Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of pyrite and chalcopyrite as sensor electrode for amperometric detection and measurement of hydrogen peroxide
Y. Wang a,
K. J. Zhaoa,
D. P. Tao*b,
F. G. Zhaia,
H. B. Yanga and
Z. Q. Zhang
a,
K. J. Zhaoa,
D. P. Tao*b,
F. G. Zhaia,
H. B. Yanga and
Z. Q. Zhang *a
*a
aSchool of Chemical Engineering, University of Science and Technology Liaoning, 185 Qianshan Middle Road, High-tech zone, Anshan, Liaoning 114501, China. E-mail: zhangzhiqiang@ustl.edu.cn
bSchool of Mining Engineering, University of Science and Technology Liaoning, 185 Qianshan Middle Road, High-tech zone, Anshan, Liaoning 114501, China. E-mail: dptao@qq.com
First published on 30th January 2018
Abstract
The sensing performance of solid-state amperometric sensors based on natural sulfide minerals, i.e., pyrite and chalcopyrite, has been characterized for the detection and measurement of hydrogen peroxide (H2O2) in aqueous medium. The sensors showed a wide linear relationship range between response current and the concentration of H2O2 from 1.0 × 10−5 mol L−1 to 1.0 × 10−2 mol L−1 and 1.0 × 10−4 mol L−1 to 3.0 × 10−2 mol L−1 for pyrite and chalcopyrite, respectively. The limit of detection (LOD) was as low as 8.6 × 10−6 mol L−1 and 5.2 × 10−5 mol L−1 (S/N = 3), respectively. The electrodes exhibited great sensitivity, repeatability and short response time (less than 5 s). The results show that pyrite and chalcopyrite can be used as a natural, low cost, reliable and sensitive sensor for hydrogen peroxide detection, creating a new and high value application for the sulfide minerals.
Introduction
Hydrogen peroxide (H2O2) is widely present in nature, particularly in waterways and various life systems. It is not only a product of the reactions catalyzed by various oxidase enzymes, but also an essential compound in industrial, clinical, pharmaceutical and environmental analyses.1,2 The sensitive, accurate, and low-cost determination of H2O2 is essential for industrial processes and clinical research. There are a number of methods for H2O2 detection and measurement, including spectrophotometry,3 titrimetry,4 X-ray absorption,5 chemiluminescence6 and fluorometric methods.7 These methods usually require expensive equipment and reagents, complicated and time-consuming procedures and skillful operators. Therefore, the electrochemical technique for H2O2 detection becomes one of the most attractive alternatives due to its simplicity, high sensitivity and selectivity.8–11 It has proved to be an effective and simple technique for H2O2 determination.12Conventional electrochemical technique for H2O2 detection and measurements is usually carried out using enzyme immobilization modified electrodes, which have gained great interest due to its unique advantage in sensitivity and selectivity.13,14 However, the enzyme-based sensors often lack of acceptable stability due to the inherent characteristics of enzymes the activity of which can readily be affected by temperature, pH, humidity, and toxic chemicals.15,16 Therefore, much attention has recently been paid to the development of non-enzymatic electrochemical H2O2 sensors for their simplicity, high reliability and sensitivity and low cost.17–19 Numerous functional materials were used for the non-enzymatic sensing of H2O2, including nanostructured materials,20–22 ionic liquid,23 polymer,24 sol–gel25 and ceramic matrix.26,27 In order to develop a better technique for detection and measurement of H2O2, electrodes made of natural pyrite and chalcopyrite have been investigated in this study for their feasibility to function as a sensor for H2O2 in aqueous medium.
