Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
LC-MS based cell metabolic profiling of tumor cells: a new predictive method for research on the mechanism of action of anticancer candidates†
Hua Wanga,
Jia-hui Hu a,
Cui-chai Liua,
Min Liua,
Zheng Liub and
Li-xin Sun
a,
Cui-chai Liua,
Min Liua,
Zheng Liub and
Li-xin Sun *a
*a
aDepartment of Pharmaceutical Analysis, School of Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China. E-mail: sunlixin67@yahoo.com; Tel: +86 02443520600
bGLP Center, School of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University, Shenyang, China
First published on 8th May 2018
Abstract
In the process of anticancer drug development, research on the mechanism of action remains a major obstacle. In the present study, a cell metabolic profiling based discriminatory model was designed to give general direction on anticancer candidate mechanisms. Firstly, ultra-performance liquid chromatography in tandem with high-definition mass spectrometry was applied to obtain a comprehensive metabolic view of 12 human tumor cells. Secondly, multivariate data analysis was used to assess the metabolites’ variations, and 42 metabolites were identified as the main contributors to the discrimination of different groups. Then a metabolite-based prediction model was constructed for the first time and verified by cross validation (R2 = 0.909 and Q2 = 0.869) and a permutation test (R2 = 0.0871 and Q2 = −0.4360). To validate if the model can be applied for mechanism prediction, 4 independent sample sets were used to train the model and the data dots of different drugs were located in different regions. Finally, the model was applied to predict the anticancer mechanism of two natural compounds and the results were consistent with several other studies. Overall, this is the first experimental evidence which reveals that a metabolic profiling based prediction model has good performance in anticancer mechanism research, and thus it may be a new method for rapid mechanism screening.
1. Instruction
Progress in early detection and treatment, especially in the research of anticancer drugs, has been gradually changing cancer from a guaranteed death sentence into a chronic illness.1 The research and development of anticancer drugs is a complicated process including drug discovery, preclinical tests, and clinical trials. In this complex process, research on mechanism of action is of great significance to an anticancer candidate being developed and reaching the market.2 The research on mechanism of action mainly focuses on revealing the main targets of candidates and the signalling pathways associated with effects, discovering potential side effects, studying drug resistant mechanisms and instructing rational drug design.3 However, research on the mechanism of anticancer drug action is a very costly and time-consuming step, and it is reported that nearly one-third of the capital lost on all drug failures, $8 billion, is attributed to the failure of drug pharmacology and toxicology.4 Therefore, more-robust and faster approaches for predicting the preliminary mechanism of action of anticancer candidates are urgently needed to point out directions for further studies and improve the efficacy of drug development.In a previous study, on the basis of nucleotide content, a discriminatory model was developed which was capable of distinguishing different types of anti-tumor agents and preliminarily predicting the possible mechanism of compounds to a certain extent.5,6 However, the model was not satisfactory, and its prediction accuracy and stability were not perfect. This result could be attributable to the limitation of the method. The previous model only investigated the changes in intracellular nucleotide content. Actually, because of the extensive interactions and biochemical consequences, most intracellular metabolites will be affected after treatment. To improve the reliability of mechanism predictions, it could be better to extend the database to cover as many intracellular metabolites as possible. Hence, a more global and powerful approach to study the correlation between changes in metabolites and mechanisms of action may be a novel method to predict the mechanism of anticancer candidates.
As an emerging discipline, cell metabolic profiling may provide full insight into integral perturbed metabolic profiles and help understand the effect caused by treatment. Thus it has been widely performed to study the mechanisms of antitumor active components with unclear modes. For instance, based on cell metabolic profiling Gao established an approach to investigate the antitumor action mechanism of a novel flavonoid derivate on HepG2 cells.7 Hu analyzed the metabolic changes induced by celastrol treatment in human cervical cancer cells and provided new evidence of the pharmacological mechanism of celastrol.8 Furthermore, some metabolic profile based discrimination models have been reported in evaluating clinical therapy, diagnosing disease,9 revealing dose effects,10 determining disease stage,11 and improving avian productivity.12 For example, a study has demonstrated the novel strategy that the metabolic profiling approach may be applicable to show the discrimination of metabolites between treated and control groups in human endothelial cells, and thus contribute to the assessment of the clinical therapeutic effect.13 However, no information is available on metabolic profiling applied to anticancer mechanism prediction. Herein, a metabolic profile, for the first time, was adopted to create an anticancer mechanism prediction model which might be beneficial for understanding the wide-ranging molecular interactions induced by agent treatment in tumor cells.
In this protocol, ultra-performance liquid chromatography in tandem with high-definition mass spectrometry was chosen as a sensitive analytical technique to reveal the biological information of different tumor cell lines. Then the metabolism changes were analyzed after incubation with clinical chemotherapeutic drugs which exhibit one of four different kinds of action mechanism, including antimetabolic agents (5-fluorouracil, methotrexate and cytarabine), agents which directly act on DNA (mitomycin C, cisplatin and etoposide), agents which affect microtubule dynamics (taxol, vincristine and docetaxel), and RNA interference agents (daunorubicin, epirubicin and dactinomycin D). With multivariate statistical analysis, drug-correlated key markers were described, and based on the metabolites, a generalizable orthogonal partial least squares discriminant analysis (OPLS-DA) prediction model was obtained for the first time. By analyzing the following new data (gemcitabine, carmustine, mitoxantrone, vindesine) that belong to the four categories previously mentioned, the prediction accuracy of the model was further validated. Finally, using the obtained model we successfully predicted the anticancer mechanism of two natural compounds (apigenin, diosgenin) that had exhibited cytotoxicity. Our study demonstrated for the first time that the cell metabolic profiling based novel prediction model may be a reliable method for preliminary mechanism research of anticancer candidates.
2. Material and methods
2.1 Chemicals and reagents
High-performance liquid chromatography (HPLC) grade methanol and acetonitrile were purchased from Fisher Scientific (Waltham, USA). Internal standard L-methionine sulfone and testosterone propionate were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Dulbecco’s modified Eagle’s cell culture media (DMEM), Roswell Park Memorial Institute (RPMI) 1640 media, fetal calf serum (FCS) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were all obtained from Gibco-BRL (Grand Island, New York, USA).2.2 Cell lines and cell culture
All cell lines were obtained from the American Type Culture Collection (ATCC), (http://www.atcc.org/). Human pulmonary carcinoma cells (A549), human rhabdomyosarcoma cells (A204), human hepatocellular carcinoma cell lines (HepG2), human ovarian carcinoma cells (SKOV3), human breast carcinoma cells (T47D), and human breast adenocarcinoma cells (MCF7) were cultured in DMEM medium containing 3.7 g L−1 NaHCO3, and 10% FCS. Human fibrosarcoma cells (HT1080), human prostatic cancer cells (DU145), human prostate carcinoma cells (PC3), Henrietta Lacks strain of cancer cells (HeLa), human mouth epidermal carcinoma cells (KB), and human gastric carcinoma cells (SGC7901) were maintained in RPMI-1640 medium supplemented with 2.4 g L−1 HEPES, 2.0 g L−1 NaHCO3, and 10% FCS. The twelve tumor cell lines were incubated at 37 °C with 5% CO2 in a humidified air atmosphere for 4 or 5 days, with the culture duration selected to maintain cells under exponential growth and to reach 80% confluence.2.3 Cell treatment and quenching
Cells were seeded at a density of 1.0 × 106 cells into each T25 flask and cultivated for 24 h. According to the IC50,14 drugs were diluted with culture medium and added to flasks of treatment groups. After treatment with or without drugs for 48 h, the culture medium was removed from the flask and the cells were quickly washed twice with 3 mL of ice-cold phosphate-buffered saline (PBS, pH 7.4). The cells were rapidly quenched by liquid nitrogen (LN2), then suspended in ice-cold PBS by cell scraping with a pre-chilled cell scraper, which was performed on ice. The quenched biomass was then centrifuged (3 min, 1500 rpm) at 4 °C, with cell pellets collected and stored at −80 °C until further use. Each condition was applied to four replicates, three of which were applied for metabolic profiling and one for cell number counting to normalize the data. Cell counts were processed according to these steps: separation of the medium, then washing with cold PBS twice, detachment from flasks using 0.25% trypsin, and the final cell number was obtained using a hemocytometer.152.4 Sample preparation
The sample preparation followed a modified protocol.16 Briefly, to extract lipophilic metabolites, 300 μL of dichloromethane and 20 μL of internal standard (50 μg mL−1 methanol solution of testosterone propionate) were used and vortexed for 3 min. Maximum lysis of cell compartments was achieved by ultrasonic treatment for 1 min, allowing release of intracellular metabolites. Then the mixture was placed in an ice-water bath for 20 min, followed by a centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The supernatant was transferred to a new Eppendorf tube and evaporated to dryness under a gentle nitrogen stream at 40 °C. To extract the hydrophilic metabolites, 300 μL of methanol and 20 μL of internal standard (50 μg mL−1 methionine sulfoxide solution) were added to the residue. After vigorously vortexing for 3 min the mixture was placed in an ice-water bath for 20 min and centrifuged at 12
000 rpm for 10 min at 4 °C. The supernatant was transferred to a new Eppendorf tube and evaporated to dryness under a gentle nitrogen stream at 40 °C. To extract the hydrophilic metabolites, 300 μL of methanol and 20 μL of internal standard (50 μg mL−1 methionine sulfoxide solution) were added to the residue. After vigorously vortexing for 3 min the mixture was placed in an ice-water bath for 20 min and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The supernatant was collected and dried with a stream of nitrogen. Following this, the samples were reconstituted in 100 μL of acetonitrile–water (1
000 rpm for 10 min at 4 °C. The supernatant was collected and dried with a stream of nitrogen. Following this, the samples were reconstituted in 100 μL of acetonitrile–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,v/v) for analysis.
1,v/v) for analysis.
2.5 LC-MS analysis
The samples were analysed by an ACQUITY UPLC system coupled to a SYNAPT-G2-Si high-definition mass spectrometer from Waters (Milford, USA). The HILIC method helped to separate highly polar metabolites whereas the traditional RPLC method provided efficient separation of nonpolar metabolites. These two methods were complementary and together, can provide enhanced coverage of metabolites.For both RP and HILIC modes, the injection volume was 5 μL. The mass spectrometry was performed in electrospray positive ionization mode with mass ranges of 124–1000 Da and 8–1000 Da. Instrument settings were set as follows: ion voltage 3.0 kV, source temperature 120 °C, desolvation temperature 350 °C, cone voltage at 30 V and 20 V respectively, the cone gas flow 50 L h−1 and desolvation gas flow 600 L h−1. The scan time was set at 0.2 s, the interscan delay at 0.1 s. The same parameters were applied for the simultaneous MS and MS/MS fragments analyses, with a collision energy ramp from 10 to 35 eV.
2.6 Method validation
In order to investigate the repeatability of the method, we prepared six SGC cell samples in parallel, and seven steady peaks including internal standard were then chosen to calculate the RSD values using retention time and relative peak area. In addition, one identical sample was stored at room temperature after preparation and analyzed at 0, 2, 4, 8, 12 and 24 h, to validate the stability. On the other hand, to check the stability of the LC-MS system while running samples, quality control (QC) samples were injected at the beginning of the run and after every six real samples. Prior to analysis, QC samples were prepared by mixing equal aliquots of all of the samples in the identical corresponding dataset.172.7 Statistical analysis
All of the raw data were imported to the MarkerLynx 4.1 software (Waters) for peak alignment, background noise subtraction, and data reduction to obtain a metabolite features list containing aligned retention time and m/z values. The main parameters were set as follows: mass range 80–1000 Da, mass window 0.05 Da, minimum intensity 5% of base peak intensity, noise elimination level 6 and RT tolerance 0.5 min. To acquire consistent variables, the data was filtered further by removing any peaks with missing values (ion intensity = 0) greater than 20% in all samples.18 Finally, the data were log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10-transformed and Paretoscaled before the statistical analysis.
10-transformed and Paretoscaled before the statistical analysis.
Raw data quality was checked by principal component analysis (PCA), an unsupervised method, with the SIMCA13.0 software (Umetrics AB, Umea, Sweden), both for each batch and for all batches together. Then a generic and powerful processing method known as OPLS-DA, which is able to effectively remove the non-correlated variations from X, was executed to identify the metabolic variations caused by treatments. Differential metabolites responsible for discrimination between the groups were selected on the basis of a threshold of variable importance in projection value from the OPLS-DA model. In parallel, one-way analysis of variance (ANOVA) along with a Bonferroni correction was applied to calculate the statistical difference of each variable whose VIP value was higher than 1.0, setting the significance level to 0.05 as a criterion with the SPSS 19.0 software. Heat map analysis was performed by MultiExperiment Viewer (version 4.8.1).
The receiver operating characteristic (ROC) curve was used to evaluate whether the differential metabolites were the independent factors of drug action. The discriminatory power of each candidate was ranked using the area under the curve (AUC). Biomarkers with AUC >0.50 were regarded as being highly sensitive and specific.
2.8 Metabolite identification
The putative identification of these discriminative metabolites was based on accurate mass, retention time, and MS/MS information. The levels of chemical identification were in accordance with the published guidelines for metabolomics studies.19,20 Level 1 was used for the direct matching of orthogonal data, such as the retention time and mass spectrum (MS and MS/MS spectra) using standard molecules. All standard molecules were solubilized in water–acetonitrile (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50,v/v). Level 2 was used for an MS/MS spectral data match of a specific compound in electronic libraries such as the HMDB database, the METLIN database and the KEGG database, but with no standards available. Level 3 was used only for chemical class assignments where the exact identity of the compound could not be verified. To further illustrate whether the observed differences in the metabolites reflect coordinated alternations in defined metabolic pathways, a pathway analysis was performed in Metabo Analyst 3.0 (http://www.metaboanalyst.ca/MetaboAnalyst/).
