Open Access Article
Open Access ArticleShunaoxin dropping pill, a Chinese herb compound preparation, attenuates memory impairment in D-galactose-induced aging mice
Hong Zhou†
ab,
Zhuo Qu†a,
Jingze Zhangc,
Bingjie Jianga,
Changxiao Liud and
Wenyuan Gao *a
*a
aTianjin Key Laboratory for Modern Drug Delivery and High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Weijin Road, Tianjin 300072, China. E-mail: pharmgao@tju,edu.cn; Fax: +86-22-87401895; Tel: +86-22-87401895
bNo. 6 Traditional Chinese Medicine Factory, Tianjin Zhongxin Pharmaceutical Group Corporation Ltd., Tianjin 300401, China
cDepartment of Pharmacy, Tianjin Key Laboratory of Cardiovascular Remodeling and Target Organ Injury, Logistics University of Chinese People's Armed Police Forces, Tianjin 300162, China
dThe State Key Laboratories of Pharmacodynamics and Pharmacokinetics, Tianjin 300193, China
First published on 13th March 2018
Abstract
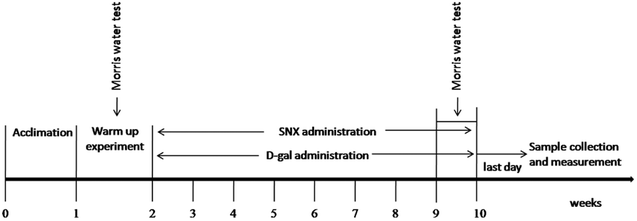
The Shunaoxin dropping pill (SNX) is derived from a traditional recipe. It has been used to treat cerebrovascular diseases in China since 2005 (approval number Z20050041). In this study, we used an in vitro H2O2-induced PC12 cell oxidative damage model and an in vivo D-gal-induced mouse memory impairment model to investigate whether SNX had neuroprotective effects. In vitro, prior to exposure to 100 μM H2O2 for 2 h, PC12 cells were pre-treated with SNX 50 μg mL−1 for 24 h. Hoechst 33258 staining was used to confirm the effect of SNX on apoptosis in the PC12 cells. Our results demonstrate that H2O2 suppresses the proliferation of PC12 cells and induces cell death. Pretreatment with SNX attenuates H2O2-induced apoptosis in PC12 cells. In vivo, D-gal was administered (100 mg kg−1, subcutaneously (s.c.)) once daily for 8 weeks to induce memory deficit and neurotoxicity in the brain of an aging mouse. Then, SNX (320 mg kg−1) was simultaneously administered orally. The present study demonstrates that SNX can alleviate aging in the mouse brain induced by D-gal via improving behavioral performance, alleviating oxidative stress, inhibiting neuroinflammation, and reducing brain cell damage in the hippocampus. Overall, these data clearly demonstrate the neuroprotective effect of SNX from the in vitro and in vivo results. SNX may be considered a novel agent for easing aging and/or age-related neurodegenerative diseases.