Pyrite and chalcopyrite represent the Earth's most abundant and widespread sulfide minerals. Pyrite, with the formula of FeS2, is a Fe(II) polysulfide with a cubic NaCl-type crystalline structure. It is the most thermodynamically stable iron sulfide found in the nature. Over the past decades, the electrochemistry of pyrite has been studied extensively.28–31 As the most abundant copper mineral, chalcopyrite (CuFeS2) is the most economically important copper resource and is always found in association with pyrite. The selective separation of chalcopyrite from pyrite is very difficult because of several electrochemical interactions that occur at the surface of minerals during grinding.32 New applications of pyrite and chalcopyrite are of great importance. Due to their excellent characteristics such as semiconductivity, non-toxicity, and availability in the nature pyrite and chalcopyrite have recently been used in various electrochemical applications in the form of solid state sensor materials.33–36
In this study, the natural minerals (pyrite and chalcopyrite) were used as the working electrode to detect the H2O2 in aqueous solution. The main purpose of this study was to further explore analytical applications of pyrite and chalcopyrite electrodes, with a focus on developing an inexpensive, rapid and convenient method for the determination of biologically important compound, H2O2. It has been found that under the optimum operational conditions, the pyrite and chalcopyrite based sensors showed good sensitivity, rapid response time and excellent operational stability for the detection and measurement of hydrogen peroxide. Compared with traditional non-enzyme biosensor that works in alkaline solution, the pyrite and chalcopyrite sensors can detect H2O2 not only in alkaline solution, but also in acidic and neutral environment with Britton–Robinson buffer, which is an amazing feature for a new analytical sensor developed from different material. This unique characteristic of mineral sensors will lead to more extensive applications of H2O2 sensors in various industries.
Experimental
Reagents and materials
The experiments were carried out with samples of natural pyrite and chalcopyrite (from Shanxi province, China) without further purification. The pyrite and chalcopyrite samples were first cut into about 5 mm × 5 mm × 5 mm in size in the university laboratory. The processed sulfide mineral samples were then sent to Tianjin AIDAhengsheng Science-Technology Development Co., Ltd. to fabricate the sensor electrodes. The fabrication process is described briefly as follows: the column-shaped mineral electrode sample was covered by Teflon with only two ends exposed to air. One end was connected to a copper wire and the other end was inserted into the solution to detect H2O2. The copper wire was connected to the electrochemical analyzer. The working area was 0.20 cm2 for both pyrite and chalcopyrite electrodes. Prior to each experiment, the working surface of an electrode was polished using alumina powder (0.05 μm) for 30 s to obtain a shiny new surface, rinsed with deionized water and dried at the room temperature.Uric acid (UA), glucose, fructose, sodium hydroxide (NaOH), phosphoric acid, glacial acetic acid, boric acid and hydrogen peroxide (H2O2) were purchased from Sinopharm Chemical Reagent Co., Ltd. A 0.1 M Britton–Robinson buffer (BR, prepared by mixing phosphoric acid, glacial acetic acid and boric acid) was used to prepare electrolyte for the acidic solution. The neutral and alkaline solutions were adjusted by blending the mixed acid and NaOH. All chemicals were of analytical grade and were used without further purification.
Apparatus
The morphology of the minerals was observed using the field-emission scanning electron microscope (FE-SEM) (ΣIGMA-HD, ZEISS). The chemical components of pyrite and chalcopyrite were obtained using an X-ray fluorescence analyzer EDX 8300 manufactured by Suzhou Precision Instrument Co., Ltd. Electrochemical measurements were performed with a CHI 750D workstation (Shanghai Chenhua, China). A conventional three electrode system with pyrite or chalcopyrite as working electrode, a thin Pt wire as counter electrode and Ag/AgCl (sat. KCl) as reference electrode was employed in this study. All measurements were performed in air at room temperature of approximately 20 °C.Results and discussion
Characterization of pyrite and chalcopyrite