50,v/v). Level 2 was used for an MS/MS spectral data match of a specific compound in electronic libraries such as the HMDB database, the METLIN database and the KEGG database, but with no standards available. Level 3 was used only for chemical class assignments where the exact identity of the compound could not be verified. To further illustrate whether the observed differences in the metabolites reflect coordinated alternations in defined metabolic pathways, a pathway analysis was performed in Metabo Analyst 3.0 (http://www.metaboanalyst.ca/MetaboAnalyst/).
2.9 Model construction
Based on the metabolomic change, we constructed a OPLS-DA model for anticancer mechanism prediction of candidates. Meanwhile, for the purpose of evaluating the robustness of the model, sevenfold cross validation was applied which was further confirmed by analysis of variance (CV-ANOVA). In addition, the model was likely to suffer from overfit even though the Q2 value satisfies the acceptable threshold, which prompted us to employ a permutation test for verifying the model given.2.10 Evaluation and application of the model
To evaluate the accuracy of the above model, the model was validated with the metabolic profiling data of gemcitabine (GEM), carmustine (BCNU), mitoxantrone (MIT) and vindesine (VDS), and the four drugs belonging to the four categories previously used to construct the model. In the next step we used the model to predict the potential mechanism of apigenin (API) and diosgenin (DIO), which have been reported to have an unclear anticancer effect.3. Results
3.1 Method validation
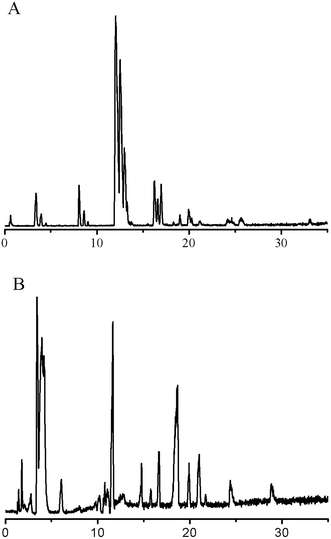
Two typical total ion chromatograms obtained from the RPLC method and HILIC method are illustrated in Fig. 1. The repeatability results are shown in Table 1; the RSD of retention time for the RPLC and HILIC analyses ranged from 0.04% to 1.2% and 0.08% to 0.10% respectively, while the RSD of relative peak area ranged from 11% to 15% and 3.0% to 8.5% respectively, indicating the good reproducibility of this method. The RSDs of stability (<15%) showed that the preparation sample stored at room temperature was stable for 24 h. In addition, we imported the QC sample data into SIMCA13.0 for PCA analysis and all of the QC samples shifted a small extent within 2 SD, suggesting excellent systematic stability during the whole experiment (Fig. S7, ESI†). These quality assessments showed that the method was reliable and that any observed differences between the test samples would reflect variations in their metabolite profiles rather than technical errors.| Mode | Metabolite | m/z | RSD/RT (%) | RSD/area (%) |
|---|---|---|---|---|
| RPLC | Methyl malonyl carnitine | 262.3071 | 1.2 | 14 |
| RPLC | 3-Methylglutaryl-carnitine | 290.3483 | 0.14 | 14 |
| RPLC | Hexadeca-sphinganine | 274.3148 | 0.30 | 15 |
| RPLC | Dihydro-sphingosine | 302.3059 | 0.09 | 12 |
| RPLC | Cyclic GMP | 346.3753 | 0.18 | 11 |
| RPLC | Testosterone propionate | 345.2750 | 0.27 | 12 |
| RPLC | DG(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)/20 1(9Z)/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(11Z)/0 1(11Z)/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
621.4358 | 0.04 | 15 |
| HILIC | L-Phenylalanine | 165.9617 | 0.10 | 8.5 |
| HILIC | L-Methionine sulfone | 182.0782 | 0.09 | 4.7 |
| HILIC | L-Glutamic acid | 148.0601 | 0.09 | 3.0 |
| HILIC | Glutamine | 147.0724 | 0.08 | 7.8 |
| HILIC | Glycerophosphocholine | 258.1533 | 0.08 | 4.9 |
| HILIC | 4-Pyridoxic acid | 184.1304 | 0.09 | 3.7 |
| HILIC | Histidine | 156.0900 | 0.08 | 7.0 |
3.2 Quality assessment of the LC-MS data
To illustrate the general trend, unsupervised PCA was performed, and the results of the PCA of the untargeted dataset are displayed in Fig. S8 (ESI†). The first two principal components accounted for only 47% of the overall variability. In the PCA score plot, each data point represented one sample, and the distance between points in the score plot was an indication of similarity between samples. The results indicated that the four groups clustered and separated respectively, which may be caused by the action of the four drugs on the twelve tumor cells. However, some sample points overlapped with the other group and any individual outlier was ruled out.3.3 Selection and identification of differential metabolites
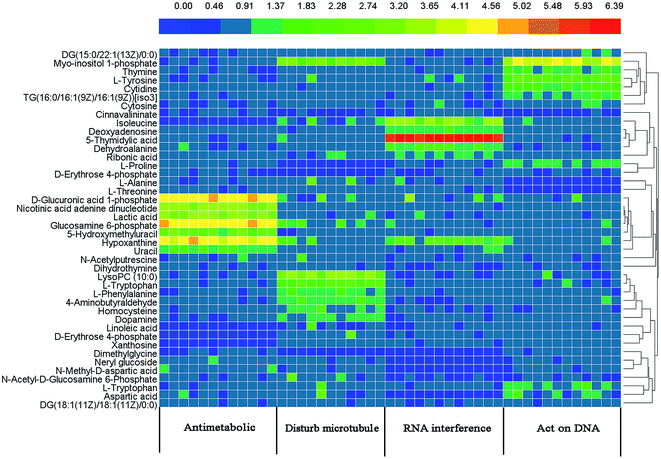
After OPLS-DA analysis, based on the VIP values (VIP > 1.0), a list of altered metabolites making the greatest contribution to the classification were screened. Among them, the concentrations of 11, 17, 12 and 12 metabolites for each drug-treated group (antimetabolic agents, agents directly acting on DNA, agents which affect microtubule dynamics, and RNA interference agents) were observed to be significantly different (p < 0.05) between the two groups.Tables 2–5 summarise the marker metabolite information and the fold change (FC), with a positive value indicating a relatively higher concentration is present in the drug-treated group, while a negative value suggests a relatively lower concentration is present in treatment group. To understand the difference between the metabolites across the four drug treated groups, the 42 metabolites were visualized in a clustered heat map (Fig. 2), with colors based on the actual FC values. The metabolites were classed into four clusters, which demonstrated the ability of metabolomics to distinguish the mechanism of action of different anticancer drugs.
| No. | RT/min | Biomarker | m/z | P value | AUC | FC | Trend | Metabolite pathway |
|---|---|---|---|---|---|---|---|---|
| a “↑” indicates that the metabolite content in the drug-treated group is higher than that in the control group; “↓” indicates that the metabolite content in the drug-treated group is lower than that in the control group; the P value was calculated using One Way ANOVA with the Bonferroni correction (significance at *P < 0.05, **P < 0.01). | ||||||||
| 1 | 35.704 | DG(15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/22 0/22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(13Z)/0 1(13Z)/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
654.5905 | **P < 0.01 | 0.674 | 1.298 | ↑ | Glycerolipids metabolism |
| 2 | 39.784 | Myo-inositol 1-phosphate | 261.0414 | *P < 0.05 | 0.805 | 3.469 | ↑ | Inositol phosphate metabolism |
| 3 | 24.220 | Cinnavalininate | 301.0518 | *P < 0.05 | 0.678 | 0.816 | ↓ | Tryptophan catabolism |
| 4 | 39.799 | LysoPC (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
413.3280 | *P < 0.05 | 0.819 | 3.592 | ↑ | Phospholipid metabolism |
| 5 | 21.039 | 4-Aminobutyraldehyde | 88.0743 | *P < 0.05 | 0.546 | 1.628 | ↑ | Arginine catabolism |
| 6 | 11.865 | Dimethylglycine | 104.0729 | *P < 0.05 | 0.561 | 0.620 | ↓ | Glycine metabolism |
| 7 | 15.049 | L-Proline | 116.0712 | *P < 0.05 | 0.582 | 0.314 | ↓ | Arginine and proline metabolism |
| 8 | 15.609 | Homocysteine | 136.0404 | **P < 0.01 | 0.655 | 1.632 | ↑ | Cysteine and methionine metabolism |
| 9 | 15.405 | Dopamine | 154.0883 | *P < 0.05 | 0.606 | 1.440 | ↑ | Tyrosine catabolism |
| 10 | 13.764 | L-Phenylalanine | 165.9617 | *P < 0.05 | 0.621 | 1.724 | ↑ | Phenylalanine metabolism |
| 11 | 12.077 | L-Tryptophan | 205.0541 | *P < 0.05 | 0.547 | 2.195 | ↑ | Tryptophan metabolite |
| 12 | 17.052 | D-Erythrose 4-phosphate | 223.0174 | **P < 0.01 | 0.501 | 0.644 | ↓ | Pentose phosphate metabolism |
| No. | RT/min | Biomarker | m/z | P value | AUC | FC | Trend | Metabolite pathway |
|---|---|---|---|---|---|---|---|---|
| a “↑” indicates that the metabolite content in the drug-treated group is higher than that in the control group; “↓” indicates that the metabolite content in the drug-treated group is lower than that in the control group; the P value was calculated using One Way ANOVA with the Bonferroni correction (significance at *P < 0.05, **P < 0.01). | ||||||||
| 1 | 24.322 | Cinnavalininate | 301.2291 | *P < 0.05 | 0.712 | 0.756 | ↓ | Tryptophan catabolism |
| 2 | 35.764 | DG(15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/22 0/22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(13Z)/0 1(13Z)/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
654.5937 | **P < 0.01 | 0.704 | 1.274 | ↑ | Glycerolipids metabolism |
| 3 | 39.875 | TG(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16 0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)/16 1(9Z)/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z))[iso3] 1(9Z))[iso3] |
820.7550 | *P < 0.05 | 0.762 | 1.564 | ↑ | Glycerolipids metabolism |
| 4 | 39.780 | Myo-inositol 1-phosphate | 261.0370 | *P < 0.05 | 0.804 | 4.177 | ↑ | Inositol phosphate metabolism |
| 5 | 40.722 | DG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(11Z)/18 1(11Z)/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(11Z)/0 1(11Z)/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
638.5786 | *P < 0.05 | 0.645 | 1.041 | ↑ | Glycerolipids metabolism |
| 6 | 20.912 | 4-Aminobutyraldehyde | 88.0744 | *P < 0.05 | 0.616 | 1.149 | ↑ | Arginine catabolism |
| 7 | 15.647 | L-Alanine | 90.0564 | *P < 0.05 | 0.539 | 0.285 | ↓ | Alanine metabolism |
| 8 | 10.820 | Cytosine | 112.0494 | *P < 0.05 | 0.524 | 1.224 | ↑ | Pyrimidine metabolism |
| 9 | 15.047 | L-Proline | 116.0708 | *P < 0.05 | 0.671 | 1.372 | ↑ | Arginine and proline metabolism |
| 10 | 11.907 | L-Threonine | 120.0678 | *P < 0.05 | 0.654 | 0.661 | ↓ | L-Threonine metabolism |
| 11 | 11.690 | Thymine | 127.0493 | *P < 0.05 | 0.522 | 1.959 | ↑ | Pyrimidine metabolism |
| 12 | 20.908 | Dihydrothymine | 129.0671 | **P < 0.01 | 0.558 | 0.965 | ↓ | Pyrimidine metabolism |
| 13 | 16.862 | N-Acetylputrescine | 131.1208 | *P < 0.05 | 0.651 | 0.968 | ↓ | Arginine catabolism |
| 14 | 15.421 | Dopamine | 154.0849 | *P < 0.05 | 0.575 | 1.166 | ↑ | Tyrosine catabolism |
| 15 | 15.584 | L-Tyrosine | 181.9096 | *P < 0.05 | 0.609 | 2.705 | ↑ | Tyrosine metabolism |
| 16 | 12.031 | L-Tryptophan | 205.0562 | *P < 0.05 | 0.533 | 1.603 | ↑ | Tryptophan metabolite |
| 17 | 15.068 | Cytidine | 244.0982 | **P < 0.01 | 0.597 | 1.835 | ↑ | Pyrimidine metabolism |
| No. | RT/min | Biomarker | m/z | P value | AUC | FC | Trend | Metabolite pathway |
|---|---|---|---|---|---|---|---|---|
| a “↑” indicates that the metabolite content in the drug-treated group is higher than that in the control group; “↓” indicates that the metabolite content in the drug-treated group is lower than that in the control group; the P value was calculated using One Way ANOVA with Bonferroni correction (significance at *P < 0.05, **P < 0.01, ***P < 0.001); “—” means that there is no corresponding metabolite pathway determined by MetaboAnalyst 3.0. | ||||||||
| 1 | 12.260 | D-Glucuronic acid 1-phosphate | 275.0116 | ***P < 0.001 | 0.696 | 4.932 | ↑ | Amino sugar and nucleotide sugar metabolism |
| 2 | 16.821 | Uracil | 113.0351 | **P < 0.01 | 0.783 | 1.808 | ↑ | Pyrimidine metabolism |
| 3 | 0.661 | Hypoxanthine | 136.9225 | ***P < 0.001 | 0.652 | 4.736 | ↑ | Pyrimidine metabolism |
| 4 | 40.628 | Nicotinic acid adenine dinucleotide | 666.1287 | *P < 0.05 | 0.722 | 3.708 | ↑ | Act as second messenger |
| 5 | 33.008 | Glucosamine 6-phosphate | 282.2731 | *P < 0.05 | 0.643 | 4.980 | ↑ | Alanine, aspartate and glutamate metabolism |
| 6 | 39.893 | Linoleic acid | 303.3405 | *P < 0.05 | 0.522 | 0.166 | ↓ | Lipid metabolism |
| 7 | 13.055 | Isoleucine | 131.9333 | ***P < 0.001 | 0.678 | 0.013 | ↓ | Valine, leucine and isoleucine biosynthesis |
| 8 | 36.194 | 5-Hydroxymethyluracil | 143.0419 | ***P < 0.001 | 0.650 | 3.027 | ↑ | — |
| 9 | 11.862 | Lactic acid | 91.0408 | ***P < 0.001 | 0.543 | 3.800 | ↑ | Glycolysis or gluconeogenesis |
| 10 | 1.879 | D-Erythrose 4-phosphate | 218.0164 | ***P < 0.001 | 0.609 | 0.019 | ↓ | Pentose phosphate pathway |
| 11 | 33.832 | Xanthosine | 307.0836 | *P < 0.05 | 0.543 | 0.169 | ↓ | Purine metabolism |
| No. | RT/min | Biomarker | m/z | P value | AUC | FC | Trend | Metabolite pathway |
|---|---|---|---|---|---|---|---|---|
| a “↑” indicates that the metabolite content in the drug-treated group is higher than that in the control group; “↓” indicates that the metabolite content in the drug-treated group is lower than that in the control group; the P value was calculated using One Way ANOVA with the Bonferroni correction (significance at *P < 0.05, **P < 0.01, ***P < 0.001); “—” means that there is no corresponding metabolite pathway determined by MetaboAnalyst 3.0. | ||||||||
| 1 | 39.952 | Ribonic acid | 167.0589 | ***P < 0.001 | 0.520 | 1.562 | ↑ | — |
| 2 | 12.269 | Deoxyadenosine | 274.1097 | ***P < 0.001 | 0.875 | 1.646 | ↑ | Purine metabolism |
| 3 | 12.759 | 5-Thymidylic acid | 340.2486 | **P < 0.01 | 0.843 | 6.795 | ↑ | Pyrimidine metabolism |
| 4 | 40.002 | Neryl glucoside | 345.0645 | **P < 0.01 | 0.807 | 0.004 | ↓ | — |
| 5 | 16.917 | Uracil | 113.0375 | **P < 0.01 | 0.521 | 1.011 | ↑ | Pyrimidine metabolism |
| 6 | 0.675 | Hypoxanthine | 136.9216 | ***P < 0.001 | 0.821 | 2.487 | ↑ | Pyrimidine metabolism |
| 7 | 12.983 | Isoleucine | 131.9327 | ***P < 0.001 | 0.912 | 3.769 | ↑ | Valine, leucine and isoleucine biosynthesis |
| 8 | 19.790 | Aspartic acid | 134.0478 | ***P < 0.001 | 0.615 | 0.411 | ↓ | Alanine, aspartate and glutamate metabolism |
| 9 | 19.792 | Dehydroalanine | 88.0386 | ***P < 0.001 | 0.812 | 1.856 | ↑ | Methylation metabolism |
| 10 | 11.429 | Dimethylglycine | 104.0691 | **P < 0.01 | 0.512 | 0.136 | ↓ | Methylation metabolism |
| 11 | 15.802 | N-Methyl-D-aspartic acid | 148.0639 | **P < 0.01 | 0.735 | 0.021 | ↓ | — |
| 12 | 1.795 | N-Acetyl-D-glucosamine 6-phosphate | 319.0641 | ***P < 0.001 | 0.631 | 0.106 | ↓ | Amino sugar and nucleotide sugar metabolism |
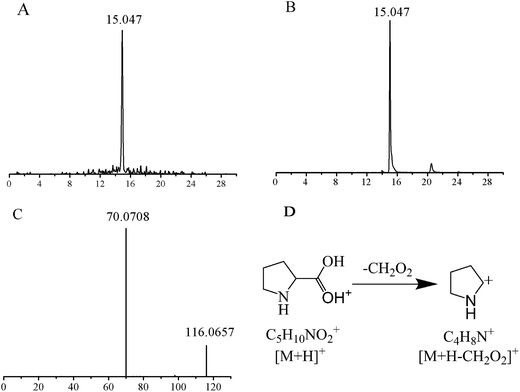
The structures of these candidates were identified using the method described above, by which 9 were identified using standard molecules, 30 were identified by matching to a database, and the rest by fragmentation pathway (shown in Table 2). For example, the identification of the candidate at m/z 116.0708 (retention time: 15.047 min) is shown in Fig. 3. First, the accordant quasimolecular ion peak was determined based on its retention time in Masslynx with m/z 116.0708 (Fig. 3A). Then the candidate was found to be L-proline by searching the HMDB and METLIN databases. Finally, the result was confirmed by comparison with the retention time (Fig. 3B) and the MS/MS spectrum of an L-proline standard (Fig. 3C). A fragmentation pathway was used to identify other metabolites with similar structures.
3.4 Construction and validation of metabolite-based prediction model
The data matrix (retention time–mass pairs, sample names and normalized peak areas) obtained by RPLC and HILIC was utilized to construct OPLS-DA models respectively. Then the data were combined together, namely RP + HI, and the quality of the model was evaluated using a number of evaluation values such as the parameter R2, which indicated the goodness of fit, and Q2, which represented the model’s predictability. It can be seen from Table S3 (ESI†) that after data integration the values of R2 (cum.) and Q2 (cum.) were 0.909 and 0.869, which were larger than the parameters of the samples analyzed in one analysis mode. The results highlighted the advantages of the method using the RPLC/HILIC method for the analysis of both polar and non-polar metabolites within a complex cell sample. Once the data structure had been chosen, the OPLS-DA prediction model (Fig. 4A) was constructed with all of the samples from the four categories.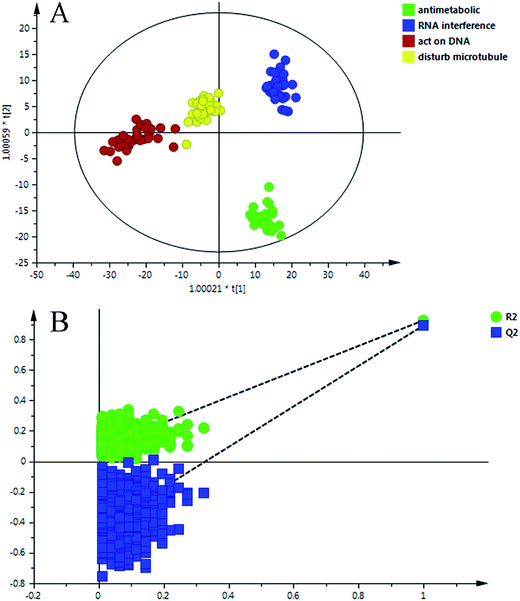 | ||
| Fig. 4 (A) OPLS-DA scores scatter plot of the four classes on the RP + HI dataset; (B) permutation test of the OPLS-DA model. | ||
To validate the stability and accuracy of the model, sevenfold cross validation was applied which was further confirmed by the analysis of variance CV-ANOVA with a p value ≪0.01, which is shown in Table S4 (ESI†). Of the 999 random permutations, all R2 (cum.) and Q2 (cum.) values computed from the permuted dataset were below those from the original dataset, and the intercept values of R2 = 0.0871 and Q2 = −0.4360 are demonstrated in Fig. 4B. Overall, these results indicated that the predictive capabilities of the OPLS-DA models of the LC-MS data were reliable.
3.5 Evaluation of prediction models with new sample sets
As shown in Fig. 4, the new data dots of different drugs are located in different regions. The four drugs were considered: disturbing microtubule dynamics (VDS), embedding between a double-stranded DNA (MIT), alkylating nucleic acid chain (BCNU) and cytidine derivatives (GEM). The dots of VDS completely overlapped with those of agents that affect microtubule dynamics, with R2 = 0.718 and Q2 = 0.674. The process was replicated, and a lot of sample points of GEM, BCNU and MIT were distributed in the corresponding area. In general, these results were in agreement with the actual antitumor mechanism. These results may partially prove that the model could accurately predict the anticancer mechanism of candidates that belonged to the four categories.3.6 Application of the prediction model
To further evaluate the performance of the model in mechanism prediction, the cell lines were treated with API and DIO in the same method described above. Fig. 5 shows that most of the data points which represent the API treatment group are located in the same area as those for agents that affect microtubule dynamics, with R2 = 0.838 and Q2 = 0.762, suggesting that the API was likely to have an anticancer effect by interfering with microtubules. However, the data dots of DIO fell in the region between those of agents that affect microtubule dynamics and those of agents that directly act on DNA. The parameters of R2 = 0.618 and Q2 = 0.589 indicated that the prediction model was feasible but not very accurate when it was applied to DIO. This explained and highlighted that DIO may have a complicated anticancer effect on tumor cells.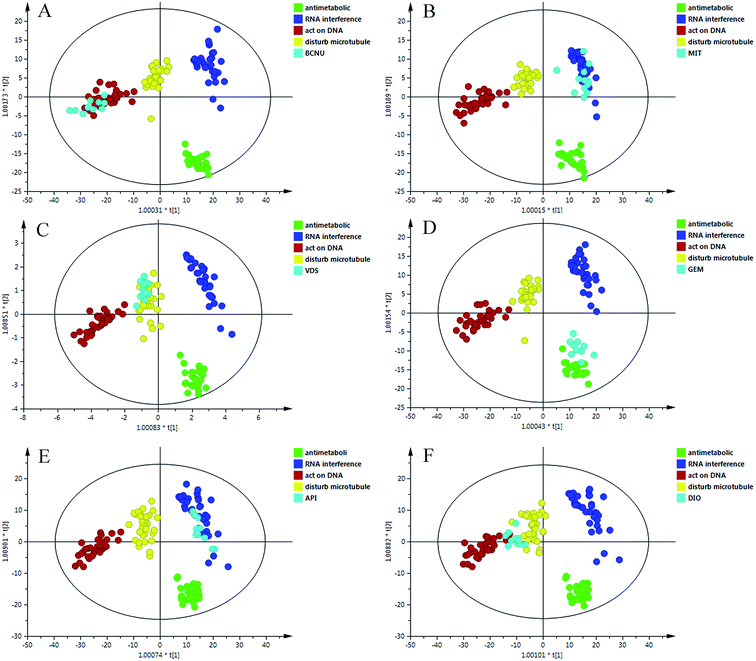 | ||
| Fig. 5 The OPLS-DA score plots of the different treated groups and four classes. (A) BCNU; (B) MIT; (C) VDS; (D) GEM; (E) API; (F) DIO. | ||
4. Discussion
4.1 Optimization of analysis method
Our study sought to analyze a great deal of metabolites to gather real-time cell metabolomic information and thus deepen the knowledge of drug effects on cancer cells. Considering that direct methanolic extraction was also preferred in many biological samples, the extraction solution was optimized according to the peak number in the TIC, which is shown in Fig. 1. At first, a liquid–liquid extraction method was applied with chloroform–methanol–water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,v/v/v). However, there was a protein membrane between the upper and lower layers of the two-phases, which caused trouble in the sampling process and introduced a large deviation to the subsequent analysis. Thus a binary solvent (methylene chloride and methanol) was utilized, which better facilitated sample handling and substituted dichloromethane for chloroform, thereby reducing harm to the operator.
2,v/v/v). However, there was a protein membrane between the upper and lower layers of the two-phases, which caused trouble in the sampling process and introduced a large deviation to the subsequent analysis. Thus a binary solvent (methylene chloride and methanol) was utilized, which better facilitated sample handling and substituted dichloromethane for chloroform, thereby reducing harm to the operator.
4.2 Effects of anticancer agents on metabolic profiles
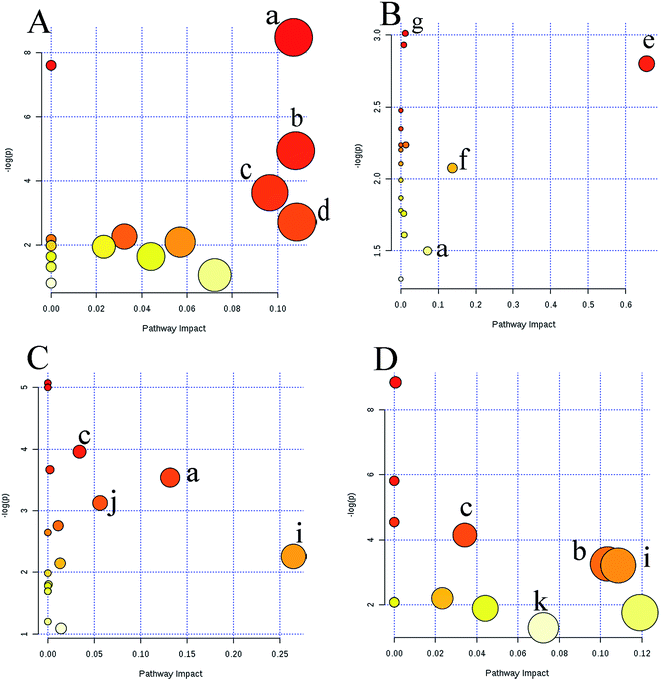
Metabolic profiling not only reveals the individual metabolite alteration but also provides an integrated view of the metabolic processes induced by anticancer candidates. With multivariate and univariate statistical analyses, 42 metabolites were tentatively identified and an OPLS-DA model was constructed to preliminarily predict the possible mechanism of candidates. Based on the metabolite information, a map of anticancer drug related metabolic pathways was constructed and is shown in Fig. 6. Several metabolic pathways were involved in the modifications between the groups. MetaboAnalyst was used to assign different pathways for the feature compounds.The dots in Fig. 6 represent the pathways that were matched using pathway impact values from pathway topology analysis and p values from pathway enrichment analysis. Pyrimidine metabolism, purine metabolism, arginine and proline metabolism, glycine, serine and threonine metabolism, tryptophan metabolism, linoleic acid metabolism, pyruvate metabolism, amino sugar and nucleotide sugar metabolism, alanine, aspartate and glutamate metabolism, phenylalanine metabolism, tyrosine metabolism, and aminoacyl-tRNA biosynthesis, were selected as the most important altered metabolic pathways. These affected pathways were highly related to amino acid metabolism, nucleotide metabolism and lipid metabolism. These intrinsic metabolism disorders may highly relate to their principal metabolic pathways, and maintain unlimited proliferation and thus are vulnerable to anticancer drugs that impair their vital metabolic pathways.21
4.3 The potential anticancer mechanism of API and DIO
Apigenin (5,7,4′-trihydroxyflavone) is a flavonoid compound, and it has been reported that apigenin inhibits tumor cell proliferation, induces tumor cell apoptosis and could inhibit tumor angiogenesis, invasion and metastasis, and also interferes with the signal transduction pathway and anti-oxidation of tumor cells.22 The metabolic profiling model was applied for the first time to explore metabolic effects of apigenin in tumor cell lines. The method predicted that API may have a similar anticancer mechanism to agents that affect microtubule dynamics. In terms of recent research on mechanisms of API, API may exert its anticancer activity by interfering with microtubule dynamics through multiple molecular pathways.First, Wen investigated that after treatment with apigenin, the amount of tubulin was reduced and was irregularly arranged in HepG2 cells.23 This phenomenon may be caused by the perturbations in microtubule dynamics, because apigenin bounded tubulin, inhibited tubulin polymerization into microtubule, distorted microtubule structure and caused apoptosis.24 In addition, some molecular interactions studies concerning apigenin influence upon microtubules have been reported, such as up-regulated p21/WAF1 expression,25 inhibition of the expression of cyclinD1 and phosphorylation of Rb protein,26 and controlling the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1 alpha (HIF-1alpha).27 Thus the prediction results gave a reliable direction of mechanisms and were consistent with all the research mentioned above.
Diosgenin is a naturally occurring steroidal sapogenin, and it has been reported that diosgenin affects various phases of tumorigenesis including tumor cell proliferation, cell migration, angiogenesis and apoptosis.28 The data dots of DIO were distributed in the region between those of agents that affect microtubule dynamics and those of agents that directly act on DNA, indicating that DIO was likely to have two anti-tumor mechanisms. On the one hand, it was proven by binding DNA, activating IjBa kinase and p65 phosphorylation, and inhibiting Akt activation, that DIO could suppress TNF-induced NF-jB activation,29 which is a requirement of cell life processes from the G phase to the S phase.30 Besides, treatment of HepG2 cells with diosgenin resulted in activating cpoly-ADP-ribose polymerase (PARP) and releasing cytochrome that is thought to be one of the early cell signals of DNA damage.31 Also, diosgenin strongly generated ROS and this oxidative stress might induce DNA damage.32 On the other hand, Akinori demonstrated that DIO led to a decrease of the α-tubulin and c-fos mRNA expression relating to the cell cycle.33 However, further study is required on the effects of DIO on microtubules in detail. The results of several other studies have shown that DIO may increase sustained phosphorylation of JNK, p38 MAPK and apoptosis signal-regulating kinase (ASK)-1, indicating that DIO may have a variety of anticancer mechanisms.34 The results presented here suggested that the constructed model might be able to achieve a better prediction of anticancer candidates with multiple mechanisms of action.
4.4 Advantages and directions
To our best of our knowledge, this is the first metabolic profile study of tumor cells exploring the possibility to construct a preliminary model for the research of anticancer mechanisms. In fact, most anticancer drugs are designed to decrease tumor cell proliferation and some anticancer drugs with different mechanisms may cause the same changes in nucleotide content. As depicted in the previous model, the two data points of antimetabolic agents and agents that directly act on DNA were overlapped partially, which may be caused by the fact that antimetabolic agents not only competitively antagonize endogenous nucleotides but also indirectly perturb the pathway of DNA synthesis. In addition, the parameters of the previous model were R2 = 0.744 and Q2 = 0.665, demonstrating that this prediction model was practical and predictive to some extent. Compared with the previous study, our present study has provided integrative insights on metabolic shifts during drug action, and the new method can yield more new biomarkers to help reveal the mechanism of anticancer drug action. The significantly perturbed metabolic pathways included energy metabolism, amino acid metabolism and lipid metabolism, which showed that the constructed model is highly related to drug action. In this new model, four groups cluster and separate respectively with R2 = 0.909 and Q2 = 0.869, and this meant that the present method was more accurate and stable than the previous one. Furthermore, the use of the RPLC/HILIC combined method for the analytes provided a comprehensive metabolite profile on the cell changes.However, our study needs further improvements. First, given that the status of adherent tumor cells was more stable than that of suspension cells, we only chose twelve typical adherent tumor cells for this study, which belonged to different tissues cells or the same cancer transferred to different organs such as DU145 and PC3. To expand the application and improve the accuracy of the method, more cell lines and more drugs should be involved. Second, using different analysis platforms together can validate and complement each other, which can provide more information on the connection between metabolites and drug actions. Third, it will be helpful to provide metabolite concentrations to support this hypothesis, not only based on the literature but also experimental data. Extra work, e.g., an assay panel and quantitative study should be done for the confirmation of the method. Certainly, the new method in this paper can predict the mechanism of action of anticancer candidates early. But for a better understanding of how compounds or drug candidates interact with wide-ranging molecular targets and the biochemical consequences of these interactions, traditional strategies for mechanism research still need to be performed, such as Flow Cytometry (FCM), Polymerase Chain Reaction (PCR), western Blotting (WB), and so on.
5. Conclusions
In conclusion, by using LC-MS based cell metabolic profiling coupled with multivariate and univariate statistical analyses, a large overview of metabolic shifts in treated groups have been presented. These 42 metabolites might be tied to several important metabolic pathways, which might be potential targets for future research on anticancer candidate mechanisms. Based on these metabolites, the first OPLS-DA prediction model has been successfully constructed for investigating the mechanism of anticancer candidates. Additionally, the results showed that the prediction model performed well when it was validated, and the results were similar to the actual anti-tumor mechanism. Finally, two natural compounds were used and the prediction model proven to be robust and accurate against biases caused by different mechanisms of action, which validated that the model was also very useful for the prediction of anticancer candidates with multiple mechanisms. Thus, our work is the first study providing a reliable rapid screening method to evaluate the preliminary efficacy of anticancer compounds on tumour cells that would hopefully facilitate the development of safer drugs and help improve the development efficiency.Compliance with ethical requirements
The study has been approved by the ethics committee of the medical faculty at the Shenyang Pharmaceutical University.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work is financially supported by the Natural Science Foundation of Liaoning Province (Liaoning, China, No. 20170540851) and the Project of Liaoning Distinguished Professor (2014).References
- M. Takahashi, Jpn. J. Clin. Oncol., 2016, 46, 599–604 CrossRef PubMed.
- K. P. Valente, S. Khetani, A. R. Kolahchi, A. Nezhad, A. Suleman and M. Akbari, Drug Discovery Today, 2017, 11, 1654–1670 CrossRef PubMed.
- Q. Deng, J. Z. Meng and C. L. Xiao, Chin. J. Antibiot., 2016, 9, 641–646 Search PubMed.
- A. C. Lee, K. Shedden, G. R. Rosania and G. M. Crippen, J. Chem. Inf. Model., 2008, 48, 1379–1388 CrossRef CAS PubMed.
- C. C. Zhang, Z. Liu, X. Liu, L. Wei, Y. J. Liu, J. Yu and L. X. Sun, Acta Pharm. Sin. B, 2013, 3, 254–262 CrossRef.
- C. C. Liu, F. Wang, X. Liu, M. Liu, Z. Liu and L. X. Sun, Anti-Cancer Agents Med. Chem., 2016, 16, 568–578 CrossRef CAS PubMed.
- D. Gao, F. Jin, H. X. Liu, Y. N. Wang and Y. Y. Jiang, Talanta, 2014, 118, 382–388 CrossRef CAS PubMed.
- Y. S. Hu, Y. P. Qi, H. Liu, G. R. Fan and Y. F. Chai, Biochim. Biophys. Acta, 2013, 1830, 2779–2789 CrossRef CAS PubMed.
- R. Barrilero, E. Llobet, R. Mallol, J. Brezmes, L. Masana, M. Zulet, J. A. Martínez, J. Ribalta, M. Bulló and X. Correig, Metabolomics, 2015, 11, 1394–1404 CrossRef CAS.
- S. Grison, G. Favé, M. Maillot, L. Manens, O. Delissen, É. Blanchardon, I. Dublineau, J. Aigueperse, S. Bohand, J. C. Martin and M. Souidi, Metabolomics, 2016, 12, 154 CrossRef PubMed.
- C. I. L. Roy, L. J. Mappley, R. M. L. Ragione, M. J. Woodward and S. P. Claus, Metabolomics, 2016, 12, 157–171 CrossRef PubMed.
- A. N. Lane, R. M. Higashi and T. W. Fan, Metabolomics, 2016, 12, 118–144 CrossRef PubMed.
- C. R. Pestana, A. C. Urbaczek, J. V. Alberici, G. J. Rodrigues, J. Gerson and E. Carrilho, Life Sci., 2016, 172, 42–47 CrossRef PubMed.
- X. Liu, C. C. Zhang, Z. Liu, L. Wei, Y. J. Liu, J. Yu and L. X. Sun, Anticancer Drugs, 2014, 25, 690–703 CAS.
- M. Belouechebabari, L. E. Jackson, N. M. Alsaffar, S. A. Eccles, F. I. Raynaud, P. Workman, M. O. Leach and S. M. Ronen, Mol. Cancer Ther., 2006, 5, 187–196 CrossRef CAS PubMed.
- G. Martano, N. Delmotte, P. Kiefer, P. Christen, D. Kentner, D. Bumann and J. A. Vorhol, Nat. Protoc., 2015, 10, 1–11 CAS.
- C. Michal, T. Joanna, M. V. Jose Luis, E. Jesús and B. Coral, PLoS One, 2012, 7, e31982 Search PubMed.
- A. K. Smilde, M. J. van, S. Bijlsma, B. J. van and R. H. Jellema, Anal. Chem., 2005, 77, 6729–6736 CrossRef CAS PubMed.
- L. W. Sumner, A. Amberg, D. Barrett, M. H. Beale, R. Beger, C. A. Daykin, T. W. M. Fan, O. Fiehn, R. Goodacre, J. L. Griffin, T. Hankemeier, N. Hard, J. Harnly, R. Higashi, J. Kopka, A. N. Lane, J. C. Lindon, P. Marriott, A. W. Nicholls, M. D. Reily, J. J. Thaden and M. R. Viant, Metabolomics, 2007, 3, 211–221 CrossRef CAS PubMed.
- D. Rago, G. Gürdeniz, G. Ravn-Haren and L. O. Dragsted, Metabolomics, 2015, 11, 27–39 CrossRef CAS.
- W. F. Yao, H. Li, Q. Liu, Y. Gao, J. Dai, B. H. Bao, L. Zhang and A. W. Ding, Int. J. Mol. Sci., 2016, 17, 1514–1527 CrossRef PubMed.
- F. Ali, N. F. Rahul, S. Jyoti and Y. H. Siddique, Int. J. Food Prop., 2017, 20, 1197–1238 CrossRef CAS.
- R. Wen, C. Zhou and Y. Jia, China J. Mod. Med., 2016, 1, 5–8 Search PubMed.
- D. Choudhury, A. Ganguli, D. G. Dastidar, B. R. Acharya, A. Das and G. Chakrabarti, Biochimie, 2013, 95, 1297–1309 CrossRef CAS PubMed.
- S. Shukla and S. Gupta, Mol. Cancer Ther., 2006, 5, 843–852 CrossRef CAS PubMed.
- S. Gupta, F. Afaq and H. Mukhtar, Biochem. Biophys. Res. Commun., 2001, 287, 914–920 CrossRef CAS PubMed.
- J. Fang, C. Xia, Z. Cao, J. Z. Zheng and B. H. Jiang, FASEB J., 2005, 19, 342–353 CrossRef CAS PubMed.
- Y. Chen, Y. M. Tang, S. L. Yu, Y. W. Han, J. P. Kou and B. L. Liu, Chin. J. Nat. Med., 2015, 13, 578–587 CAS.
- S. Shishodia and B. B. Aggarwal, Oncogene, 2006, 25, 1463–1473 CrossRef CAS PubMed.
- H. Matsushime, M. F. Roussel, R. A. Ashmun and C. J. Sherr, Cell, 1991, 65, 701–713 CrossRef CAS PubMed.
- D. S. Kim, B. K. Jeon, Y. E. Lee, W. H. Woo and Y. J. Mun, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 1–8 Search PubMed.
- M. J. Liu, Z. Wang, Y. Ju, R. N. Wong and Q. Y. Wu, Cancer Chemother. Pharmacol., 2005, 55, 79–90 CrossRef CAS PubMed.
- A. Haratake, D. Watase, S. Setoguchi, N. Nagata-Akaho, K. Matsunaga and J. Takata, Life Sci., 2017, 174, 77–82 CrossRef CAS PubMed.
- C. Corbiere, B. Liagre, F. Terro and J. L. Beneytout, Cell Res., 2004, 14, 188–196 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00242h |
| This journal is © The Royal Society of Chemistry 2018 |