Introduction
Alzheimer's disease (AD), the most common type of age-related dementia, is characterized by memory deterioration and behavior disorder. The World Alzheimer Report 2016 estimated that there were 46.8 million people worldwide living with dementia in 2015 and this number will reach 131.5 million in 2050. Histopathological features of AD include the presence of extracellular deposits of β-amyloid peptides (plaques) and intracellular aggregates of the microtubule binding protein tau (tangles), which form the two distinguishing hallmark pathological features of AD.1 Moreover, one of the strongest risk factors for the AD is brain aging.2 Reactive oxygen species (ROS) and oxidative stress are known to be associated with several age-associated neuronal disorders. D-galactose (D-gal) is a physiological nutrient; however, accumulated D-gal in vivo is metabolized by D-gal oxidases, which is accompanied by H2O2 generation, resulting in an increased oxidative damage.3 Moreover, a growing body of evidence indicates that D-gal overdose in animals causes neurotoxicity including oxidative stress, neuronal apoptosis, and astrocyte activation.4 Therefore, injection of D-gal is a model for brain aging, which induces and accelerates senescence in rodents to develop AD-like symptoms.5The Shunaoxin dropping pill (SNX) is derived from a traditional recipe, which is composed of 2 herbs: Chuanxiong (Ligusticum chuanxiong Hort, Umbelliferae) and Danggui (Angelica sinensis radix, Umbelliferae). Both Chuanxiong and Danggui have been widely used in China, Japan, Korea, and other Asian countries for centuries. SNX is produced by supercritical fluid extraction as a solid dispersion and has been used to treat cerebrovascular diseases in China since 2005 (approval number Z20050041). Our previous research demonstrated that the six major chemical compounds in SNX extract were ferulic acid, senkyunolide H, senkyunolide I, senkyunolide A, ligustilide, and levistolide A.6 Moreover, our previous results suggested that ligustilide-rich total lactones derived from the Shunaoxin dropping pill could alleviate the cognition decline triggered by diabetes.7 The reported studies showed that ligustilide exerted neuroprotective effects against ischemia/reperfusion-induced brain injury by minimizing oxidative stress and anti-apoptosis.8,9 Ligustilide inhibits Aβ1-40 toxicity on PC12 cells and ameliorates memory impairment induced by scopolamine in mice.10 This compound could be developed into a therapeutic agent to prevent and treat ischaemic disorders. In addition, ferulic acid inhibits Aβ1-40 toxicity on PC12 cells, provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury in rats, and ameliorates memory impairment in a D-gal-induced aging mouse model.11,12 Therefore, we suspect that SNX has a neuroprotective effect. In this study, we used an in vitro H2O2-induced PC12 cell oxidative damage model and an in vivo D-gal-induced mouse memory impairment model to investigate whether SNX had neuroprotective effects.
Materials and methods
Materials
SNX was obtained from Zhongxin Pharmaceuticals (Tianjin, China). D-gal and vitamin E (VE) were purchased from YiFang Technology Co., Ltd. (Tianjin, China). Donepezil (DON) was purchased from a drug store (Tianjin, China). Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GPx) assay kits were obtained from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). Anti-cleaved caspase-3, anti-nuclear factor κB (NF-κB), and anti-glial fibrillary acidic protein (GFAP) antibodies were purchased from Boster Biological Engineering Co., Ltd. (Wuhan, China). Biotinylated goat anti-rabbit secondary antibody and 3,3′-diaminobenzidine tetrahydrochloride (DAB) were purchased from ZSGB-BIO (Beijing, China). The other reagents were commercially available and of analytical purity.Neuroprotective effects of SNX on H2O2-induced cytotoxicity
Neuroprotective effects of SNX on D-gal-induced impairment of memory and learning of aging mice
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in the blocking solution. Following incubation with the primary antibody, the sections were incubated for 1 h in biotinylated goat anti-rabbit secondary antibody diluted 1
100 in the blocking solution. Following incubation with the primary antibody, the sections were incubated for 1 h in biotinylated goat anti-rabbit secondary antibody diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in PBS and subsequently incubated with an avidin–biotin–horseradish peroxidase complex for 15 min at 37 °C. The sections were washed twice with PBS and incubated in DAB, and then, the sections were washed with distilled water. The staining of caspase-3, NF-κB, and GFAP proteins were measured using the Image Pro Plus software (IPP 6.0, Media Cybernetics). Brown staining on the cell membrane or in the cytoplasm represented positive staining, and the staining density indicated the expression levels of caspase-3, NF-κB, and GFAP proteins.