Field emission scanning electron micrograph (FE-SEM) was used to investigate the structure and morphology of pyrite electrode surface (A), chalcopyrite electrode surface (B), pyrite middle section (C) and chalcopyrite middle section (D) as shown in Fig. 1. The black parts (II, V, VI, VIII) in Fig. 1 are attributed to the impurities. Since chalcopyrite always coexists with pyrite in natural minerals, it is not unusual to observe the dark grey parts (I, IV, VII, IX) which represent the pyrite mineral. The light grey (part III, X) is the morphology of chalcopyrite. The SEM characterization was performed with the middle section of mineral sample after fracture and the results is in good agreement with pyrite and chalcopyrite electrode surfaces, implying the uniformity in composition of the minerals along the electrode length. The FE-SEM energy spectra showed that the FeS2 content of pyrite and chalcopyrite are 91.2% and 63.6%, respectively. Comparing the SEM image and energy spectrum indicates that half of chalcopyrite mineral is composed of pyrite.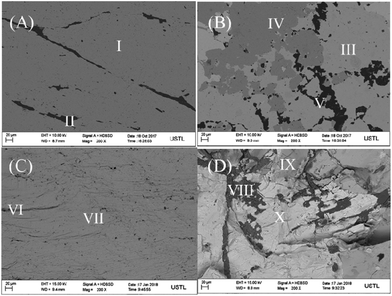 | ||
| Fig. 1 The SEM images of pyrite electrode surface (A), chalcopyrite electrode surface (B), pyrite middle section (C) and chalcopyrite middle section (D). | ||
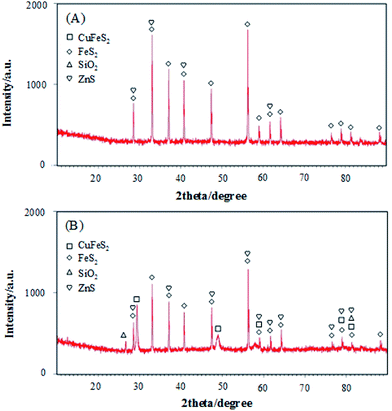
The phase characteristics and structures of pyrite and chalcopyrite were identified from the X-ray diffraction (XRD) patterns shown in Fig. 2. The broad diffraction peaks observed in Fig. 2A can be assigned to FeS2 structure which is the main content of pyrite sample. FeS2 is also a major component in Fig. 2B, confirming pyrite is closely associated with chalcopyrite. The five diffraction peaks observed with 2θ values of 29.44, 49.11, 59.06, 79.06 and 81.52 are attributed to CuFeS2. Fig. 2 suggests both pyrite and chalcopyrite contain impurities such as SiO2 and ZnS which is in accordance with the XRF data shown below.
To quantify the components of pyrite and chalcopyrite minerals, X-ray fluorescence (XRF) analysis was carried out and the results are shown in Table 1. The impurities in pyrite can introduce significant variations in its bulk semi-conducting properties which can directly affect the reactivity of pyrite surfaces.37 Table 1 shows that the powder pyrite sample contained a very low content of Cu element. Thus, iron sulfide FeS2 plays a dominant role in the detection of H2O2. The XRF data obtained with pyrite powder showed a lower purity than that of the FE-SEM energy spectra. It is believed that the purity of electrode surface used to detect H2O2 was enhanced to some extent by polishing and soaking in alkaline solutions.
| Mineral | Fe | Cu | S | Impurities |
|---|---|---|---|---|
| Pyrite | 33.05 | 0.11 | 10.95 | 10.47 |
| Chalcopyrite | 22.16 | 11.72 | 8.20 | 10.45 |
Electrocatalytic performance of H2O2 on pyrite and chalcopyrite
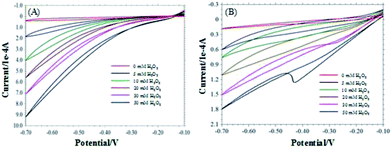
The CVs of the pyrite and chalcopyrite with and without H2O2 in 0.1 M NaOH are shown in Fig. 3A and B. The reduction peak current increased with increasing the concentration of H2O2, indicating the pyrite and chalcopyrite based sensors have good sensitivity for H2O2. However, the sensitivity of chalcopyrite is lower than that of pyrite. The large content of impurity in chalcopyrite resulted in the irregular CV curves and lower sensitivity.Fig. 4A shows the typical current plot for the pyrite based sensor on successive additions of 5 mM H2O2 in 25 mM NaOH under stirring at the working potential of −0.2 V. Fig. 4B is the typical current response for chalcopyrite electrode on successive additions of 5 mM H2O2 in 25 mM NaOH at the working potential of −0.4 V. Well-defined current responses for H2O2 were obtained at both pyrite and chalcopyrite electrodes. The pyrite sensor exhibited good repeatability with a relative standard deviation (RSD) of 4.2% for 10 successive measurements. The chalcopyrite sensor also showed rather good repeatability with a RSD of 5.3% for 8 incremental additions of H2O2. These results are better than certain H2O2 biosensors that undergo the complicated fabrication procedures.39,40 The response time is one of the most important parameters for describing sensor characteristics.41 The response current achieved the steady state in less than 3 seconds for pyrite and within 4 seconds for chalcopyrite, representing rapid response rates for natural minerals as compared with other materials such as ceramic carbon composite,42 carbon felt,11 glassy carbon,43 iron nanoparticles,44 and carbon nanotubes.45 Based on the above results it is reasonable to conclude that pyrite and chalcopyrite are excellent natural mineral sensors for detection and measurements of H2O2.
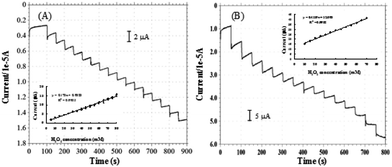 | ||
| Fig. 4 Amperometric responses of H2O2 on pyrite ((A), applied potential −0.2 V, [H2O2] = 5 mM) and chalcopyrite ((B), applied potential −0.4 V, [H2O2] = 5 mM). | ||
The same component FeS2 exists in both pyrite and chalcopyrite minerals. It is speculated that the electro-catalytic reaction happened between FeS2 and H2O2 in the two electrodes. The results in Fig. 5C and D indicate that even in acidic and neutral solution, pyrite and chalcopyrite still can detect H2O2 in the negative potential range. This suggests hydroxyl group (OH–) is not essential for the electro-catalytic reaction. FeS2 was oxidized by H2O2 to produce Fe3+ and S0. Previous studies have found that Fe3+ can be reduced to Fe2+ at −0.4 V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and the reduction of S0 to HS− happened at −0.6 V.38 Fig. 3 reveals that the reduction current increased with decreasing the applied potential, as more elemental sulphur was reduced to HS− at lower potentials.
30 and the reduction of S0 to HS− happened at −0.6 V.38 Fig. 3 reveals that the reduction current increased with decreasing the applied potential, as more elemental sulphur was reduced to HS− at lower potentials.
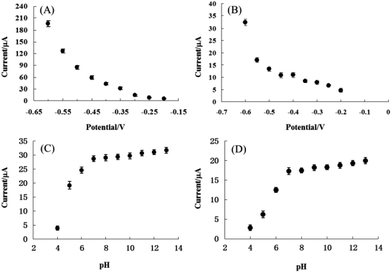
In order to identify the optimum conditions for pyrite and chalcopyrite electrodes used for detection of H2O2, the applied potential and the pH dependency of electrolyte were varied in aqueous solutions with 10 mM H2O2. The response currents were measured as a function of applied potentials between −0.2 V and −0.6 V with a step of 0.05 V and the results are shown in Fig. 5A and B for pyrite and chalcopyrite sensors, respectively. The reduction current of H2O2 increased consistently with decreasing the applied potential. Because the baseline also increased significantly with decreasing the applied potential, the lower potential value was employed in the subsequent experiments to avoid the reduction of soluble oxygen in solution. The effect on reduction current of pH from 4 to 13 was also studied and the results are shown in Fig. 5C and D for pyrite and chalcopyrite, respectively. The amperometric response increased from pH 4 to pH 7, reached a plateau at pH 7, and maintained essentially constant until pH 13 for both pyrite and chalcopyrite. This wide pH range is preferred not only for the commercial applications, but also for future study in combination with enzymes. These results show that the OH− group is not essential for the detection of H2O2. It should be pointed out that strongly alkaline solution is not desirable either from the viewpoint of sensor's practical applications. The finding is very encouraging for commercial applications of mineral sensors in complicated environment although the operational stability is not satisfying in the neutral and acid conditions with the current design of electrodes and refinement in electrode fabrication is needed for better sensing performance.
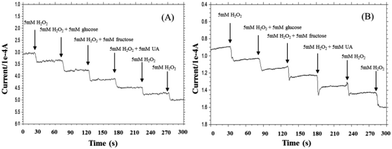
Selectivity is another important parameter for non-enzymatic H2O2 sensor since a good selectivity ensures high accuracy.46 To investigate the selectivity of pyrite and chalcopyrite for H2O2, the effect of interfering reagents on the response current has been studied. The typical amperometric response on successive additions of 5 mM H2O2, and 5 mM interference species (UA, glucose, fructose) under stirring is shown in Fig. 6. It is important to minimize the effect of interfering species possibly existing in real aqueous solutions for practical applications of amperometric sensors. As shown in Fig. 6, a clear current step was observed with the addition of H2O2 and negligible effects on the current response were observed when glucose, fructose and UA were added into the solution, demonstrating that the natural minerals can be used to detect H2O2 in the presence of these interferents at the same concentration. The results seemed acceptable in comparison with conventional H2O2-detected biosensors.47–49 The good anti-interference ability may largely be attributed to the low working potential used in the determination of H2O2.50
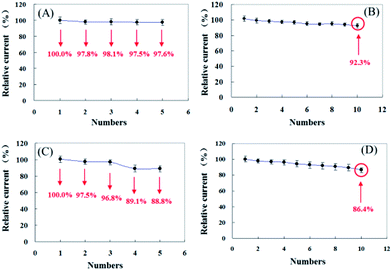
The repeatability and reproducibility of pyrite and chalcopyrite sensors were examined by detecting 5 mM H2O2 five times with an interval of 24 hours and the results are shown in Fig. 7. In comparison pyrite shows better repeatability and reproducibility than chalcopyrite sensor. The relative standard deviation (RSD) of repeatability and reproducibility is 2.77% and 0.95% for pyrite and 4.27% and 4.98% for chalcopyrite, which represent very good performance, especially for natural mineral sensors.
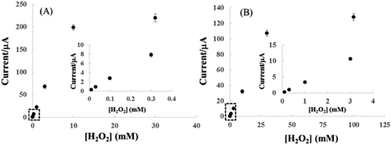
Fig. 8 shows the correlation between cathodic peak current and H2O2 concentration for pyrite (A) and chalcopyrite (B) electrode in 25 mM NaOH aqueous solution at the applied potential of −0.6 V. The pyrite and chalcopyrite sensors show a wide range of linear relationship between the response current and H2O2 concentration from 1.0 × 10−5 mol L−1 to 1.0 × 10−2 mol L−1 with a correlation coefficient of 0.997 and from 1.0 × 10−4 mol L−1 to 3.0 × 10−2 mol L−1 with a correlation coefficient of 0.991 for pyrite and chalcopyrite, respectively. The limit of detection (LOD) was as low as 8.6 × 10−6 mol L−1, with a sensitivity of 19.61 μA mM−1 for pyrite (S/N = 3) whereas the LOD was 5.2 × 10−5 mol L−1 with a sensitivity of 3.21 μA mM−1 for chalcopyrite (S/N = 3). In comparison with other H2O2-based sensors including enzyme and non-enzyme system shown in Table 2, the characteristics of pyrite and chalcopyrite sensors were rather impressive, especially considering the natural minerals were directly used without any purification. Although certain nanomaterial modified electrodes possess better performance but they are much more costly and require a complex fabrication process.
| Electrode material | Linear range (mol L−1) | LOD (μmol L−1) | Reference |
|---|---|---|---|
| a SGCCN sol–gel-derived ceramic-carbon nanotube, GCE glass carbon electrode, MWCNT multiwalled carbon nanotubes, GO graphene oxide, nanoCoPc cobalt phthalocyanine nanorods Gr graphene, Cu2O cuprous oxide nanowires, CuNi copper/nickel, υ-CuNWs vertically aligned copper nanowires, pTB–HRP–GOx/RGO poly(toluidine blue)–horseradish peroxidase–glucose oxidase/reduced graphene oxide. | |||
| HRP/SGCCN/GCE | 5.0 × 10−4 to 1.0 × 10−2 | 12.89 | 51 |
| HRP/sol–gel/MWCNT/GCE | 7.0 × 10−5 to 3.0 × 10−3 | 14 | 52 |
| GO–Ag nanocomposite | 1.0 × 10−4 to 1.1 × 10−3 | 28.3 | 53 |
| nanoCoPc-Gr | 1.0 × 10−5 to 6.0 × 10−4 | 10.1 | 54 |
| Cu2O/Au | 2.5 × 10−7 to 5.0 × 10−3 | 0.12 | 55 |
| CuNi/MWCNT/GCE | 1.0 × 10−7 to 5.0 × 10−3 | 0.0025 | 56 |
| υ-CuNWs | 5.0 × 10−7 to 8.0 × 10−4 | 0.4 | 57 |
| pTB–HRP–GOx/RGO | −3.0 × 10−5 | 0.2 | 58 |
| Pyrite | 1.0 × 10−5 to 1.0 × 10−2 | 8.6 | This work |
| Chalcopyrite | 1.0 × 10−4 to 3.0 × 10−2 | 52 | This work |
To further investigate the selectivity and applicability of pyrite and chalcopyrite towards H2O2, disinfector samples and drinking water were used as real samples. The results are presented in Table 3. The results were consistent with the conventional potassium permanganate titration method.59 RSD and recovery were less than 3.5% and 98.0–103.8%, respectively for pyrite sensor and less than 4.8% and 97.4–107.0% for chalcopyrite sensor, suggesting that both pyrite and chalcopyrite can be used to detect and measure the concentration of H2O2 in real samples with reasonably good performance.
| Sensors | Samples | Number | Measured by proposed H2O2 biosensor | Measured by KMnO4-titrated method | ||||
|---|---|---|---|---|---|---|---|---|
| Detected (μM) | Added (μM) | Found (μM) | RSDa (%) | Recovery (%) | ||||
| a RSD (%) calculated from three separate experiments. | ||||||||
| Pyrite | Drinking water | 1 | Not found | 50 | 51.9 | 3.5 | 103.8 | Not found |
| 2 | Not found | 100 | 98.0 | 2.6 | 98.0 | Not found | ||
| 3 | Not found | 200 | 202.1 | 2.5 | 101.1 | Not found | ||
| Disinfector samples | 1 | 100.2 | 50 | 151.9 | 2.1 | 101.1 | 99.9 | |
| 2 | 200.5 | 100 | 302.3 | 2.7 | 100.6 | 200.2 | ||
| 3 | 299.3 | 200 | 494.5 | 1.9 | 99.0 | 299.5 | ||
| Chalcopyrite | Drinking water | 1 | Not found | 50 | 53.5 | 4.8 | 107.0 | Not found |
| 2 | Not found | 100 | 97.4 | 3.7 | 97.4 | Not found | ||
| 3 | Not found | 200 | 203.9 | 4.0 | 102.0 | Not found | ||
| Disinfector samples | 1 | 99.6 | 50 | 153.1 | 3.8 | 102.3 | 99.3 | |
| 2 | 200.1 | 100 | 305.0 | 3.5 | 101.6 | 199.8 | ||
| 3 | 300.5 | 200 | 492.0 | 4.2 | 98.3 | 300.1 | ||
Conclusions
Electrochemical characteristics of the solid-state amperometric sensors based on the natural sulfide minerals of pyrite and chalcopyrite are investigated in aqueous medium. The electrodes were conveniently prepared using inexpensive natural sulfide minerals that originated from the natural environment. In 25 mM NaOH solution at −0.6 V (vs. Ag/AgCl) the pyrite electrode and chalcopyrite electrode displayed a current response that is in linear relationship with H2O2 concentration in a wide range from 1.0 × 10−5 mol L−1 to 1.0 × 10−2 mol L−1 and 1.0 × 10−4 mol L−1 to 3.0 × 10−2 mol L−1, respectively. Moreover, the sulfide mineral electrodes exhibited good repeatability and rapid response time (about 3–4 s). The pyrite and chalcopyrite can be used over a wide pH range. Based on this study, it is believed that other sulfide minerals may also work as the sensor for the detection of H2O2. Future studies will be focused on practical usage of natural sulfide minerals as sensitive electrochemical amperometric sensors in aqueous and non-aqueous solution for detection and concentration measurements of some chemical compounds important for pharmaceutical and environmental applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the Natural Science Foundation of Liaoning Province (No. 20170540464), the Department of Education of Liaoning (No. 2017LNQN05) and the Foundation of University of Science and Technology, Liaoning (No. 2015QN08 and No. 2016RC12). We would like to express our grateful appreciation to Dr X. X. Gao of University of Science and Technology Liaoning for her assistance in XRF analyses.References
- B. Halliwella, M. V. Clementb and L. H. Longvsdfa, FEBS Lett., 2000, 486, 10 CrossRef
.
- T. Ruzgas, E. Csöregi, J. Emneus, L. Gorton and G. Marko-Varga, Anal. Chim. Acta, 1996, 330, 123 CrossRef CAS
.
- C. Matsubara, N. Kawamoto and K. Takamura, Analyst, 1992, 117, 1781 RSC
.
- N. V. Klassen, D. Marchington and C. E. McGowan, Anal. Chem., 1994, 66, 2921 CrossRef CAS
.
- M. Giorgetti, D. Tonelli, M. Berrettoni, G. Aquilanti and M. Minicucci, J. Solid State Electrochem., 2014, 18, 965 CrossRef CAS
.
- S. Hanaoka, J. M. Lin and M. Yamada, Anal. Chim. Acta, 2001, 426, 57 CrossRef CAS
.
- T. R. Holm, G. K. George and M. J. Barcelona, Anal. Chem., 1987, 59, 582 CrossRef CAS
.
- H. Razmi and R. Mohammad-Rezaei, Microchim. Acta, 2010, 171, 257 CrossRef CAS
.
- L. Y. Ning, Y. Z. Liu, J. W. Ma, X. B. Fan, G. L. Zhang, F. B. Zhang, W. C. Peng and Y. Li, Ind. Eng. Chem. Res., 2017, 56, 4327 CrossRef CAS
.
- Y. Y. Zhang, X. Y. Bai, X. M. Wang, K. K. Shi, Y. L. Zhu and H. Jiang, Anal. Chem., 2014, 86, 9459 CrossRef CAS PubMed
.
- Y. Wang and Y. Hasebe, Sens. Actuators, B, 2011, 155, 722 CrossRef CAS
.
- F. Ricci and G. Palleschi, Biosens. Bioelectron., 2005, 21, 389 CrossRef CAS PubMed
.
- D. Y. Tang, B. Y. Xia and Y. Q. Zhang, Microchim. Acta, 2008, 160, 367 CrossRef CAS
.
- H. L. Zhang, G. S. Lai, D. Y. Han and A. M. Yu, Anal. Bioanal. Chem., 2008, 390, 971 CrossRef CAS PubMed
.
- Y. Xiao, H. X. Ju and H. Y. Chen, Anal. Chim. Acta, 1999, 391, 73 CrossRef CAS
.
- C. X. Lei, S. Q. Hu, G. L. Shen and R. Q. Yu, Talanta, 2003, 59, 981 CrossRef CAS PubMed
.
- J. F. Ping, S. P. Ru, K. Fan, J. Wu and Y. B. Ying, Microchim. Acta, 2010, 171, 117 CrossRef CAS
.
- J. Y. Jin, W. Q. Wu, H. Min, H. M. Wu, S. F. Wang, Y. Ding and S. J. Yang, Microchim. Acta, 2017, 184, 1389 CrossRef CAS
.
- R. J. Toh, C. C. Mayorga-Martinez, J. Han, Z. Sofer and M. Pumera, Anal. Chem., 2017, 89, 4978 CrossRef CAS PubMed
.
- X. H. Shu, Y. Chen, H. Y. Yuan, S. F. Gao and D. Xiao, Anal. Chem., 2007, 79, 3695 CrossRef CAS PubMed
.
- F. A. Wang, X. Q. Liu, C. H. Lu and I. Willner, ACS Nano, 2013, 7, 7278 CrossRef CAS PubMed
.
- Z. Zhang, S. Q. Gu, Y. P. Ding, F. F. Zhang and J. D. Jin, Microchim. Acta, 2013, 180, 1043 CrossRef CAS
.
- V. S. Joshi, J. Kreth and D. Koley, Anal. Chem., 2017, 89, 7709 CrossRef CAS PubMed
.
- H. Wei, J. D. Xie, X. M. Jiang, T. Ye, A. P. Chang and W. T. Wu, Macromolecules, 2014, 47, 6067 CrossRef CAS
.
- F. Doroftei, T. Pinteala and A. Arvinte, Microchim. Acta, 2014, 181, 111 CrossRef CAS
.
- K. Thenmozhi and S. Sriman Narayanan, Anal. Bioanal. Chem., 2007, 387, 1075 CrossRef CAS PubMed
.
- D. R. Shankaran, N. Uehera and T. Kato, Anal. Bioanal. Chem., 2002, 374, 412 CrossRef PubMed
.
- E. Ahlberg, K. S. E. Forssberg and X. Wang, J. Appl. Electrochem., 1990, 20, 1033 CrossRef CAS
.
- Y. Y. Huai, C. Plackowski and Y. J. Peng, Miner. Eng., 2017, 111, 131 CrossRef CAS
.
- D. P. Tao, P. E. Richardson, G. H. Luttrell and R. H. Yoon, Electrochim. Acta, 2003, 48, 3615 CrossRef CAS
.
- A. P. Chandra and A. R. Gerson, Surf. Sci. Rep., 2010, 65, 293 CrossRef CAS
.
- N. P. Finkelstein, Int. J. Miner. Process., 1997, 52, 81 CrossRef CAS
.
- Z. Simić, Z. Stanić and M. Antonijević, J. Braz. Chem. Soc., 2011, 22, 709 CrossRef
.
- R. Mihajlović, Z. Stanić and M. Antonijević, Electrochim. Acta, 2006, 51, 3707 CrossRef
.
- Z. Stanić and J. Stepanović, Monatsh. Chem., 2010, 141, 137 CrossRef
.
- Z. Stanić and J. Stepanović, J. Solid State Electrochem., 2016, 20, 2879 CrossRef
.
- P. K. Abraitis, R. A. D. Pattrick and D. J. Vaughan, Int. J. Miner. Process., 2004, 74, 41 CrossRef CAS
.
- X. Zhu, M. E. Wadsworth, D. M. Bodily and A. M. Riley, Processing and Utilization of High Sulfur Coals IV, Elsevier, Amsterdam, 1991, pp. 205–222 Search PubMed
.
- Y. Wang and Y. Hasebe, Anal. Sci., 2011, 27, 401 CrossRef CAS PubMed
.
- H. Y. Song, C. H. Ma, L. Y. You, Z. Y. Cheng, X. H. Zhang, B. S. Yin, Y. N. Ni and K. Q. Zhang, Microchim. Acta, 2015, 182, 1543 CrossRef CAS
.
- Y. Wang, T. Hosono and Y. Hasebe, Microchim. Acta, 2013, 180, 1295 CrossRef CAS
.
- K. Thenmozhi and S. S. Narayanan, Anal. Bioanal. Chem., 2007, 387, 1075 CrossRef CAS PubMed
.
- J. B. Jia, Microchim. Acta, 2008, 163, 237 CrossRef CAS
.
- G. S. Lai, H. L. Zhang and D. Y. Han, Microchim. Acta, 2009, 165, 159 CrossRef CAS
.
- H. J. Jiang, C. Du, Z. Q. Zou, X. W. Li, D. L. Akin and H. Yang, J. Solid State Electrochem., 2009, 13, 791 CrossRef CAS
.
- L. Y. Chen, T. Fujita, Y. Ding and M. W. Chen, Adv. Funct. Mater., 2010, 20(14), 2279 CrossRef CAS
.
- J. F. Ping, S. P. Ru, K. Fan, J. Wu and Y. B. Ying, Microchim. Acta, 2010, 171, 117 CrossRef CAS
.
- H. A. Zhong, R. Yuan and Y. Q. Chai, Bioprocess Biosyst. Eng., 2011, 34(8), 923 CrossRef CAS PubMed
.
- L. Q. Luo, L. M. Zhu, Y. H. Xu, L. Y. Shen, X. Wang, Y. P. Ding, Q. X. Li and D. M. Deng, Microchim. Acta, 2011, 174, 55 CrossRef CAS
.
- F. Chekin, L. Gorton and I. Tapsobea, Anal. Bioanal. Chem., 2015, 407, 439 CrossRef CAS PubMed
.
- X. H. Kang, J. Wang, Z. W. Tang, H. Wu and Y. H. Lin, Talanta, 2009, 78, 120 CrossRef CAS PubMed
.
- S. X. Xu, J. L. Li, Z. L. Zhou and C. X. Zhang, Anal. Methods, 2014, 6, 6310 RSC
.
- M. M. Shahid, P. Rameshkumar and N. M. Huang, Microchim. Acta, 2016, 183, 911 CrossRef
.
- H. H. Wang, Y. Bu, W. L. Dai, K. Li, H. D. Wang and X. Zuo, Sens. Actuators, B, 2015, 216, 298 CrossRef CAS
.
- Z. K. Yan, J. W. Zhao, L. R. Qin, F. Mu, P. Wang and X. N. Feng, Microchim. Acta, 2013, 180, 145 CrossRef CAS
.
- W. Yi, J. Liu, H. B. Chen, Y. Gao and H. M. Li, J. Solid State Electrochem., 2015, 19, 1511 CrossRef CAS
.
- L. G. Carmona, M. M. Guzmán, A. Martín, S. B. Martínez, A. B. F. Martínez, M. C. González, J. L. Cazaña and A. Escarpa, Biosens. Bioelectron., 2017, 96, 146 CrossRef PubMed
.
- F. Wang, W. C. Gong, L. L. Wang and Z. L. Chen, Microchim. Acta, 2015, 182, 1949 CrossRef CAS
.
- L. M. Li, Z. F. Du, S. Liu, Q. Y. Hao, Y. G. Wang, Q. H. Li and T. H. Wang, Talanta, 2010, 82, 1637 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |