition reaction processes. The detailed reaction pathways with lower energy barriers as well as the geometrical structures of the stationary points are displayed in Fig. 2–4. The other reaction pathways with much higher energy barriers are presented in the ESI (Fig. S1 and S2†). | ||
| Fig. 2 CCSD(T)/aug-cc-pVTZ//M06-2X/6-311++G(3df,3pd) + ZPE energy profile and the optimized geometries of the stationary points for the reaction of H2SO3 + O3 (distances are in angstroms). | ||
 | ||
| Fig. 3 CCSD(T)/aug-cc-pVTZ//M06-2X/6-311++G(3df,3pd) + ZPE energy profile and the optimized geometries of the stationary points for the reaction of H2SO3 + O3 (distances are in angstroms). | ||
 | ||
| Fig. 4 CCSD(T)/aug-cc-pVTZ//M06-2X/6-311++G(3df,3pd) + ZPE energy profile and the optimized geometries of the stationary points for the reaction of H2SO3 + O3 (distances are in angstroms). | ||
For the second pathway, Int2 undergoes an isomerization process to form Int3 via the rotation of the dihedral angle O8–O9–S–O2 (TS2-3), and the energy barrier is 8.2 kcal mol−1. This rotation of the dihedral angle leads to distortion of the six-membered ring; accordingly, Int3 is less stable than Int2 by 3.8 kcal mol−1. Then, Int3 decomposes to produce mono-hydrated sulfur trioxide and 1O2 via the transition state TS3-4, in which the cleavage of O8–O9 bond and transfer of H5 from O7 to O2 take place simultaneously. The energy barrier of TS3-4 is 20.7 kcal mol−1. The hydrated sulfur trioxide can hydrolyze to form sulfuric acid via a four-membered proton transfer transition state (TS4-P) with an energy barrier of 23.1 kcal mol−1. Although the energy barrier of the SO3 hydrolysis reaction is slightly high, this reaction has been proven to be a catalytic reaction, and the energy barrier becomes evidently low or nearly disappears when water molecules or atmospheric acids act as catalysts in the hydrolysis reaction.62 Therefore, this reaction pathway will be increasingly important together with the environmental conditions such as high humidity or haze. Thus, the oxidation of H2SO3 by O3 starting from Int1 mainly occurs via the pathway reactants → Int1 → Int2 → P with the overall energy barrier of 18.3 kcal mol−1.
With respect to the second reaction channels, Int7 undergoes a rearrangement process to produce Int8, which needs to pass through the transition state TS7-8 with an energy barrier of 26.0 kcal mol−1. In TS7-8, H5 transfers from O7 to O9, H6 transfers from O9 to O3, and simultaneously, O7 adds to the S atom. Once Int8 is formed, there are two possible pathways connecting to P. For one pathway, Int8 undergoes an isomerization process (TS8-9), yielding the intermediate Int9. The energy barrier of this isomerization process is 9.0 kcal mol−1. Then, proton transfer and O7–O8 bond cleavage take place simultaneously via the transition state TS9-P to generate P. Otherwise, Int8 can connect to Int4 via the concerted transition state TS8-4 with an energy barrier of 16.9 kcal mol−1, and the following reaction pathways are the same as those in path 1. With regard to the third reaction channel initiating from Int7, it proceeds to produce Int9 via the transition state TS7-9. The computed energy barrier of TS7-9 is 21.2 kcal mol−1.
On the other hand, Int5 can also isomerize to produce Int10 via the seven-membered ring transition state TS5-10. Following Int10, the reaction proceeds to produce Int11, which finally decomposes to produce H2SO4 + 1O2 via TS11-P (see Fig. S1†). The energy barrier of this reaction path is as high as 35.7 kcal mol−1. In addition, Int10 can connect to Int4 via a continuous isomerization reaction process. These reaction pathways are not competitive due to their high energy barriers, and the details of these reaction pathways are presented in the ESI (see Fig. S1†). To summarize, among all the pathways discussed in this section, the reaction path: reactants → Int5 → Int6 → Int7 → P is the most favorable with the total energy barrier of 21.1 kcal mol−1.
The terminal oxygen atoms in O3 can also add to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond; this yields the five-membered structure Int15 (path 4). Then, Int15 dissociates to produce P via TSR-15, with an energy barrier (TSR-15) of 52.3 kcal mol−1. Thus, this reaction channel is not competitive.
O bond; this yields the five-membered structure Int15 (path 4). Then, Int15 dissociates to produce P via TSR-15, with an energy barrier (TSR-15) of 52.3 kcal mol−1. Thus, this reaction channel is not competitive.
For path 5, the reaction initiates from the hydrogen-bonded intermediate Int16, which is followed by oxygen addition via the transition state TS16-P to produce H2SO4 + 1O2. The energy barrier of TS16-P is 29.1 kcal mol−1 at the CCSD(T)/aug-cc-pVTZ level. However, the T1 diagnostic value of TS16-P is 0.068. Therefore, the CASPT2//CASSCF/aug-cc-pVTZ method was employed to further verify the energy barrier of TS16-P, and the computed energy barrier of TS16-P was 44.1 kcal mol−1 (see Fig. S2†). This further confirms that this reaction channel is kinetically unfavorable. To conclude, among the reactions discussed in this section, the reaction pathway reactants → Int14 → P is the most feasible with the total energy barrier of 19.0 kcal mol−1.
3.2 Mechanism of the HSO3− + O3 reaction
As discussed in the introduction, SO2 can easily hydrolyze to produce bisulfite in the gas phase, and the detailed mechanisms for the oxidation of HSO3− by O3 are presented in Fig. 5. The intramolecular interaction of HSO3− and O3 leads to the formation of the intermediate Int1′ with the binding energy of 4.6 kcal mol−1 and then Int1′ decomposes to the bisulfate ion (HSO4−) and 1O2 products via the transition state TS1-P′. In TS1-P′, O6 adds to the sulfur atom, and simultaneously, the O6–O7 bond breaks. The barrier height of this process is only 0.3 kcal mol−1. The reaction is exothermic by as much as 60.4 kcal mol−1. | ||
| Fig. 5 CCSD(T)/aug-cc-pVTZ//M06-2X/6-311++G(3df,3pd) + ZPE energy profile and the optimized geometries of the stationary points for the reaction of HSO3− + O3 (distances are in angstroms). | ||
The intermediate Int1′ can connect to Int2′ via the transition state TS1-2′. Similar to TS1-2, H5-abstraction and oxygen addition take place simultaneously in TS1-2′. The energy barrier of TS1-2′ is 0.3 kcal mol−1 relative to that of Int1′. Then, the reaction proceeds by the concerted transfer of H5 from O8 to O1 and O6–O7 bond cleavage via the transition state TS2-P′ to produce the terminal products P′ (HSO4− + 1O2). The energy barrier of TS2-P′ is 11.9 kcal mol−1.
In addition, the cycloaddition reaction can take place either by the transition state TSR-6′ or TS3-4′ to form the five-membered ring intermediate Int6′ or seven-membered ring intermediate Int4′, respectively. These two cycloaddition reactions are similar to the reaction processes via the transition states TSR-15 and TS5-10 (Fig. S2†). Int6′ decomposes into HSO4− and 1O2 by O–O bond cleavage via the transition state TS6-P′. Int4′ can connect to Int2′ via a continuous isomerization reaction via TS4-5′ and TS5-2′ and then decompose to HSO4− and 1O2 via the transition state TS2-P′. However, the two reaction pathways discussed in this section are not competitive due to their relatively high energy barriers (23.1 and 28.0 kcal mol−1). Therefore, among the reactions of HSO3− + O3, the reaction pathway reactant (HSO3− + O3) → Int1′ → TS1-P′ → P′(HSO4− + 1O2) is the most feasible with the total energy barrier of only 0.3 kcal mol−1.
3.3 Comparison of the molecular orbitals of the intermediates
When a H2SO3 molecule dissociates one proton forming the HSO3− anion, the reaction mechanisms are entirely different. Oxygen addition from Int1′ in the HSO3− + O3 reaction is the most favorable reaction pathway with an energy barrier of only 0.3 kcal mol−1 (Fig. 5), whereas the similar reaction path from Int16 in the H2SO3 + O3 reaction is unfeasible due to is high energy barrier of over 29 kcal mol−1 (see Fig. S2†). To further explain the huge differences in the energy barriers of these two reaction channels, the molecular orbital analysis is a good choice. It is generally recognized that the kinetic stability of reactants or reactant intermediates can be quantitatively determined using HOMO–LUMO energy separation based on the simple Hückel theory.63 The smaller the HOMO–LUMO gap, the more energetically favorable the electrons to add to a high-lying LUMO from a low-lying HOMO. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the intermediates Int1′ and Int16 are drawn in Fig. 6.As can be seen from Fig. 6, for both Int1′ and Int16, their HOMOs are mainly occupied on the sulfur atom, and the LUMOs are mainly distributed in the anti-bonding π* orbital of O3. The HOMO–LUMO gap in Int1′ (4.17 eV) is smaller than that in Int16 (5.77 eV); this indicates that the electrons transfer easily from the HOMO orbital to the LUMO orbital in Int1′. Furthermore, there are some electron distributions in the π*(O–O) orbital of O3 in Int1′ as well as some overlap between the orbital of lone pair electrons in the sulfur atom and the π*(O–O) anti-bonding orbital of O3. The electron distributions in π*(O–O) lead to an increase in the electron population of π*(O–O); this will ultimately facilitate O–O bond activation. The O–O bond activation can also be reflected by the different O–O bond lengths in Int1′ and Int16. As shown in Fig. 6 and S2,† the O–O bond lengths of O3 in Int1′ are 1.244 and 1.240 Å, which are larger than those in Int16 (1.227 and 1.229 Å, respectively). Therefore, the smaller HOMO–LUMO gap combined with the electron distributions of π*(O–O) in O3 may be one reason for the lower energy barrier of TS1-P′ in the HSO3− + O3 reaction.
3.4 Kinetics and implication in atmospheric chemistry
The rate constants for the main pathways of the H2SO3/HSO3− + O3 reactions were calculated in terms of the transition state theory (TST) with the Wigner tunneling correction. There are two competitive reaction pathways for the H2SO3 + O3 reaction. The reaction pathway reactants → Int1 → Int2 → P with the total energy barrier of 18.3 kcal mol−1 is the most favorable reaction pathway. The reaction pathway reactants → Int14 → P may be a competitive reaction path with the total energy barrier of 19.0 kcal mol−1. Therefore, both reaction pathways were considered. The reaction pathways can be depicted as follows, and the computational details are presented in the ESI.†
 | (R1) |
 | (R2) |
 | (R3) |
Considering the atmospheric temperature range from 212 to 298 K in the troposphere and stratosphere of the Earth, since the altitude changes from 0 km to 50 km,62 the rate constants have been calculated in the temperature range of 200–320 K. The calculated rate constants are presented in Table 1. For the reaction R1, the rate constants stay within the range of 3.94 × 10−31–8.68 × 10−25 cm3 per molecule per s. The rate constants of reaction R2 are about 4–5 orders of magnitude smaller than those of reaction R1; this highlights that the oxidation of H2SO3 by O3 takes place mainly via reaction R1 within the studied temperature range. The rate constants of R3 are in the range of 8.89 × 10−11–1.08 × 10−10 cm3 per molecule per s, which are 15–19 orders of magnitude larger than those of reaction R1. Vahedpour et al.49 previously reported the reaction mechanism and kinetics of the SO2 + O3 reaction. The rate constant of SO2 + O3 is 2.30 × 10−23 cm3 per molecule per s at room temperature, which is approximately 13 orders of magnitude smaller than that of reaction R3. Although SO3 produced from the SO2 + O3 reaction can further transform into H2SO4 via the atmospheric hydrolysis reaction, this reaction plays a minor role in atmospheric sulfate formation. Conversely, SO2 may first hydrolyze to form sulfite in the atmosphere; then, it is easily oxidized to produce sulfate in the presence of O3. This means that sulfite may be a key intermediate in the atmospheric production of sulfate, and O3 is a potentially important oxidant besides OH for atmospheric sulfate formation.
| Reaction | T/K | 200 | 220 | 240 | 260 | 280 | 298 | 300 | 320 |
|---|---|---|---|---|---|---|---|---|---|
| H2SO3 + O3 (R1) | Keq1 | 1.37 × 10−22 | 8.55 × 10−23 | 5.92 × 10−23 | 4.44 × 10−23 | 3.54 × 10−23 | 3.01 × 10−23 | 2.96 × 10−23 | 2.58 × 10−23 |
| kTS1-2 | 2.87 × 10−9 | 1.51 × 10−7 | 4.08 × 10−6 | 6.59 × 10−5 | 7.12 × 10−4 | 4.60 × 10−3 | 5.58 × 10−3 | 3.37 × 10−2 | |
| κ(kTS1-2) | 1.84 | 1.70 | 1.58 | 1.50 | 1.43 | 1.38 | 1.37 | 1.33 | |
| kTS2-P | 6.56 × 10−1 | 9.92 × 100 | 9.55 × 101 | 6.49 × 102 | 3.36 × 103 | 1.22 × 104 | 1.40 × 104 | 4.88 × 104 | |
| κ(kTS2-P) | 3.18 | 2.80 | 2.51 | 2.29 | 2.11 | 1.98 | 1.97 | 1.85 | |
| kuni | 2.87 × 10−9 | 1.51 × 10−7 | 4.08 × 10−6 | 6.59 × 10−5 | 7.12 × 10−4 | 4.60 × 10−3 | 5.58 × 10−3 | 3.37 × 10−2 | |
| k | 3.94 × 10−31 | 1.29 × 10−29 | 2.41 × 10−28 | 2.93 × 10−27 | 2.52 × 10−26 | 1.38 × 10−25 | 1.65 × 10−25 | 8.68 × 10−25 | |
| H2SO3 + O3 (R2) | kTSR-14 | 7.13 × 10−37 | 5.44 × 10−35 | 2.05 × 10−33 | 4.47 × 10−32 | 6.37 × 10−31 | 5.17 × 10−30 | 6.43 × 10−30 | 4.92 × 10−29 |
| κ(kTSR-14) | 1.24 | 1.20 | 1.17 | 1.14 | 1.12 | 1.11 | 1.11 | 1.09 | |
| kTS14-P | 6.42 × 10−6 | 3.03 × 10−4 | 7.62 × 10−3 | 1.18 × 10−1 | 1.24 × 100 | 7.96 × 100 | 9.65 × 100 | 5.84 × 101 | |
| κ(kTS14-P) | 2.23 | 2.01 | 1.85 | 1.73 | 1.63 | 1.55 | 1.55 | 1.48 | |
| k | 7.13 × 10−37 | 5.44 × 10−35 | 2.05 × 10−33 | 4.47 × 10−32 | 6.37 × 10−31 | 5.17 × 10−30 | 6.43 × 10−30 | 4.92 × 10−29 | |
| HSO3− + O3 (R3) | K′eq | 5.37 × 10−20 | 2.22 × 10−20 | 1.09 × 10−20 | 6.10 × 10−21 | 3.78 × 10−21 | 2.64 × 10−21 | 2.54 × 10−21 | 1.82 × 10−21 |
| K′1 | 8.91 × 10−11 | 9.76 × 10−11 | 9.34 × 10−11 | 1.02 × 10−10 | 1.05 × 10−10 | 1.09 × 10−10 | 1.09 × 10−10 | 1.13 × 10−10 | |
| k′−1 | 1.66 × 109 | 4.40 × 109 | 8.58 × 109 | 1.67 × 1010 | 2.79 × 1010 | 4.12 × 1010 | 4.29 × 1010 | 6.18 × 1010 | |
| k′TS1-P | 8.77 × 1011 | 9.65 × 1011 | 1.05 × 1012 | 1.12 × 1012 | 1.18 × 1012 | 1.24 × 1012 | 1.25 × 1012 | 1.30 × 1012 | |
| κ(k′TS1-P) | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 | |
| k′ | 8.89 × 10−11 | 9.72 × 10−11 | 9.26 × 10−11 | 1.00 × 10−10 | 1.03 × 10−10 | 1.05 × 10−10 | 1.05 × 10−10 | 1.08 × 10−10 |
Speculating that this oxidation reaction is responsible for the atmospheric removal of sulfite species, the atmospheric lifetime (τ) of sulfite can be estimated by the expression τ = (k′tot × [O3])−1 according to the reaction of HSO3− + O3. When the O3 average concentration is 7.0 × 1011 molecule per cm−3 in the atmosphere,64 the lifetime of sulfite is estimated to be 0.014 s at 298 K. This result indicates that once the atmospheric sulfur dioxide transforms into sulfite species, it can be oxidized to sulfate immediately. It has been reported that the production of sulfite from SO2 is almost a barrierless process in atmospheric aerosols in the presence of water, ammonia or atmospheric acids.33,34 Consequently, the oxidation of sulfite by O3 may provide a new reaction mechanism for the missing source of atmospheric sulfate.
It is worth mentioning that the concentration of O3 is quite different in the stratosphere. The ozonosphere exits at an altitude of 20–25 km in the stratosphere, where the O3 concentration reaches its maximum (∼2.7 × 1014 molecule per cm−3).65,66 Under this condition, the lifetime of sulfite is estimated to be approximately 4.0 × 10−3 s. Thus, sulfite pollutants reaching the stratosphere may contribute to the destruction of the ozone layer.
4. Conclusion
The reaction mechanism and kinetic investigations on the reaction of H2SO3/HSO3− + O3 were performed theoretically. According to our results, it is clear that the oxidation of H2SO3 by O3 plays a minor role in the formation of sulfuric acid, and the energy barrier of the favorable reaction channel is 18.3 kcal mol−1. For the oxidation reaction of HSO3− + O3, the energy barrier of the most feasible pathway is only 0.3 kcal mol−1. In addition, the kinetic analysis verified that the oxidation of HSO3− played an important role in the formation of atmospheric sulfate. Accordingly, in this study, the reaction mechanism of the atmospheric oxidation of tetravalent sulfur species (sulfurous acid/sulfite) to produce hexavalent sulfur species (sulfuric acid/sulfate) was proposed, which might be responsible for the missing source of sulfate and particulate matter in the atmosphere. In the stratosphere with a high concentration of O3, the lifetime of HSO3− decreases to 4.0 × 10−3 s; this means that the atmospheric sulfite species may have a potential effect on the destruction of the ozone layer. Our results predict that the sulfite species may be important intermediates in the oxidation of sulfur dioxide to produce sulfate, and O3 is an important potential oxidant besides OH for the formation of atmospheric sulfate.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (Grants 21373098) for providing the financial support. We are grateful to the Computing Center of Jilin Province and High Performance Computing Center of Changchun Normal University for providing essential support.Notes and references
- I. Barnes, J. Hjorth and N. Mihalopoulos, Chem. Rev., 2006, 106, 940–975 CrossRef CAS PubMed.
- A. Mellouki, T. J. Wallington and J. Chen, Chem. Rev., 2015, 115, 3984–4014 CrossRef CAS PubMed.
- R. Zhang, A. Khalizov, L. Wang, M. Hu and W. Xu, Chem. Rev., 2012, 112, 1957–2011 CrossRef CAS PubMed.
- J. G. Calvert, A. Lazrus, G. L. Kok, B. G. Heikes, J. G. Walega, J. Lind and C. A. Cantrell, Nature, 1985, 317, 27–35 CrossRef CAS.
- B. L. Nie, J. Wang, B. H. Qu, L. X. Sun and S. H. Yan, J. Aerosol Sci., 2017, 114, 169–179 CrossRef CAS.
- J. W. DePalma, B. R. Bzdek, D. J. Doren and M. V. Johnston, J. Phys. Chem. A, 2012, 116, 1030–1040 CrossRef CAS PubMed.
- R. Y. Zhang, Science, 2010, 328, 1366–1367 CrossRef CAS PubMed.
- R. J. Charlson, S. E. Schwartz, J. M. Hales, R. D. Cess, J. A. Coakley, J. E. Hansen and D. J. Hofmann, Science, 1992, 255, 423–430 CAS.
- J. Chang, D. Y. C. Leung, C. Z. Wu and Z. H. Yuan, Renewable Sustainable Energy Rev., 2003, 7, 453–468 CrossRef.
- S. Guo, M. Hu, M. L. Zamora, J. Peng, D. Shang, J. Zheng, Z. Du, Z. Wu, M. Shao, L. Zeng, M. J. Molina and R. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17373–17378 CrossRef CAS PubMed.
- Y. Wang, G. Zhuang, Y. Sun and Z. An, Atmos. Environ., 2006, 40, 6579–6591 CrossRef CAS.
- H. He, Y. Wang, Q. Ma, J. Ma, B. Chu, D. Ji, G. Tang, C. Liu, H. Zhang and J. Hao, Sci. Rep., 2014, 4, 4172 CrossRef PubMed.
- W. K. Li and M. L. McKee, J. Phys. Chem. A, 1997, 101, 9778–9782 CrossRef CAS.
- H. Tachikawa, J. Phys. Chem. A, 2014, 118, 3230–3236 CrossRef CAS PubMed.
- M. Goodarzi, M. Vahedpour and M. Solimannejad, Struct. Chem., 2012, 23, 1609–1615 CrossRef CAS.
- T. Berndt, T. Jokinen, M. Sipila, R. L. Mauldin III, H. Herrmann, F. Stratmann, H. Junninen and M. Kulmala, Atmos. Environ., 2014, 89, 603–612 CrossRef CAS.
- G. Sarwar, H. Simon, K. Fahey, R. Mathur, W. S. Goliff and W. R. Stockwell, Atmos. Environ., 2014, 85, 204–214 CrossRef CAS.
- B. S. Wang and H. Hou, Chem. Phys. Lett., 2005, 410, 235–241 CrossRef CAS.
- I. Kerezsi, G. Lente and I. Fabian, Dalton Trans., 2006, 955–960, 10.1039/b511363f.
- M.-Y. Nie, C. Gu, K.-L. Zhong and Y.-J. Fang, Energy Fuels, 2012, 26, 5590–5595 CrossRef CAS.
- J. Zhang, R. Zhang, X. Chen, M. Tong, W. Kang, S. Guo, Y. Zhou and J. Lu, Ind. Eng. Chem. Res., 2014, 53, 6450–6456 CrossRef CAS.
- H. Meng, Y. Zhu, G. J. Evans, C.-H. Jeong and X. Yao, J. Environ. Sci., 2015, 30, 90–101 CrossRef PubMed.
- T. Kurten, J. R. Lane, S. Jorgensen and H. G. Kjaergaard, J. Phys. Chem. A, 2011, 115, 8669–8681 CrossRef CAS PubMed.
- S. A. Penkett, Nat. Phys. Sci., 1972, 240, 105–106 CrossRef CAS.
- D. Stone, M. Blitz, L. Daubney, N. U. M. Howes and P. Seakins, Phys. Chem. Chem. Phys., 2014, 16, 1139–1149 RSC.
- L. Vereecken, H. Harder and A. Novelli, Phys. Chem. Chem. Phys., 2012, 14, 14682–14695 RSC.
- R. L. Mauldin III, T. Berndt, M. Sipilae, P. Paasonen, T. Petaja, S. Kim, T. Kurten, F. Stratmann, V. M. Kerminen and M. Kulmala, Nature, 2012, 488, 193–196 CrossRef PubMed.
- J. M. Anglada, G. J. Hoffman, L. V. Slipchenko, M. M. Costa, M. F. Ruiz-Lopez and J. S. Francisco, J. Phys. Chem. A, 2013, 117, 10381–10396 CrossRef CAS PubMed.
- S. M. Kunen, A. L. Lazrus, G. L. Kok and B. G. Heikes, J. Geophys. Res.: Oceans, 1983, 88, 3671–3674 CrossRef CAS.
- M. Kulmala, T. Petaja, M. Ehn, J. Thornton, M. Sipila, D. R. Worsnop and V. M. Kerminen, in Annual Review of Physical Chemistry, ed. M. A. Johnson and T. J. Martinez, 2014, vol. 65, pp. 21–37 Search PubMed.
- R. Y. Zhang, I. Suh, J. Zhao, D. Zhang, E. C. Fortner, X. X. Tie, L. T. Molina and M. J. Molina, Science, 2004, 304, 1487–1490 CrossRef CAS PubMed.
- Y. F. Cheng, G. J. Zheng, C. Wei, Q. Mu, B. Zheng, Z. B. Wang, M. Gao, Q. Zhang, K. B. He, G. Carmichael, U. Poschl and H. Su, Sci. Adv., 2016, 2, e1601530 Search PubMed.
- J. Liu, S. Fang, W. Liu, M. Wang, F.-M. Tao and J.-y. Liu, J. Phys. Chem. A, 2015, 119, 102–111 CrossRef CAS PubMed.
- J. Liu, S. Fang, Z. Wang, W. Yi, F.-M. Tao and J.-y. Liu, Environ. Sci. Technol., 2015, 49, 13112–13120 CrossRef CAS PubMed.
- G. H. Wang, R. Y. Zhang, M. E. Gomez, L. X. Yang, M. L. Zamora, M. Hu, Y. Lin, J. F. Peng, S. Guo, J. J. Meng, J. J. Li, C. L. Cheng, T. F. Hu, Y. Q. Ren, Y. S. Wang, J. Gao, J. J. Cao, Z. S. An, W. J. Zhou, G. H. Li, J. Y. Wang, P. F. Tian, W. Marrero-Ortiz, J. Secrest, Z. F. Du, J. Zheng, D. J. Shang, L. M. Zeng, M. Shao, W. G. Wang, Y. Huang, Y. Wang, Y. J. Zhu, Y. X. Li, J. X. Hu, B. Pan, L. Cai, Y. T. Cheng, Y. M. Ji, F. Zhang, D. Rosenfeld, P. S. Liss, R. A. Duce, C. E. Kolb and M. J. Molina, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13630–13635 CrossRef CAS PubMed.
- T. M. Townsend, A. Allanic, C. Noonan and J. R. Sodeau, J. Phys. Chem. A, 2012, 116, 4035–4046 CrossRef CAS PubMed.
- H. Akimoto, in Atmospheric Reaction Chemistry, 2016, pp. 1–433, DOI:10.1007/978-4-431-55870-5.
- C. R. Hoyle, C. Fuchs, E. Jarvinen, H. Saathoff, A. Dias, I. El Haddad, M. Gysel, S. C. Coburn, J. Trostl, A. K. Bernhammer, F. Bianchi, M. Breitenlechner, J. C. Corbin, J. Craven, N. M. Donahue, J. Duplissy, S. Ehrhart, C. Frege, H. Gordon, N. Hoppel, M. Heinritzi, T. B. Kristensen, U. Molteni, L. Nichman, T. Pinterich, A. S. H. Prevot, M. Simon, J. G. Slowik, G. Steiner, A. Tome, A. L. Vogel, R. Volkamer, A. C. Wagner, R. Wagner, A. S. Wexler, C. Williamson, P. M. Winkler, C. Yan, A. Amorim, J. Dommen, J. Curtius, M. W. Gallagher, R. C. Flagan, A. Hansel, J. Kirkby, M. Kulmala, O. Mohler, F. Stratmann, D. R. Worsnop and U. Baltensperger, Atmos. Chem. Phys., 2016, 16, 1693–1712 CrossRef CAS.
- I. Faloona, Atmos. Environ., 2009, 43, 2841–2854 CrossRef CAS.
- C. Brandt and R. Vaneldik, Chem. Rev., 1995, 95, 119–190 CrossRef CAS.
- A. Simon and K. Waldmann, Z. Anorg. Allg. Chem., 1955, 281, 113–224 CrossRef CAS.
- A. Simon and K. Waldmann, Z. Anorg. Allg. Chem., 1955, 281, 135–150 CrossRef CAS.
- A. Simon and K. Waldmann, Z. Anorg. Allg. Chem., 1956, 284, 36–46 CrossRef CAS.
- A. Simon and K. Waldmann, Z. Anorg. Allg. Chem., 1956, 284, 47–59 CrossRef CAS.
- A. Simon, K. Waldmann and E. Steger, Z. Anorg. Allg. Chem., 1956, 288, 131–147 CrossRef CAS.
- W. A. H. Asman and A. J. Janssen, Atmos. Environ., 1987, 21, 2099–2119 CrossRef CAS.
- D. Sulzle, M. Verhoeven, J. K. Terlouw and H. Schwarz, Angew. Chem., Int. Ed. Engl., 1988, 27, 1533–1534 CrossRef.
- R. Atkinson and W. P. L. Carter, Chem. Rev., 1984, 84, 437–470 CrossRef CAS.
- M. Vahedpour, M. Goodarzi, N. Hajari and F. Nazari, Struct. Chem., 2011, 22, 817–822 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
- L. Liu, X. Zhang, Z. Li, Y. Zhang and M. Ge, Chemosphere, 2017, 186, 430–437 CrossRef CAS PubMed.
- J. Wurmel and J. M. Simmie, J. Phys. Chem. A, 2017, 121, 8053–8060 CrossRef CAS PubMed.
- B. Baidya, M. Lily and A. K. Chandra, Comput. Theor. Chem., 2017, 1119, 1–9 CrossRef CAS.
- S. R. Hashemi and V. Saheb, Comput. Theor. Chem., 2017, 1119, 59–64 CrossRef CAS.
- Y. Lan, S. E. Wheeler and K. N. Houk, J. Chem. Theory Comput., 2011, 7, 2104–2111 CrossRef CAS PubMed.
- E. Miliordos and S. S. Xantheas, J. Am. Chem. Soc., 2014, 136, 2808–2817 CrossRef CAS PubMed.
- T. J. Lee and P. R. Taylor, Int. J. Quantum Chem., 1989, 199–207, DOI:10.1002/qua.560360824.
- J. Peiro-Garcia and I. Nebot-Gil, ChemPhysChem, 2003, 4, 843–847 CrossRef CAS PubMed.
- P. A. Malmqvist and B. O. Roos, Chem. Phys. Lett., 1989, 155, 189–194 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schuetz, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 242–253 CrossRef CAS.
- M. Torrent-Sucarrat, J. S. Francisco and J. M. Anglada, J. Am. Chem. Soc., 2012, 134, 20632–20644 CrossRef CAS PubMed.
- J. Aihara, J. Phys. Chem. A, 1999, 103, 7487–7495 CrossRef CAS.
- J. Bai, X. Sun, C. Zhang, C. Gong, J. Hu and J. Zhang, J. Environ. Sci., 2014, 26, 181–188 CrossRef CAS.
- R. Stolarski, R. Bojkov, L. Bishop, C. Zerefos, J. Staehelin and J. Zawodny, Science, 1992, 256, 342–349 CAS.
- M. Gupta and Abhishek, Indian J. Dent. Sci., 2012, 4, 47–51 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00411k |
| This journal is © The Royal Society of Chemistry 2018 |