Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of novel nanocomposite-modified electrodes for identifying rice wines of different brands†
Zhenbo Wei,
Yanan Yang,
Luyi Zhu,
Weilin Zhang and
Jun Wang *
*
Department of Biosystems Engineering, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, PR China. E-mail: jwang@zju.edu.cn
First published on 10th April 2018
Abstract
In this paper, poly(acid chrome blue K) (PACBK)/AuNP/glassy carbon electrode (GCE), polysulfanilic acid (PABSA)/AuNP/GCE and polyglutamic acid (PGA)/CuNP/GCE were self-fabricated for the identification of rice wines of different brands. The physical and chemical characterization of the modified electrodes were obtained using scanning electron microscopy and cyclic voltammetry, respectively. The rice wine samples were detected by the modified electrodes based on multi-frequency large amplitude pulse voltammetry. Chronoamperometry was applied to record the response values, and the feature data correlating with wine brands were extracted from the original responses using the ‘area method’. Principal component analysis, locality preserving projections and linear discriminant analysis were applied for the classification of different wines, and all three methods presented similarly good results. Extreme learning machine (ELM), the library for support vector machines (LIB-SVM) and the backpropagation neural network (BPNN) were applied for predicting wine brands, and BPNN worked best for prediction based on the testing dataset (R2 = 0.9737 and MSE = 0.2673). The fabricated modified electrodes can therefore be applied to identify rice wines of different brands with pattern recognition methods, and the application also showed potential for the detection aspects of food quality analysis.
1. Introduction
An ideal analytical tool should be rugged, simple and reasonably affordable when applied to detect samples. In this regard, electrochemical sensors can offer the straightforward advantage of being able to distinguish one specific species in complex mixtures.1–3 In previous studies, glassy carbon electrode-modified chemically active materials showed high sensitivity to organic substances.4–6 Metal nanoparticle materials have attracted much research interest because of their good electrical conductivity and excellent catalytic properties, and electrochemical sensor-modified metal nanoparticles worked well in the determination of DNA, proteins, neurotransmitters, phytohormones and organic pollutants.7–10 However, these metal nanoparticles are unstable due to their high activity, easy oxidation and easy agglomeration due to van der Waals interactions.Recently, numerous studies have focused on the synthesis of composite materials combining a polymer skeleton with incorporated inorganic structures.11–13 Polymers with conjugated chains are frequently used because of their low oxidation potential, electronic and ionic conductivity and porous morphology.14,15 Due to these advantages, a scheme was proposed using polymers as carriers to deposit metal nanoparticles in order to fabricate polymer/metal nanocomposite-modified electrodes. These modified electrodes can enhance the stability of the combination of the nanometre-sized material and electrode base, lessen the tendency for the particles to reunify and improve the catalytic properties of the nanocomposites.16,17
Wine brand is one of the most important attributes which highly influences wine selection,18–20 and many powerful brands such as Moët & Chandon have played an important role in building the reputation of wines. Wine dealers understand that this reputation building tends to originate from the special quality and taste of the wines, and brands can be identified as an indicator of the quality, reliability, security and safety of the product.
Chinese rice wine is a sweet, golden wine made from glutinous rice and wheat with a unique craft, and it is also one of the three most ancient alcoholic beverages in the world.21–23 During the last 10 years, a common fraudulent practice in the commercialization of Chinese rice wine is the sale of fake brands as real wine. The fake products, with no quality assurance, are dishonest and unfair for customers. Moreover, they can disrupt the normal market because of their lower price, and also cause customers to lose faith in the quality and taste of real wine. Traditional methods for the identification of wine brands mainly include sensory evaluation, physicochemical detection and precision instrument analysis.24–26 However, all of these methods have some limitations. Sensory evaluation is easily influenced by subjective and environmental factors, physicochemical detection is complicated and has high technical requirements, and the use of precision instruments requires complex pre-processing and has a high cost. Therefore, a simple, convenient and fast method is urgently needed for the identification of wine brands.
In past research, the applications of polymer/metal nanocomposite-modified electrodes focused on analyzing trace chemicals in complex mixtures.27,28 However, research into the use of nanocomposite-modified electrodes for identifying wines of different brands was rare. Through analyzing the characteristics and applications of polymer/metal nanocomposite-modified electrodes, they can be applied as a potential tool for identifying wine brands.
The previous research indicated that the three chemical substances, ascorbic acid (Asc, sour), tyrosine (Tyr, astringent) and gallic acid (GA, astringent), have good correlation with wine brands.29,30 In this study, three types of polymer/metal nanocomposite-modified electrode (PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE), which have high sensitivity to Asc, Tyr and GA, respectively, were fabricated for the first time to identify rice wine brands. The characteristics of the modified materials were investigated using scanning electron microscopy (SEM). The working parameters of the modified electrodes were optimized by cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Two types of multi-frequency large pulse voltammetry (MLPV), multi-frequency rectangle pulse voltammetry (MRPV) and multi-frequency staircase pulse voltammetry (MSPV), were applied to obtain the wine brand information using the chronoamperometric method. MRPV and MSPV were comprised of many frequency segments of rectangle and staircase pulses, respectively, and the principle of each frequency segment of MRPV and MSPV was the same as that of large pulse voltammetry. When a potential was stepped to the final potential from the base potential (normally 0 V), a sharp oxidized or reduced transient current from the Helmholz double layer next to the electrode surface would flow to the working electrode, until only the limited diffusion faradic current remained. The complete oxidation and reduction of the electroactive compounds next to the electrode surface needs a wide potential, and one shape of the pulse cannot meet this requirement. Using two types of pulse shape can provide a wider potential and more information on the oxidation (or reduction) of the electroactive compounds in the liquid samples. With the help of pattern recognition methods, the aims of this study were (1) to evaluate the performance of the three self-fabricated polymer/metal nanocomposite-modified electrodes for analyzing Asc, Tyr and GA, and (2) to identify rice wines of different brands using the three types of modified electrode based on classification and prediction methods.
2. Experimental
2.1. Reagents and instrumentation
ASC, GA, Tyr, acid chrome blue K (ACBK), sulfanilic acid (ABSA) and glutamate (GA) were purchased from Aladdin Chemical Co. Ltd., (Shanghai, China). HAuCl4 and CuSO4 were obtained from Sinopharm Chemical Reagent Co. Ltd., China. Phosphate buffer solution (PBS, 0.1 M) was prepared by mixing stock solutions of 0.2 M Na2HPO4·12H2O and NaH2PO4·2H2O. All reagents were of analytical grade and were used directly without purification, and all solutions were prepared with double-distilled water.A pH meter (FE28-FiveEasy Plus, METTLER TOLEDO), electronic analytical balance (BS224S, Sartorius), ultrasonic cleaner (SK1200H, KUDOS), scanning electron microscope (SU8010, HITACHI, Japan) and magnetic stirrer (DF-101Z) were used. The fabrication of modified electrodes and rice wine identified experiments were performed on a PARSTAT 3000 A electrochemical workstation (AMETEK. Inc, USA). A conventional three-electrode system consisted of a working modified electrode, a platinum wire auxiliary electrode and a saturated Ag/AgCl reference electrode. A saturated KCl solution was used as the reference solution.
2.2. Rice wine samples
Rice wines of different brands, which include three types of Guyue Longshan (Dida (G-DD), Gold Five (G-GF) and Kucang (G-KC)), three types of Tapai (Techun (T-TC), Shougong (T-SG) and Laojiu (T-LJ)) and one type of Kuaijishan (K-HD), were all produced in Shaoxing (a city of Zhejiang province (120° 58′ E, 30° 01′ N), China). These rice wine samples were acquired from the manufacturers, and were kept in a cool and dry place (25 ± 1 °C) before analysis.2.3. Preparation of the modified electrodes
In the study, three types of polymer/metal nanocomposite-modified electrode (PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE), which have high sensitivity to ASC, GA, and Tyr, respectively, were fabricated for the first time.Prior to modification, the GCEs were polished with 0.3 and 0.05 μm pasty slurry on chamois leather until a mirror-like surface was obtained, then the electrodes were rinsed gradually with absolute ethyl alcohol and deionized water for 2 min each. Afterwards, the pretreated GCEs were dipped into 0.5 M H2SO4 and scanned by repeated CV (at a scan rate of 100 mV s−1) ranging from −0.5 to 1.2 V until a steady state was established. Finally, the electrode surfaces were thoroughly washed with deionized water and dried in air.
For the fabrication of PACBK/AuNP/GCE, gold nanoparticles were electrochemically deposited onto the GCE surface in 1.2 mM HAuCl4 solution containing 0.1 M KNO3 for 240 s at a fixed potential of −0.2 V. The acquired Au/GCE was then electrodeposited in 0.1 M PBS (pH 6.0) containing 0.5 mM acid chrome blue K with a cycling potential from −1.6 to +2 V at a scan rate of 100 mV s−1 for 26 cycles.
For the fabrication of PABSA/AuNP/GCE, Au/GCE was made based on the steps described above. The Au/GCE was then electrodeposited in 2 mM sulfanilic acid containing 0.1 M PBS (pH 7.0) with a cycling potential from −1.5 to +2.5 V at a scan rate of 100 mV s−1 for 12 cycles.
For the fabrication of PGA/CuNP/GCE, gold nanoparticles were electrochemically deposited onto the GCE surface in 0.01 mM CuSO4 solution containing 0.1 M Na2SO4 with a cycling potential from −1.5 to 0 V at a scan rate of 20 mV s−1 for 10 cycles. The acquired Cu/GCE was then electrodeposited in 0.01 M glutamic acid solution containing 0.1 M PBS (pH 7.0) with a cycling potential from −0.8 to +2 V at a scan rate of 100 mV s−1 for 12 cycles.
2.4. Characterization of the modified electrodes and experimental procedure for the identification of wine brands
The general morphology of the modified electrodes was observed using a field-emission scanning electron microscope (SEM, SU8010, HITACHI). The electrochemical characterization of the modified electrodes in their various states (PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE) was carried out using a traditional three-electrode system with CV. The modified electrodes acted as the working electrode, and a platinum counter electrode (purity 99.95%, length 10 mm, diameter 1 mm) and Ag/AgCl reference electrode (3.5 M saturated KCl, diameter 2 mm) were also included in the system.During the experiment, a 40 ml sample of each type of rice wine was analyzed, and 0.1 M PBS (pH 2.0) was added in order to adjust the pH of the rice wine for the best detection conditions. The sample mixtures were analyzed by the modified electrodes with multi-frequency rectangle pulse voltammetry (MRPV) and multi-frequency staircase pulse voltammetry (MSPV). Both of the two potential waveforms, which were composed of three frequencies of 1 Hz, 10 Hz and 100 Hz, were increased from 0 V to the final potential (1.5 V) in steps of 0.25 V. For each sample, 3 replicates were prepared for analysis, and the average values of the replicates were used for the data evaluation. All experiments mentioned in this paper were performed at room temperature (25 ± 1 °C).
2.5. Pattern recognition methods
In the study, principal component analysis (PCA), locality preserving projection (LPP) and linear discriminant analysis (LDA) were applied for classification.31–33 Extreme learning machine (ELM), the library for support vector machines (LIB-SVM) and the backpropagation neural network (BPNN) were applied for prediction.34–36PCA explains the maximum variance of the data set with the least number of major components without a significant loss of information.37 Supported by multivariate data analysis software, the samples receive new coordinates (scores) according to principal components describing the resumed sensor information.
LPP is a linear operation, and similarly to PCA, it can be very easy to determine the projection space of LPP by solving a generalized eigenvalue. As a result, the new samples can be directly projected onto this space, and it can maintain the maximum local characteristics of the original data.38
LDA is used as a supervised learning technique to extract features which preserve class separability. It provides a classification model, characterized by a linear dependence of the classification scores with respect to the descriptors.39
ELM was proposed by Huang et al.40 for reducing the time required to train single-layer feed-forward neural networks. It is only needed to set the number of hidden layer neurons without adjustment over the course of training to get the only optimal solution.41
LIB-SVM, which was proposed by Lin,42 is a library for support vector machines (SVMs). It is an optimized version based on standard SVMs and it is powerful for solving problems in nonlinear classifications, function estimation and density estimation.
BPNN is a multi-layer feed-forward network trained by the error backpropagation algorithm. It can learn and store a lot of input–output model mapping without revealing the mathematical equations of the mapping relationship in advance. BPNN has a strong nonlinear mapping ability and is especially suitable for classification or approximation.43
PCA, LPP and LDA were performed using SAS v8 (SAS Institute, Cary, NC, USA). ELM, LIB-SVM and BPNN were performed using Matlab 6.0. (MathWorks, Inc., USA).
3. Results and discussion
3.1. Characterization of the modified electrodes
SEM was used to observe the microstructure of the modified electrode surface. Fig. 1(a) and (b) show the typical SEM images of AuNP/GCE and PACBK/AuNP/GCE, respectively. As shown in Fig. 1(a), gold nanoparticles were clearly deposited on the GCE surface, but the nanoparticles varied greatly in size and obvious agglomeration had occurred. It can be seen from Fig. 1(b) that the PACBK film aggregated successfully on the surface of AuNP/GCE. Due to its catalytic properties, PACBK made the sizes of the gold nanoparticles homogeneous, and the agglomeration almost disappeared. Fig. 1(c) and (d) show the typical SEM images of AuNP/GCE and PABSA/AuNP/GCE. Although the AuNPs had deposited on the GCE surface successfully, the diameter of the agglomerated particles was up to 200 mm, which exceeded the general cognitional range of 1 to 100 nm for the AuNP diameter (Fig. 1(c)). Meanwhile, some agglomeration also occurred. After modification, it was obvious that the PABSA film had coated the electrode surface successfully. Moreover, the deposition optimized the configuration of the AuNPs, and there was no agglomeration of the AuNPs on the electrode surface (Fig. 1(c)). Fig. 1(e) and (f) show the typical SEM images of CuNP/GCE and PGA/CuNP/GCE. PGA had coated the surface of CuNP/GCE successfully. It not only performed its catalytic role but also improved the physical morphology of the CuNPs, changing their shape from grainy to willow leaf-shaped. The CuNPs with their new shape were more easily assembled on the surface of the electrode.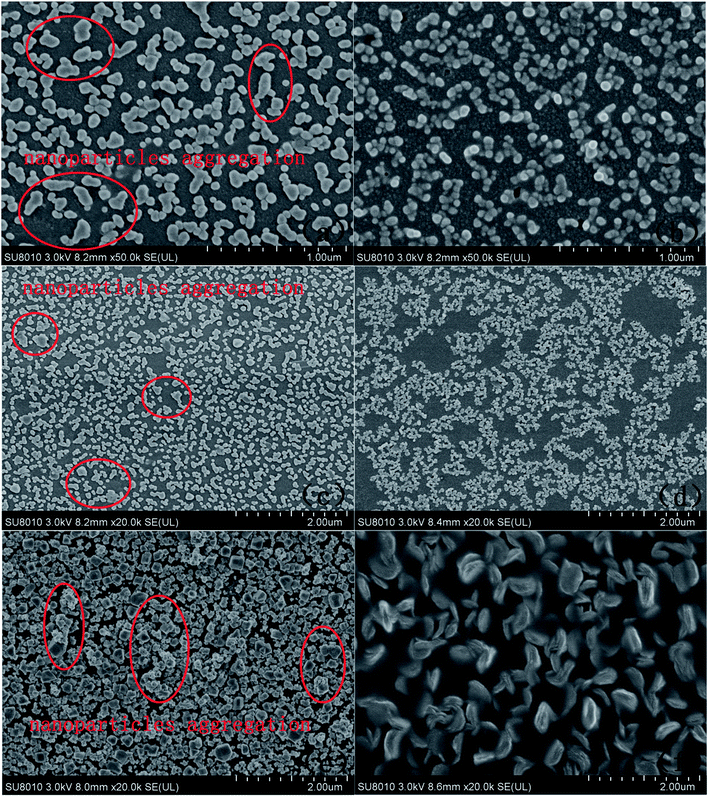 | ||
| Fig. 1 SEM images of AuNP/GCE (a), PACBK/AuNP/GCE (b), AuNP/GCE (c), PABSA/AuNP/GCE (d), CuNP/GCE (e) and PGA/CuNP/GCE (f). | ||
3.2. Electrochemical behavior of chemical substances at the modified electrodes
The electrochemical behavior of Asc, Try and GA at the corresponding GCEs was investigated by cyclic voltammetry (CV). Fig. 2(a) shows the CV responses of Asc (0.32 mM) on different electrodes. The bare GCE presented no chemical response to Asc based on CV scanning, but there were electrochemical oxidation reactions on AuNP/GCE and PACBK/AuNP/GCE. Considering that the peak currents on the PACBK/AuNP/GCE surface were significantly larger than those on the AuNP/GCE surface, PACBK/AuNP/GCE presented better catalytic properties towards Asc.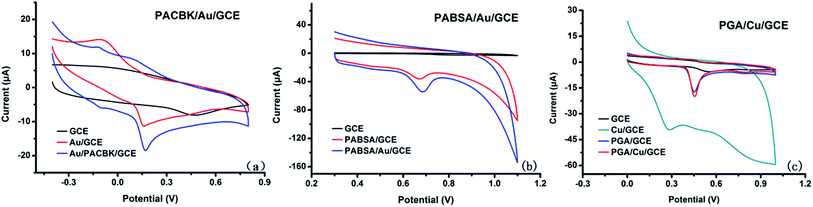 | ||
| Fig. 2 Cyclic voltammetry responses of (a) Asc sensors, (b) Tyr sensors and (c) GA sensors, modified with different materials. | ||
Fig. 2(b) shows the CV responses of Tyr (0.1 mM) on different electrodes. GCE has a weak catalytic effect on Tyr, and there were no response currents on the GCE surface. After modification, the responses of PABSA/GCE and PABSA/AuNP/GCE showed an obvious increase. By comparing the oxidation peak currents of PABSA/GCE and PABSA/AuNP/GCE, it was found that the oxidative catalytic performance of PABSA/AuNP/GCE for Tyr is much better. Because Au nanoparticles have the advantages of a large surface area, good biocompatibility and high activity, the PABSA/AuNP composite film showed better catalytic properties for Tyr than the PABSA film.
Fig. 2(c) shows the CV responses of GA (0.1 mM) on different electrodes. There was no peak current for GA on the GCE and CuNP/GCE surface based on CV measurements, but the response current of CuNP/GCE was much larger than that of the GCE due to the large surface area and good conductivity of Cu nanoparticles. Irreversible oxidation peaks of PGA/GCE and PGA/CuNP/GCE were presented due to the deposition of PGA film. The peak current of PGA/CuNP/GCE was a little larger than that of PGA/GCE, which indicated that PGA/CuNP/GCE showed better catalytic properties for GA.
3.3. Optimization of electrochemical parameters
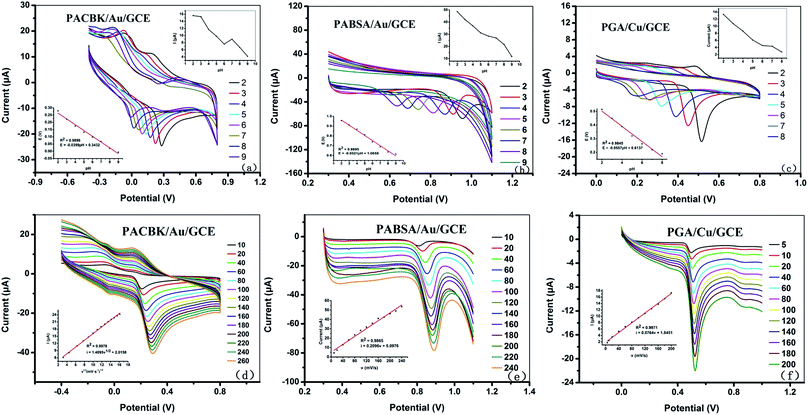
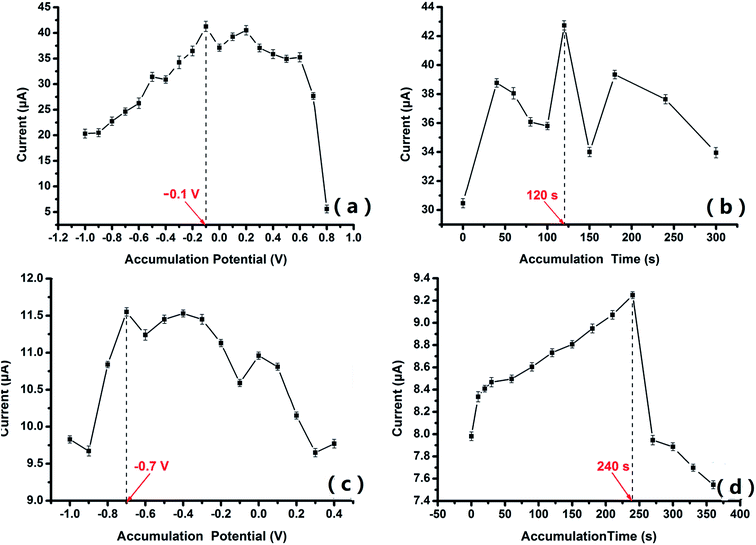
The electrochemical behavior at the surface of the modified electrodes has a high correlation with pH, scan rate and the accumulation step. The electrochemical features of electroactive molecules are dependent on the pH value of the medium. The kinetics of the electrode reaction can be investigated by studying the effects of the scan rate on the redox reaction occurring at the electrode surface. The accumulation step is an effective way to increase the efficiency of detection, especially for adsorption-controlled electrochemical reactions. The optimization of the experimental parameters for PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE is therefore discussed as follows.3.4. Quantitative detection of chemical substances
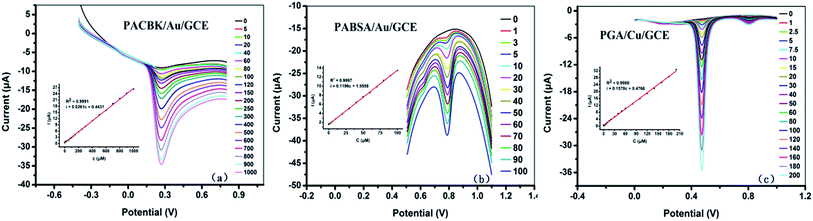
As shown in Fig. 5(a), the Asc solution (PBS solution, pH = 3) from 5 × 10−6 to 1 × 10−3 M was detected quantitatively with differential pulse voltammetry (DPV) based on these optimal conditions. The corresponding oxidation peak current increased with the increase of Asc concentration. The inset illustrates a good linear relationship between the peak current and concentration. The linear regression equation was ip (μA) = 0.0261c (μM) + 0.4431 (R2 = 0.9991), and the limit of detection (LOD) was calculated to be 0.35 mg L−1 based on a signal-to-noise ratio of 3 (S/N = 3).The Tyr solution from 1 × 10−6 to 1 × 10−4 M was detected quantitatively by DPV at a potential range of 0.5 V to 1.1 V (Fig. 5(b)). The peak current also increased with the increasing Tyr concentration. The inset shows a good linear relationship between the peak current and concentration. The linear regression equation was ip (μA) = 0.1196c (μM) + 1.5558 (R2 = 0.9997), and the LOD was calculated to be 0.07 mg L−1 (S/N = 3).
The GA solution from 1 × 10−6 to 2 × 10−4 M was detected quantitatively by DPV at a potential range of 0 to 1.0 V (Fig. 5(c)). The peak current increased with the increase of solution concentration, and the inset shows a good linear relationship between the peak current and concentration. The linear regression equation was ip (μA) = 0.1570c (μM) + 0.4766 (R2 = 0.9994), and the LOD was calculated to be 0.05 mg L−1 (S/N = 3). According to the linear equations above, the concentration of the chemical substance solution could be calculated. It therefore provided a theoretical basis for the three types of modified electrode to be applied to the analysis of rice wine samples.
3.5. Electrochemical features of the modified electrodes for rice wine of different brands
In this paper, the chronoamperometry method included MRPV and MSPV, which were composed of three frequencies of 1 Hz, 10 Hz and 100 Hz, and they were applied as the scanning potential waveforms (Fig. 6). Fig. S1† shows the response current curves obtained by PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE from rice wines of 7 brands. Fig. S1(a) and (b)† shows the response current curves obtained using PACBK/AuNP/GCE. The response curves could not be distinguished from each other because the rice wines were all produced in Shaoxing with the same marked age, and the Asc content was lower in these rice wines. Fig. S1(c) and (d)† show the response current curves obtained by PABSA/AuNP/GC. The response curves of the rice wines could be distinguished from each other. The response currents from large to small were G-DD, G-GF, T-SG, G-KC, T-LJ, T-TC and K-HD. The corresponding response currents of rice wine at the tenth second were −307.9, −147.4, −90.8, −78.3, −62.2, −44.1 and −31.9 μA (Fig. S1(c)†), respectively. The corresponding response currents at the sixth second were −191.0, −102.6, −65.8, −57.1, −45.6, −32.2 and −24.4 μA (Fig. S1(d)†), respectively.
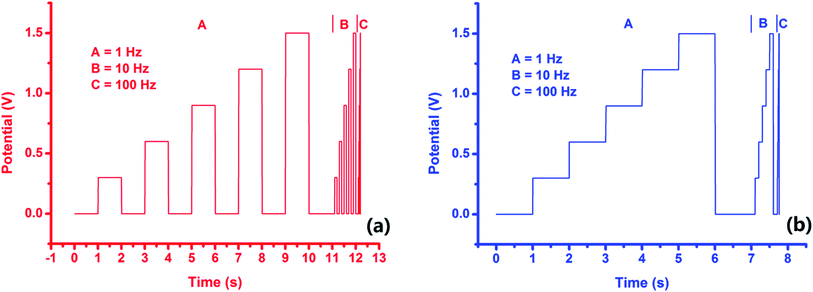 | ||
| Fig. 6 The applied multi-frequency and potential step scanning waveforms for rectangle voltammetry (a) and staircase voltammetry (b). | ||
Fig. S1(e) and (f)† show the response current curves obtained by PGA/CuNP/GCE based on the optimal conditions. All of the curves could not be distinguished obviously from each other, except for the larger response current of G-DD. The information obtained by each modified electrode with a single scanning waveform provided the complete brand information for the rice wines, and this information was applied for classification and prediction based on pattern recognition methods.
To evaluate the effect of the waveforms and modified electrodes on the response values, respectively, two-factor analysis of variance of the scanning waveforms (rectangle wave and staircase wave) and modified electrodes (PACBK/AuNP/GCE, PABSA/AuNP/GCE and PGA/CuNP/GCE) was performed based on the area feature data. The results of the analysis of samples of Kuaijishan are shown in Table 4. Smaller P values (P < 0.05) and larger F values indicated a more significant effect on the corresponding response variables. The F values of the scanning waveform and modified electrode were 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757.624 and 18
757.624 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174.932, respectively, and 3478.538 when the scanning waveform and modified electrode interacted. All of the P values were below 0.05. ANOVA analysis showed that the waveforms and electrodes, and the interaction of both, had a significant effect on the response, and that a detection system composed of two waveforms and three modified electrodes could be applied to analyze rice wine samples.
174.932, respectively, and 3478.538 when the scanning waveform and modified electrode interacted. All of the P values were below 0.05. ANOVA analysis showed that the waveforms and electrodes, and the interaction of both, had a significant effect on the response, and that a detection system composed of two waveforms and three modified electrodes could be applied to analyze rice wine samples.
3.6. The classification and prediction of rice wines of different brands
The area surrounded by the response curve and the abscissa axis was applied as feature data which was used for the classification and prediction of rice wine brands using pattern recognition methods. The processing procedures were as follows. Firstly, the correlation of the feature data with the wine brand was determined using the area method from the original responses. Secondly, the feature data were reduced to two dimensions by different dimensional reduction methods, and the efficiency of the dimensional reduction methods was compared using classification plots. Finally, the data compressed by the most efficient dimensional reduction method were applied as the input data in the prediction models for wine brand prediction. In this paper, PCA (an unsupervised method),44 LPP (a semi-supervised method)45 and LDA (a supervised method)46 were applied to reduce the dimensions of the original feature data, and these methods also displayed the classification results of the different brands of rice wine in two-dimensional or three-dimensional graphs. ELM,47 LIBSVM48 and BPNN49 were applied to predict the rice wine brands. The best method for this experiment was selected according to the effect of various models.| No. | Brand | Marked age (years) | Number of samples | Reference value for prediction |
|---|---|---|---|---|
| 1 | Guyue Longshan Dida (G-DD) | 5 | 40 | 1 |
| 2 | Guyue Longshan Gold Five (G-GF) | 5 | 40 | 2 |
| 3 | Guyue Longshan Kucang (GKC) | 5 | 40 | 3 |
| 4 | Tapai Techun (T-TC) | 5 | 40 | 4 |
| 5 | Tapai Laojun (T-LJ) | 5 | 40 | 5 |
| 6 | Tapai Shougong (T-SG) | 5 | 40 | 6 |
| 7 | Kuaijishan Huadiao (K-HD) | 5 | 40 | 7 |
| Electrode | Detected substance | LOD (mg L−1) | Content (mg L−1) |
|---|---|---|---|
| PACBK/AuNP/GCE | Asc | 0.35 | 5.71–43.20 |
| PABSA/AuNP/GCE | Tyr | 0.07 | 70–1979.45 |
| PGA/CuNP/GCE | Glc | 0.05 | 8246–43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 195 |
| Electrode | Rice wine | Rectangle wave | Staircase wave | Average variable coefficient | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Variable coefficient | Mean | Standard deviation | Variable coefficient | |||
| PACBK/AuNP/GCE | G-DD | 0.0888 | 0.0045 | 5.03% | 0.0356 | 0.0022 | 6.28% | 4.64% |
| G-GF | 0.0756 | 0.0032 | 4.27% | 0.0310 | 0.0017 | 5.53% | ||
| G-KC | 0.1100 | 0.0064 | 5.84% | 0.0426 | 0.0027 | 6.28% | ||
| T-TC | 0.0662 | 0.0022 | 3.30% | 0.0272 | 0.0012 | 4.59% | ||
| T-LJ | 0.0624 | 0.0019 | 3.05% | 0.0258 | 0.0011 | 4.37% | ||
| T-SG | 0.0705 | 0.0023 | 3.32% | 0.0290 | 0.0014 | 4.95% | ||
| K-HD | 0.0599 | 0.0021 | 3.59% | 0.0249 | 0.0011 | 4.55% | ||
| PABSA/AuNP/GCE | G-DD | 1.3599 | 0.1032 | 7.59% | 0.4778 | 0.0389 | 8.13% | 6.85% |
| G-GF | 0.8039 | 0.0329 | 4.10% | 0.2966 | 0.0229 | 7.74% | ||
| G-KC | 0.5082 | 0.0543 | 10.69% | 0.1873 | 0.0246 | 13.11% | ||
| T-TC | 0.4091 | 0.0148 | 3.62% | 0.1552 | 0.0118 | 7.63% | ||
| T-LJ | 0.2918 | 0.0111 | 3.81% | 0.1122 | 0.0086 | 7.66% | ||
| T-SG | 0.5693 | 0.0202 | 3.55% | 0.2134 | 0.0162 | 7.58% | ||
| K-HD | 0.2153 | 0.0077 | 3.56% | 0.0857 | 0.0061 | 7.10% | ||
| PGA/CuNP/GCE | G-DD | 0.1261 | 0.0202 | 15.99% | 0.0434 | 0.0031 | 7.15% | 6.12% |
| G-GF | 0.0756 | 0.0032 | 4.25% | 0.0303 | 0.0015 | 5.03% | ||
| G-KC | 0.0691 | 0.0113 | 16.36% | 0.0282 | 0.0043 | 15.25% | ||
| T-TC | 0.0610 | 0.0010 | 1.59% | 0.0252 | 0.0010 | 3.78% | ||
| T-LJ | 0.0579 | 0.0009 | 1.54% | 0.0239 | 0.0009 | 3.62% | ||
| T-SG | 0.0658 | 0.0013 | 2.00% | 0.0271 | 0.0011 | 4.13% | ||
| K-HD | 0.0560 | 0.0008 | 1.39% | 0.0233 | 0.0008 | 3.57% | ||
| Variation source | Quadratic sum | Degree of freedom | Mean square value | F | P |
|---|---|---|---|---|---|
| a P < 0.05 level, R2 = 0.996, correction R2 = 0.996. | |||||
| Calibration model | 1.021 | 5 | 0.204 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 612.913 612.913 |
0.000 |
| Intercept | 1.442 | 1 | 1.442 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 948.320 948.320 |
0.000 |
| Waveform | 0.260 | 1 | 0.260 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757.624 757.624 |
0.000 |
| Electrode | 0.639 | 2 | 0.320 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 174.932 174.932 |
0.000 |
| Waveforma electrode | 0.122 | 2 | 0.061 | 3478.538 | 0.000 |
| Error | 0.004 | 234 | 0.000 | ||
| Total variation | 2.467 | 240 | |||
| Correction variation sum | 1.026 | 239 | |||
The results of prediction using ELM, LIBSVM and BPNN were evaluated by the correlation coefficient (R2) and root mean square error (RMSE) (a good result should have a low RMSE, close to zero, and a high correlation coefficient, close to one). The radial basis function (RBF) was chosen as the kernel function of ELM for prediction, and the number of neurons of the hidden layer was set from 5 to 100. As shown in Fig. S3(a),† the neuron number of the hidden layer was 45 when the prediction model presented the best results, and the results of wine brand prediction based on the training and testing datasets were R2 = 0.9665, RMSE = 0.3720 and R2 = 0.9620, RMSE = 0.4060, respectively (Table 5). Although both R2 values met the requirements, both RMSE values were not good enough. RBF was also applied as the kernel function of LIBSVM, and the important parameter g and the penalty factor c were auto calculated (g = 1 and c = 32, Fig. S3(b)†). As shown in Table 2, the results of wine brand prediction based on the training and testing datasets were R2 = 0.9762, RMSE = 0.3134 and R2 = 0.9621, RMSE = 0.4147, respectively. The prediction result was similar to that of ELM, whose RMSE was not good enough. Although all of the correlation coefficients (R2) of ELM and LIBSVM based on the training and testing datasets were over 0.96, the values of the RMSE were large, and all of the RMSE values of the testing dataset were 0.40. According to the analyses above, both prediction models were not the best choice. For a better result, BPNN, with three layers, was applied to predict wine brands. The network topology of BPNN was 6–30–7, i.e. the node number in the input layer was 6 (6 types of feature data), in the hidden layer was 30 and in the output layer was 7. The results using BPNN based on the training dataset and testing dataset were R2 = 0.9952, RMSE = 0.1378 and R2 = 0.9827, RMSE = 0.2673, respectively (Table 2). It was obvious that the results of prediction using BPNN showed the highest R2 values and the lowest RMSE values, which indicated that BPNN was the best method to predict wine brands, better than ELM and LIBSVM.
| ELM | LIBSVM | BPNN | ||||
|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| Training set | 0.9665 | 0.3720 | 0.9762 | 0.3134 | 0.9952 | 0.1378 |
| Testing set | 0.9620 | 0.4060 | 0.9621 | 0.4177 | 0.9827 | 0.2673 |
4. Conclusions
Three types of polymer/metal nanocomposite-modified electrode were self-fabricated for classifying and predicting wines of different brands. SEM images exhibited clearly that the synthetic process of the composite could inhibit the aggregation of the metallic nanoparticles, and the electrochemical parameters of the three modified electrodes (pH, scan rate, accumulation potential and time) were optimized using CV and LSV. The modified electrodes showed stable performance for detecting rice wines based on MRPV and MSPV. With the help of pattern recognition methods, all of the samples could be separated using PCA, LPP and LDA, and the samples containing similar chemicals could be grouped together in the score plots. BPNN performed better than ELM and LIBSVM for the prediction of wine brands, and the prediction results were R2 = 0.9952, RMSE = 0.1378 (training set) and R2 = 0.9827, RMSE = 0.2673 (testing set). The results of the study show that the polymer/metal nanocomposite-modified electrode is a promising and practical tool for the classification of rice wines. In the future, more effort should be put into fabricating more sensitive electrodes and optimizing the signal processing algorithm.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of the Chinese National Foundation of Nature and Science through Project 31570005 and 31560477.References
- M. Alagiri, P. Rameshkumar and A. Pandikumar, Microchim. Acta, 2017, 184, 3069–3092 CrossRef CAS.
- A. N. Kawde, N. Baig and M. Sajid, RSC Adv., 2016, 6, 91325–91340 RSC.
- N. E. El Hassani, K. Tahri, E. Llobet, B. Bouchikhi, A. Errachid, N. Zine and N. El Bari, Food Chem., 2018, 243, 36–42 CrossRef PubMed.
- W. X. Cheng, P. Liu, M. Zhang, J. Z. Huang, F. L. Cheng and L. S. Wang, RSC Adv., 2017, 7, 47781–47788 RSC.
- V. Kumar, P. Guleria and S. K. Mehta, Environ. Chem. Lett., 2017, 2, 165–177 CrossRef.
- P. Devi, R. Jain, A. Thakur, K. Manish, K. L Nitin, N. Manoj and K. Praveen, Trends Anal. Chem., 2017, 95, 69–85 CrossRef CAS.
- D. Valera, P. J. Espinoza-Montero, J. Alvarado, P. Carrera, P. Bonilla, L. Cumbal and L. Fernández, Sens. Actuators, B, 2017, 253, 1170–1179 CrossRef CAS.
- J. Parra-Barranco, J. R. Sanchez-Valencia, A. Barranco and A. R. Gonzalez-Elipe, Nanotechnology, 2017, 28, 485602 CrossRef CAS PubMed.
- S. Shikha, K. G. Thakur and M. S. Bhattacharyya, RSC Adv., 2017, 7, 42845–42855 RSC.
- J. Han, M. G. Wang, Y. M. Hu, C. Q. Zhou and R. Guo, Prog. Polym. Sci., 2017, 70, 52–91 CrossRef CAS.
- R. Pandey, N. Jian, A. Inberg, R. E. Palmer and Y. Shacham-Diamand, Electrochim. Acta, 2017, 246, 1210–1216 CrossRef CAS.
- Y. Ma, X. L. Shen, Q. Zeng and L. S. Wang, Microchim. Acta, 2017, 184, 4469–4476 CrossRef CAS.
- Y. G. Kim, T. I. Ryu, K. H. Ok, M. G. Kwak, S. M. Park, N. G. Park, C. J. Han, B. S. Kim, M. J. Ko, H. J. Son and J. W. Kim, Adv. Funct. Mater., 2015, 25, 4580–4589 CrossRef CAS.
- A. Ghoorchian and N. Alizadeh, Sens. Actuators, B, 2018, 255, 826–835 CrossRef CAS.
- T. Kangkamano, A. Numnuam and W. Limbut, Biosens. Bioelectron., 2017, 102, 217–225 CrossRef PubMed.
- S. Amidi, Y. H. Ardakani, M. Amiri-Aref, E. Ranjbari, Z. Sepehri and H. Bagheri, RSC Adv., 2017, 7, 40111–40118 RSC.
- A. Sanmugam, A. R. Jeyaraman, S. Venkatesan, Y. Anbalagan and D. Vikraman, Ionics, 2017, 23, 1249–1257 CrossRef CAS.
- K. J. Kshirasagar, U. S. Markad, A. Saha, K. K. K. Sharma and G. K. Sharma, Mater. Res., 2017, 4, 15–25 Search PubMed.
- D. Priilaid, G. Human and K. Pitcher, S. Afr. J. Bus. Manag., 2017, 48, 45–54 Search PubMed.
- A. J. Blair, C. Atanasova, L. Pitt, A. Chan and A. Wallstrom, J. Prod. Brand Manag., 2017, 26, 447–452 CrossRef.
- L. Carsana and A. Jolibert, J. Prod. Brand Manag., 2017, 26, 80–90 CrossRef.
- Z. B. Wei, W. L. Zhang and J. Wang, Microchim. Acta, 2017, 184, 3441–3451 CrossRef CAS.
- Z. B. Wei, J. Wang and L. S. Ye, Biosens. Bioelectron., 2011, 26, 4767–4773 CrossRef CAS PubMed.
- Y. Y. S. Rahayu, Y. Yoshizaki, K. Yamaguchi, K. Okutsu, T. Futagami, H. Tamaki, Y. Sameshima and K. Takamine, Food Chem., 2017, 224, 398–406 CrossRef CAS PubMed.
- Q. Peng, W. H. Xing, Q. X. Xu, J. Y. Chen, H. Yu, F. R. Chen, B. B. Li, X. Xu, Z. Y. Wang and R. G. Tian, J. Chem. Soc. Pakistan, 2017, 39, 484–487 Search PubMed.
- W. D. Bai, S. G. Sun, W. H. Zhao, M. Qian, X. Y. Liu and W. X. Chen, Food Anal. Method., 2017, 10, 2068–2077 CrossRef.
- Z. B. Wei, J. Wang and S. Q. Cui, Anal. Methods, 2016, 8, 6361–6371 RSC.
- C. Minnai, M. Di Vece and P. Milani, Nanotechnology, 2017, 28, 355702 CrossRef PubMed.
- A. Javadi, J. Z. Zhao, C. Z. Cao, M. Pozuelo, Y. C. Yang, I. Hwang, T. C. Lin and X. C. Li, Sci. Rep., 2017, 7, 7098 CrossRef PubMed.
- F. Shen, Methods and devices for assessing aging characteristics and fingerprint quality of Shaoxing wines, Zhejiang University, Hangzhou, 2012 Search PubMed.
- E. T. Haroon, X. B. Zou, X. W. Huang, J. Y. Shi and A. M. Abdalbasit, Food Chem., 2016, 206, 37–43 CrossRef PubMed.
- P. Y. Zhu, J. Du, B. L. Xu and M. L. Lu, Anal. Methods, 2017, 9, 1806–1815 RSC.
- T. Shunsuke and Y. Keitaro, Chem. Commun., 2013, 49, 10430–10432 RSC.
- I. M. Apetrei and C. Apetrei, Sens. Actuators, B, 2017, 234, 371–379 CrossRef.
- W. R. Chen, J. Bin, H. M. Lu, Z. M. Zhang and Y. Z. Liang, Analyst, 2016, 141, 1973–1980 RSC.
- F. N. Azad, M. Ghaedi, A. Asfaram, A. Jamshidi, G. Hassani, A. Goudarzi, M. H. A. Azqhandi and A. Ghaedi, RSC Adv., 2016, 6, 19768–19779 RSC.
- Z. B. Wei, Y. N. Yang, J. Wang, W. L. Zhang and Q. F. Ren, J. Food Eng., 2018, 217, 75–92 CrossRef.
- G. F. Lu, Y. Wang, J. Zou and Z. Q. Wang, Neural Network., 2018, 97, 127–136 CrossRef PubMed.
- S. S. Qiu, J. Wang and L. P. Gao, J. Agric. Food Chem., 2014, 62, 6426–6434 CrossRef CAS PubMed.
- G. B. Huang, Q. Y. Zhu and C. K. Siew, Neurocomputing, 2006, 70, 489–501 CrossRef.
- L. Zhang and D. Zhang, IEEE T. Neur. Net. Lear., 2017, 28, 3045–3060 CrossRef PubMed.
- R. E. Fan, P. H. Chen and C. J. Lin, J. Mach. Learn. Res., 2005, 6, 1889–1918 Search PubMed.
- X. Y. Yang, G. A. Xu, Q. Li, Y. H. Guo and M. Zhang, PLoS One, 2017, 12, 0187204 Search PubMed.
- P. Alessio, C. S. Martin, J. A. de Saja and M. L. Rodriguez-Mendez, Sens. Actuators, B, 2016, 233, 654–666 CrossRef CAS.
- S. S. Qiu, J. Wang and D. D. Du, Innovative Food Sci. Emerging Technol., 2017, 42, 33–41 CrossRef CAS.
- X. Ceto, J. Capdevila, A. Puig-Pujol and M. del Valle, Electroanalysis, 2014, 26, 1504–1512 CrossRef CAS.
- M. M. Elkhoudary, R. A. A. Salam and G. M. Hadad, RSC Adv., 2017, 7, 20936–20946 RSC.
- X. Z. Hong and J. Wang, Anal. Methods, 2014, 6, 3133–3138 RSC.
- R. Merindol and A. Walther, Chem. Soc. Rev., 2017, 46, 5588–5619 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00164b |
| This journal is © The Royal Society of Chemistry 2018 |