500 in PBS and subsequently incubated with an avidin–biotin–horseradish peroxidase complex for 15 min at 37 °C. The sections were washed twice with PBS and incubated in DAB, and then, the sections were washed with distilled water. The staining of caspase-3, NF-κB, and GFAP proteins were measured using the Image Pro Plus software (IPP 6.0, Media Cybernetics). Brown staining on the cell membrane or in the cytoplasm represented positive staining, and the staining density indicated the expression levels of caspase-3, NF-κB, and GFAP proteins.Results
SNX improved the viability of PC12 cells damaged by H2O2
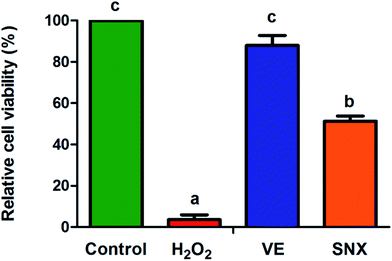
To verify the cytotoxic and neuroprotective effects of SNX for H2O2-induced PC12 cell oxidative injury, a cell viability assay was performed by the MTT method. The results indicate that SNX has no significant cytotoxicity (data not shown herein). As Fig. 2 illustrates, the cell viability was markedly decreased in PC12 cells damaged by H2O2, whereas SNX effectively protected the cells as compared to those in the model.SNX prevented apoptosis in PC12 cells induced by H2O2
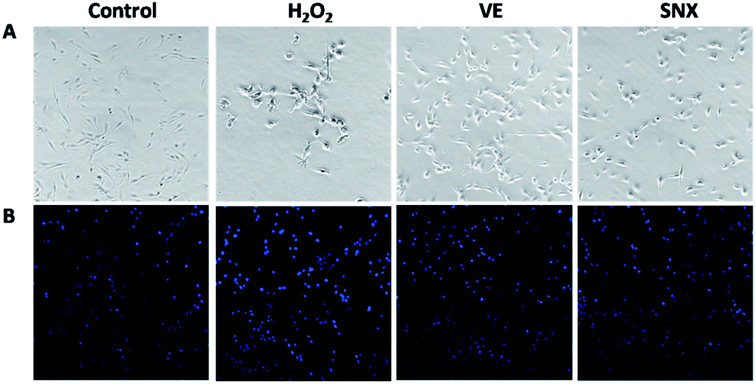
We observed the effect of SNX on the morphology of PC12 cells treated with H2O2. The PC12 cells in the control group grew well and maintained normal cell morphology (Fig. 3A). However, exposure to H2O2 for 2 h resulted in an obvious reduction in the number of viable cells and cell body shrinkage and disruption of the dendritic networks (Fig. 3A). However, cells in cultures pre-treated with SNX displayed normal morphology without remarkable cell number reduction and cell congregation.The morphological changes were further confirmed using the DNA fluorescent dye Hoechst 33258. As illustrated in Fig. 3B, cells in the control group exhibited a high rate of healthy nuclei. However, cells treated with H2O2 alone contained a lot of small bright blue dots, representing chromatin condensation or nuclear fragmentation. Pretreatment with SNX could reverse these abnormalities, and a significant decrease in nuclear condensation and fragmentation was observed.
SNX improved learning and memory of D-gal treated mice
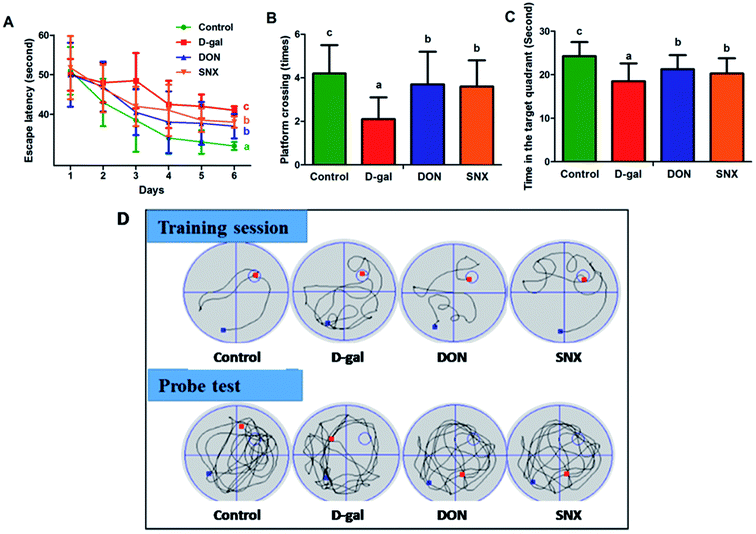
To investigate the effect of D-gal and SNX on mice behavior and memory function, the Morris water maze test was performed. As shown in Fig. 4A, the escape latency declined progressively during the 6 training days. Moreover, the escape latency in the D-gal group was much longer than that in the other groups for all training days. These results revealed that the D-gal-treated mice had significant memory impairment. After the trial session on day 7, the hidden platform was removed, and the probe test was performed. As illustrated in Fig. 4B and C, the number of platform crossings and the time spent in the target quadrant were significantly increased by SNX treatment in the D-gal-treated mice as compared to those in the case of the D-gal-alone-treated mice. Compared with the vehicle control group, D-gal-alone-treated mice spent less time (P < 0.05) in the target quadrant and crossed to the platform fewer times (P < 0.05). Interestingly, there was a significant difference between the SNX-treated groups and the D-gal-alone-treated group, indicating that SNX could restore the D-gal-induced deficit of memory and learning ability. In addition, the swimming speed of the mice in the model group was lower than that in mice in the other groups, but there was no significant difference (data was not presented).SNX alleviated oxidative stress in the brain of D-gal-treated mice
We have further examined several key antioxidants, including the enzymatic antioxidants SOD and GPx, which can scavenge ROS, GSH levels in the brain of D-gal-induced mice, and the oxidative stress-induced MDA levels. As illustrated in Table 1, compared to the control group, mice treated with D-gal showed a significant decrease in the content of GSH, activities of SOD, and GPx, whereas the MDA level was significantly increased (P < 0.05). Administration of SNX resulted in antioxidant effects in D-gal-treated mice, as evidenced by an increase in the GSH content, SOD and GPx activities along with a decline of the MDA level in D-gal-alone-treated mice.| Groups | MDA (nM mg−1 protein) | GSH (mg g−1 protein) | GPx (mU mg−1 protein) | SOD (U mg−1 protein) |
|---|---|---|---|---|
| a The results are presented as mean ± standard errors (n = 6). Different letters represent significantly different values as assessed by One-way ANOVA and LSD tests with P < 0.05. | ||||
| Control | 85.33 ± 10.51 a | 29.31 ± 2.18 c | 16.41 ± 2.01 b | 0.81 ± 0.07 b |
| D-gal | 117.67 ± 11.39 c | 15.44 ± 1.98 a | 11.59 ± 1.99 a | 0.67 ± 0.05 a |
| DON | 90.38 ± 9.81 b | 23.45 ± 3.27 b | 14.37 ± 2.78 b | 0.77 ± 0.05 b |
| SNX | 99.29 ± 12.67 b | 22.76 ± 3.12 b | 15.44 ± 1.88 b | 0.75 ± 0.03 b |
SNX attenuated the caspase-3 protein expression in the hippocampus of D-gal-treated mice
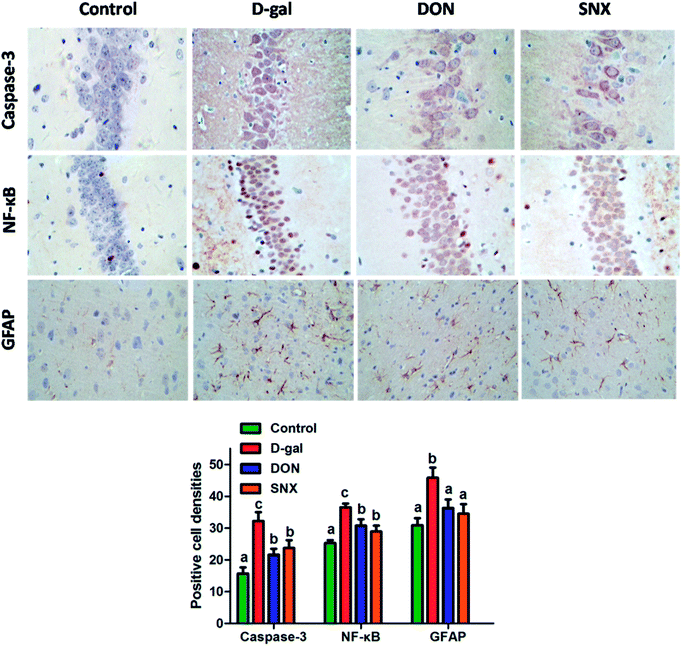
To further investigate the neuroprotective mechanism of SNX, we observed the effect of SNX on the expression of caspase-3 protein in the CA1 region of the hippocampus through immunohistochemistry. The results shown in Fig. 5 demonstrated that D-gal treatment induced an increase in the activation of caspase-3 in the CA1 region of the hippocampus that was reversed by SNX, and a decrease in the number of active caspase-3-positive cells as compared to that in the D-gal treated group was observed (P < 0.05).SNX reduced the expression of the activated NF-κB in the brain of D-gal-treated mice
The previous study demonstrated that D-gal activated NF-κB that led to neuroinflammation. According to the present immunohistochemistry analysis, the hippocampus NF-κB expression in the D-gal-alone-treated group was significantly higher than that in the other group (P < 0.05). D-gal induced the increase of NF-κB expression in the hippocampus that was markedly restored by SNX administration (P < 0.05, Fig. 5). These results suggest that the anti-amnesic effects of SNX may be due to the inhibition of the NF-κB signaling pathway in the hippocampus.SNX inhibited the activation of astrocytes in the cortex of D-gal-treated mice
To analyze the expression of GFAP, we performed an immunohistochemistry technique. Our results shown in Fig. 5 indicated that the mice in the D-gal-alone-treated group had obviously more GFAP levels in the cortex than those in the control group. SNX significantly reduced the number of GFAP immunoreactive astrocytes from 45.8 ± 3.2% in the cortex of D-gal-treated mice to 34.5 ± 3.0%, whereas DON (positive control) reduced the same to 36.3 ± 2.7%.Histopathological examination
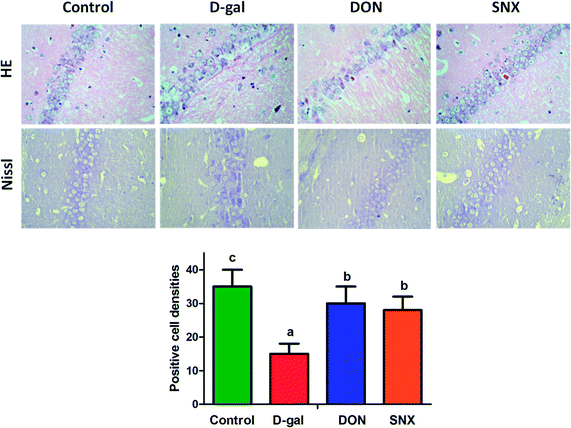
Next, we investigated the frontal brain atrophy in D-gal mice. As shown in Fig. 6, the frontal portion of the cerebrum showed atrophy-prone changes in D-gal mice. Administration with DON and SNX could alleviate the frontal brain atrophy induced by D-gal treatment. Moreover, we observed that the brain weight in the D-gal-alone-treated group decreased. However, there was no significant difference among all the groups. Histological features of the CA1 region of mice hippocampus are depicted in Fig. 7. There are no remarkable hippocampal neuronal abnormalities in the control group. CA1 pyramidal cells were arranged neatly and tightly with no noticeable cell loss. Compared with the case of the control group, D-gal induced obvious neuropathological changes in the CA1 region of the hippocampus, and extensively damaged neurons in the hippocampus CA1 region were observed. The CA1 layer neurons showed pronounced shrinkage of their neuronal bodies with their nuclei losing regular outlines and becoming hyperchromatic. However, SNX administration significantly attenuated the severity of neuronal pathomorphological changes. These data indicate that SNX protects the brain against D-gal induced injury, similar to the effects of DON.Nissl staining
Nissl body staining is a marker of mature neurons and can be used to detect neuronal apoptosis. As illustrated in Fig. 7, the Nissl-stained slices of mouse brain showed intense, rich Nissl bodies in the control group, with clearly depicted axons and no obvious abnormalities in neurons. In the D-gal-alone-treated group, Nissl bodies were significantly reduced in the region of the hippocampus CA1. Treatment with SNX protected the neurons from the D-gal-induced damage. The quantification of Nissl-positive cell densities in the hippocampus was determined, as shown in Fig. 7. The number of survival neurons in the CA1 region of the hippocampus was reduced in D-gal-treated mice as compared to that in the vehicle-treated mice. After SNX and DON treatment, the number of survival neurons significantly increased in the CA1 region of the hippocampus in SNX-treated mice as compared to that in the D-gal-alone-treated group. These findings indicate that SNX protects against neuronal apoptosis in the hippocampus of D-gal treated mice.Discussion
During natural aging, the brain undergoes progressive morphologic and functional changes resulting in the observed behavioral retrogression such as decline in motor and cognitive performance. It will be of significant value to find out drugs against neurodegeneration to delay brain senescence. In this study, we demonstrated that SNX treatment protected the PC12 cells against H2O2-induced oxidative injury and apoptosis and the hippocampus against abnormalities using a well-characterized aging mouse model with D-gal administration. The results showed that SNX afforded significant cytoprotection against H2O2-induced apoptosis and delayed the senescence of the hippocampus induced by D-gal via anti-oxidation, anti-inflammation, and anti-apoptosis.At first, in vitro model system with H2O2-induced PC12 cell oxidative injury was used to illustrate the neuroprotective effect of SNX. It is widely accepted that oxidative stress can cause DNA damage. Hoechst 33258 staining was used to monitor the changes in the DNA and nuclear structure of H2O2-treated PC12 cells during growth in the presence or absence of SNX. Our present results demonstrate that H2O2 suppresses the proliferation of PC12 cells and induces cell death, as evidenced by Hoechst 33258 staining. Pretreatment with SNX attenuates H2O2-induced apoptosis in PC12 cells. We further carried out the in vivo experiments to confirm the neuroprotective effect of SNX.
D-gal is a reducing sugar and can be metabolized at normal concentrations. However, accumulated D-gal in vivo is metabolized by the D-gal oxidases accompanied by the generation of H2O2, resulting in the increase of oxidative damage. Mice administered with D-gal (100 mg kg−1 intraperitoneally) for 60 days have neuroinflammation, elevation of ROS, and synaptic disorder.14 Therefore, we carried out this research to investigate the neuroprotective effect of SNX on the D-gal-induced aging mouse model from the perspective of anti-oxidation, anti-neuroinflammation, and anti-apoptosis.
Oxidative stress plays an important role in the aging process.15 MDA is a major marker of oxidative damage in cell membranes. SOD and GPx are the most important antioxidant enzymes that inhibit free radical formation.16 Mice treated with D-gal show decreased activities of SOD and GPx as well as decreased levels of GSH.17 In our present study, mice were given D-gal for 8 weeks. Brain MDA levels increased; however, SOD and GPx activities as well as GSH levels were found to decrease following D-gal treatment. These findings clearly show that D-gal treatment causes significant oxidative stress in the brain, as indicated by the increased MDA levels and diminished activity of the antioxidant enzymes. Interestingly, SNX could effectively restore these antioxidant enzymes and attenuate MDA production in the brain of D-gal mice; this suggested that SNX protected the mice against D-gal-induced brain oxidative stress by decreasing ROS and the lipid peroxide levels. These findings supported the view that aging was closely associated with an ROS attack, and SNX could obviously strengthen the anti-oxidative defense against free radicals and abate oxidative damage in the brain of D-gal-induced mice. However, further research is needed to reveal in greater detail the precise molecular mechanism of action.
Numerous mechanisms are associated with oxidative stress-induced neurodegeneration in the D-gal induced aging mouse model.18 Recent studies have reported that D-gal administration involves cell apoptosis and caspase-3 activation in mice hippocampal neurons.19,20 To further investigate the possible mechanism of SNX in reversing D-gal induced memory decline, the level of caspases-3, one of the major apoptotic mediators, was examined. The present results demonstrate that treatment with SNX can reverse caspase-3 protein expression in the CA1 region, suggesting the anti-apoptotic capability of SNX.
Recent reports demonstrate that D-gal induces astrocyte dysfunction and inflammatory response.21 The increased GFAP expression in the cerebral cortex is an indicator of brain aging. NF-κB, a family of DNA-binding proteins, is activated under physiological and pathological conditions and involved in regulating many aspects of cellular activity in stress, injury, and especially in pathways of inflammatory response.20 Therefore, in the present study, we observed the expression of GFAP in the cerebral cortex and NF-κB in the CA1 region. Interestingly, SNX attenuated a D-gal-mediated decrease in the astrocyte plasticity marker GFAP. The excessive increase in the GFAP expression resulting in astrogliosis implicates inflammation, increase in reactive oxygen species, and transition to a neurodegenerative state.22 Few studies propose the involvement of the transcription factors NF-κB in regulation of the transcription of GFAP. In our present study, the results show that SNX prevents the D-gal-induced memory impairment associated with the activation of NF-κB. SNX treatment significantly reduced the expression of NF-κB as compared to the case of the D-gal administered group; this suggested that SNX could protect the hippocampus from age-induced chronic inflammation.
Conclusion
In general, the present study demonstrates the neuroprotective effect of SNX from the results of in vitro and in vivo experiments. SNX can alleviate aging in the mouse brain induced by D-gal by improving behavioral performance, alleviating oxidative stress, inhibiting neuroinflammation, and reducing brain cell damage in the hippocampus. SNX may be considered as a novel agent for easing aging and/or age-related neurodegenerative diseases.Conflicts of interest
We have no conflict of interest in this research.Abbreviation
| AD | Alzheimer's disease |
| CMC-Na | Carboxymethylcellulose sodium |
| DAB | 3,3′-Diaminobenzidine tetrahydrochloride |
| D-gal | D-galactose |
| DON | Donepezil |
| ROS | Reactive oxygen species |
| VE | Vitamin E |
| NF-κB | Nuclear factor κB |
| GFAP | Glial fibrillary acidic protein |
| PBS | Phosphate buffer saline |
| HE | Hematoxylin and eosin |
| MDA | Malondialdehyde |
| SNX | Shunaoxin dropping pill |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
Acknowledgements
The work was supported by the Special Financial Grant received from the China Postdoctoral Science Foundation (No. 2015T81140), PhD Research Startup Foundation of Logistics University of Chinese People's Armed Police Forces (No. WHB201509), and Science and Technology Support Program Foundation of Tianjin China (No. 15CZDSY01020).References
- S. W. Pimplikar, J. Clin. Immunol., 2014, 34, 64–69 CrossRef CAS PubMed.
- J. H. Zhu, X. Y. Mu, J. Zeng, C. Y. Xu, J. Liu, M. S. Zhang, C. P. Li, J. Chen, T. Y. Li and Y. P. Wang, PLoS One, 2014, 9, e101291 Search PubMed.
- A. F. Aydın, J. Çoban, I. Doğan-Ekici, E. Betül-Kalaz, S. Doğru-Abbasoğlu and M. Uysal, Metab. Brain Dis., 2016, 31, 337–345 CrossRef PubMed.
- C. X. Chen, L. Y. Huang, Z. H. Nong, Y. X. Li, W. Chen, J. P. Huang, X. R. Pan, G. W. Wu and Y. Z. Lin, Neurochem. Res., 2017, 1–14 Search PubMed.
- T. Ali, H. Badshah, T. H. Kim and M. O. Kim, J. Pineal Res., 2015, 58, 71–85 CrossRef CAS PubMed.
- L. Huo, J. Z. Zhang, Z. Qu, H. Chen, Y. M. Li and W. Y. Gao, J. Ethnopharmacol., 2015, 173, 352–360 CrossRef CAS PubMed.
- H. Zhou, Z. Qu, J. Z. Zhang, Y. X. Liu, H. G. Yang, H. Chen, Y. M. Li, C. X. Liu and W. Y. Gao, RSC Adv., 2016, 6, 109132 RSC.
- X. M. Wu, Z. M. Qian, L. Zhu, F. Du, W. H. Yung, Q. Gong and Y. Ke, Br. J. Pharmacol., 2011, 164, 332–343 CrossRef CAS PubMed.
- H. Y. Peng, J. R. Du, G. Y. Zhang, X. Kuang, Y. X. Liu, Z. M. Qian and C. Y. Wang, Biol. Pharm. Bull., 2007, 30, 309–312 CAS.
- L. L. Cheng, X. N. Chen, Y. Wang, L. Yu, X. Kuang, L. L. Wang, W. Yang and J. R. Du, Fitoterapia, 2011, 82, 1128–1132 CrossRef CAS PubMed.
- C. Y. Cheng, S. Y. Su, N. Y. Tang, T. Y. Ho, S. Y. Chiang and C. L. Hsieh, Brain Res., 2008, 1209, 136–150 CrossRef CAS PubMed.
- H. G. Yang, Z. Qu, J. Z. Zhang, L. Q. Huo, J. Gao and W. Y. Gao, Int. J. Food Sci. Nutr., 2016, 67, 806–817 CrossRef CAS PubMed.
- Z. Qu, H. G. Yang, J. Zhang, L. Q. Huo, H. Chen, Y. M. Li, C. X. Liu and W. Y. Gao, Neurochem. Res., 2016, 41, 2199–2214 CrossRef CAS PubMed.
- H. C. Huang, B. W. Zheng, Y. Guo, J. Zhao, J. Y. Zhao, X. W. Ma and Z. F. Jiang, J. Alzheimers Dis., 2016, 52, 899–911 CrossRef CAS PubMed.
- G. Paradies, G. Petrosillo, V. Paradies and F. M. Ruggiero, Neurochem. Int., 2011, 58, 447–457 CrossRef CAS PubMed.
- S. J. Tsai and M. C. Yin, Eur. J. Pharmacol., 2012, 689, 81–88 CrossRef CAS PubMed.
- Z. Qu, J. Z. Zhang, H. G. Yang, L. Q. Huo, J. Gao, H. Chen and W. Y. Gao, Physiol. Behav., 2016, 154, 114–125 CrossRef CAS PubMed.
- A. Kumar, A. Prakash and S. Dogra, Int. J. Alzheimer's Dis., 2011, 2011, 1–9 CrossRef PubMed.
- X. Cui, P. P. Zuo, Q. Zhang, X. K. Li, Y. Z. Hu, J. G. Long, L. Packer and J. Liu, J. Neurosci. Res., 2006, 83, 1584–1590 CrossRef CAS PubMed.
- J. Lu, Y. L. Zheng, D. M. Wu, L. Luo, D. X. Sun and Q. Shan, Biochem. Pharmacol., 2007, 74, 1078–1090 CrossRef CAS PubMed.
- M. Lei, X. Hua, M. Xiao, J. Ding, Q. Han and G. Hu, Biochem. Biophys. Res. Commun., 2008, 369, 1082–1087 CrossRef CAS PubMed.
- E. C. Phillips, C. L. Croft, K. Kurbatskaya, M. J. O'Neill, M. L. Hutton, D. P. Hanger, C. J. Garwood and W. Noble, Biochem. Soc. Trans., 2014, 42, 1321–1325 CrossRef CAS PubMed.
Footnote |
| † These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |