Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reduced graphene oxide-supported Ag-loaded Fe-doped TiO2 for the degradation mechanism of methylene blue and its electrochemical properties†
Dhayanantha Prabu Jaihindh,
Ching-Cheng Chen and
Yen-Pei Fu *
*
Department of Materials Science and Engineering, National Dong Hwa University, Shou-Feng, Hualien 97401, Taiwan. E-mail: ypfu@gms.ndhu.edu.tw
First published on 9th February 2018
Abstract
Graphene oxide-based composites have been developed as cheap and effective photocatalysts for dye degradation and water splitting applications. Herein, we report reduced graphene oxide (rGO)/Ag/Fe-doped TiO2 that has been successfully prepared using a simple process. The resulting composites were characterized by a wide range of physicochemical techniques. The photocatalytic activities of the composite materials were studied under visible light supplied by a 35 W Xe arc lamp. The rGO/Ag/Fe-doped TiO2 composite demonstrated excellent degradation of methylene blue (MB) in 150 min and 4-nitrophenol (4-NP) in 210 min under visible light irradiation, and trapping experiments were carried out to explain the mechanism of photocatalytic activity. Moreover, electrochemical studies were carried out to demonstrate the oxygen evolution reaction (OER) activity on rGO/Ag/Fe-doped TiO2 in 1 M of H2SO4 electrolyte, with a scan rate of 50 mV s−1. The reductions in overpotential are due to the d-orbital splitting in Fe-doped TiO2 and rGO as an electron collector and transporter.
1. Introduction
In recent years, the increasing release of dye wastewater from various industries, such as textiles, printing, food, and cosmetics, has become a major threat to humans and ecology owing to the toxicity and non-biodegradability of dye wastewater.1,2 Industrially exuded wastewater contains many harmful contaminants such as heavy metals and dyes, which are considered carcinogenic.3–7 Moreover, the need for clean and renewable energy has inspired researchers to investigate semiconductors, such as TiO2, as photocatalysts for water splitting, the oxygen evolution reaction (OER), the oxygen reduction reaction (ORR), as well as environmental clean-up because industrial waste often contains notable concentrations of synthetic organic dyes.8–10 The large band gap of TiO2 restricts its practical applications under visible-light illumination, which covers a wide range of the solar spectrum. To overcome this problem, several attempts have been made such as (1) doping a transition metal ion into an anatase TiO2 lattice; in this regard, Fe3+ ions have attracted significant attention due to their half-filled d-electronic configuration and identical ionic radius to Ti4+; this implies that the Fe3+ ion may be incorporated into the structure of the TiO2 lattice.11 Moreover, doping of Fe3+ ions reduces the recombination of the photogenerated electrons and holes; this leads to the narrowing of the band gap energy of TiO2.12–15 (2) Depositing noble metal nanoparticles, such as Ag nanoparticles, as electron-transfer co-catalysts, which have a broad range of visible spectra absorption as well as strong localized surface plasmon resonance (LSPR).16 (3) Graphene composites and TiO2 nanoparticles have the advantages of increasing the absorptivity of pollutants, extended light absorption range, and facile charge transportation and separation.17,18Graphene, a two-dimensional layer of sp2-hybridized carbon atoms, has been widely used in sensors, electronics, drug delivery, supercapacitors, and catalysis due to its unique electrical properties,19,20 high thermal conductivity,21 mechanical strength, and specific surface area.22 However, graphene has some limitations for wide use in wastewater treatment due to its water-insoluble properties.23 Recently, several studies have contributed to the investigation of the catalytic or photocatalytic properties of graphene or reduced graphene oxide (RGO).17,24–26 The oxidized derivative of graphene, such as graphene oxide (GO), contains various functional groups such as carboxyl, hydroxyl and epoxide on its surface, which makes it highly hydrophilic and water soluble. This makes it applicable for supporting metal/metal oxide particles.27 Compared with GO, reduced graphene oxide (rGO) has higher electrical conductivity and thermal stability, which may promote electron transport speed in the Fenton process.28,29 Also, these rGO/TiO2-based composites have been used in many applications such as photocatalysis, solar cells, and hydrogen evolution.30–33 TiO2-based photocatalysts are poorly used in oxygen evolution reactions (OER). To date, expensive noble metals such as Pt, RuO2 and IrO2 electrocatalysts are widely used because of their excellent characteristics.34,35 These include low over-potential, excellent reaction kinetics and an outstanding long-term durability in acidic media for the oxygen reduction reaction (ORR), which is important for developing new noble metal free electrocatalysts and exhibiting good electrochemical activity and stability in acidic operating conditions.36
In the present study, we report a Ag loaded Fe-doped TiO2 on rGO (rGO/Ag/Fe-doped TiO2) synthesized for the removal of toxic methylene blue (MB) dye from wastewater, and OER in the acidic electrolyte. During the in situ synthesis of GO/metal oxide nanocomposites, GO was reduced to rGO. The rGO/Ag/Fe-doped TiO2 composite consists of unique properties for each constituent; e.g., TiO2 particles degrade organic pollutants, Fe3+ acts as an electro-transfer cocatalyst and rGO provides an effective pathway to increase the surface area as well as suppress the recombination of charge carriers in TiO2. Ag nanoparticles also act as electro-transfer cocatalysts and active reaction sites on the graphene surface to improve the interfacial catalytic performance. Herein we report MB and 4-nitrophenol degradation, and photoelectrochemical properties of photocatalysts, such as TiO2, Fe-doped TiO2, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2, using a 35 W Xe arc lamp.
2. Experimental
2.1. Materials
All the chemicals used in the study were analytical grade. Titanium dioxide powders (TiO2), ferric oxide (Fe2O3), sulfuric acid (H2SO4), and sodium nitrate (NaNO3) were purchased from Shimakyu's Pure Chemicals, Japan. Potassium permanganate (KMnO4) and hydrogen peroxide (H2O2, 30 vol%) were purchased from Choneye Pure Chemicals, China, and 4-nitrophenol was purchased from Acros Organics, U.S.A. All chemicals were used without further purification.2.2. Preparation of rGO/Ag/Fe-doped TiO2
The synthesis of Fe-doped TiO2 has been elaborated on in a previous contribution, and GO was prepared from graphite powder according to a modified Hummers' method.37–41 Briefly, the Ag-loaded Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2 were prepared by a simple chemical reduction and the hydrothermal method as follows. Fe-doped TiO2 (0.4 g of 2 mol%) was sonicated with 20 mL of deionized water for 10 min to get a good dispersion of materials and then water was removed by centrifugation. In another beaker, 0.1 M of AgNO3 (1 wt% of Ag) and 5 M of NH4OH were dissolved in 10 mL of DI water and then Fe-doped TiO2 powder was added to the silver nitrate solution, the mixture was sonicated for 10 min and then 0.03 g mL−1 of glycerin was added to reduce AgNO3 to Ag nanoparticles. The solution was stirred for 3 h at room temperature. The final product obtained was washed with ethanol a few times and dried in a hot air oven at 70 °C for 24 h. The rGO/Ag/Fe-doped TiO2 composite was prepared as follows. First, 5 wt% (0.025 g) of GO was taken with 20 mL of ethanol and was ultrasonicated to get a better dispersion and then 0.5 g of Ag loaded Fe-doped TiO2 powder was added followed by 10 mL of DI water. This mixture was stirred for 15 min to obtain a homogeneous solution, which was then transferred into a Teflon sealed autoclave and heated at 120 °C for 12 h. Afterwards, the precipitate was washed with DI water a few times and dried in an oven at 70 °C for 24 h. Finally, the hydrothermally reduced graphene oxide was connected to the Ag loaded Fe-doped TiO2 (Fig. 1).2.3. Characterization
A computerized X-ray powder diffractometer (XRD) with Cu Kα radiation (λ = 0.15406 nm) (Rigaku D/Max-II) was used to identify the GO, rGO, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2. Their particle-size, morphology, and composition were observed and analyzed using a scanning electron microscopy (SEM; Hitachi 3400N) equipped with an energy dispersive spectrometer (EDS). Electron probe microanalysis (EPMA) was employed to study the elements on the rGO/Ag/Fe-doped TiO2 composite using an electron probe X-ray microanalyzer (JEOL JXA-8200). The particle size, shape and selected area electron diffraction (SAED) pattern were observed by transmission electron microscopy (TEM; JEOL JEM-3010, Tokyo, Japan). X-ray photoelectron spectroscopy (XPS; VGS Thermo K-Alpha) with an Al Kα radiation as the exciting source was adapted to assist us in figuring out the chemical composition. All the binding energies referenced the C 1s peak at 284.3 eV. The UV-vis spectra were obtained using a UV-vis recording spectrophotometer with an integrating sphere (Jasco, V-600). Photoluminescence spectra were measured using a PerkinElmer LS 45 with Xe lamp. The properties of GO, rGO, and other TiO2-based photocatalysts were characterized by micro-Raman (Renishaw, 1000B). Fourier transform infrared (FTIR) spectroscopy studies were carried out using a PerkinElmer Spectrum. Samples for analysis were prepared by the KBr pellet method. The weight ratio of sample/KBr was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
2.4. Photocatalytic activity
The catalytic reaction for the degradation of aqueous methylene blue (MB) was carried out in a 200 mL Pyrex glass vessel with magnetic stirring. The initial concentration of the methylene blue was set to 20 mg L−1 (20 ppm). The photocatalyst (10 mg) was mixed with 50 mL of MB solution. The photocatalytic activities were evaluated by measuring the photocatalytic degradation of MB using a 35 W Xe arc lamp and color temperature of 6000 K as the visible-light source. Illumination was implemented after the suspension was ultrasonicated for 10 min, then it was left in a dark room for 20 min to reach adsorption–desorption equilibrium. At specific time intervals (every 30 min), 5 mL of the sample was taken from the suspensions and centrifuged to remove photocatalyst particles prior to spectral measurement. MB was monitored by measuring the absorbance at a wavelength of 664 nm, characteristic of MB. To understand the mechanism of photocatalytic degradation, the trapping experiment was carried out by using different kinds of scavengers such as isopropyl alcohol (IPA) as the hydroxyl radical scavenger and formic acid (CH2O2) as the holes scavenger and the addition of tetrachloromethane (CCl4) and para-benzoquinone (PBQ) as the electron and superoxide radical scavenger. The scavenger concentrations were set at 1 mmol for the trapping experiment. The same procedure was followed for 4-NP degradation; the initial concentration of 4-NP was set to 20 mg L−1 (20 ppm), and 10 mg of photocatalyst was mixed with 50 mL of 4-NP solution. The adsorption of 4-NP was evaluated in the dark and it was found negligible after 1 h for all of the photocatalysts. 4-NP was monitored by measuring the absorbance at a wavelength of 315 nm characteristic of 4-NP.2.5. Photoelectrochemical properties
Photoelectrochemical properties was determined by using a three electrode cell consisting of a working electrode (WE), Pt as the counter electrode (CE), and Ag/AgCl (in 3 M KCl) as the reference electrode (RE). H2SO4 solution (1 M) was used as an electrolyte. The electrochemical measurements were performed using a potentiostat/galvanostat (CHI, 6273D) at room temperature. The catalysts inks were prepared by ultrasonication of a turbid solution containing 20 mg of photocatalytic materials with 300 μL of deionized water and 30 μL of 5% Nafion for 20 min. A known amount of the catalyst ink was taken and placed on a glassy carbon electrode (GCE) with an active surface area of 0.071 cm2, which acted as the working electrode in the three electrode cell system. The oxygen evolution reaction (OER) was carried out using a 35 W Xe arc lamp with color temperature 6000 K and emissions in the range of 360–1000 nm were used to irradiate the samples.3. Results and discussion
3.1. Composition and morphology characterization
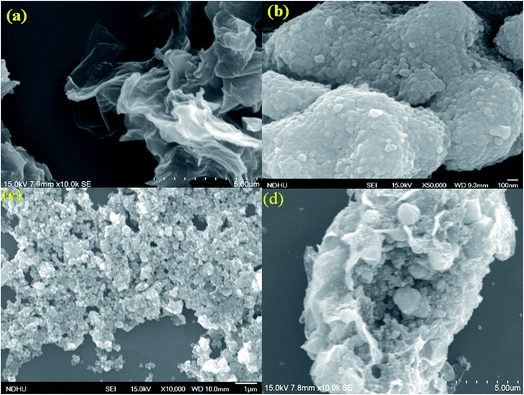
The morphology and grain size of TiO2, Fe-doped-TiO2, rGO and rGO/Ag/Fe-doped TiO2 composite were investigated by SEM. The SEM image of rGO in Fig. 2(a) reveals a platelet-like interlinked structure. Fig. 2(b) depicts agglomerated TiO2 particles with the individual grain size of around 100 nm. Fig. 2(c) shows the Fe-doped TiO2, which shows that there is no significant difference between TiO2 and Fe-doped TiO2, because the Fe ions were totally incorporated into the crystal structures of TiO2. Fig. 2(d) shows the rGO/Ag/Fe-doped TiO2 composite; the Ag/Fe-doped TiO2 particles were well wrapped and connected to reduced graphene oxide. The good connection between Ag/Fe-doped TiO2 and rGO therefore facilitated the transfer of photo-induced electrons during the photoexcitation process, which enhanced photocatalytic activity of the composite. To further identify the element distribution in the rGO/Ag/Fe-doped TiO2 composite, EPMA was employed to characterize Ti, O, Fe, Ag, and C elements in the specimens. Fig. 3(a) shows the rGO/Ag/Fe-doped TiO2 image, which was pressurized into a tablet for EPMA analysis and led to the agglomeration of the composite. Fig. 3(b) and (c) show the element mapping for Ti and O, which were homogeneously distributed. Clearly, there was interaction between Ti and O due to the formation of TiO2. Fig. 3(d) and (e) show the element mapping for Fe and Ag; the contents of Fe and Ag were significantly lower than Ti and O, as was expected. The lowest element content was C from rGO, which was ascribed to the fact that carbon is very light and is not easy to detect accurately and therefore the C distribution amount may not be a real reflection in the mapping image. The colored scale bar shows the relative concentration of elements in the scanned area (20 μm). In the colored scale bar, the relative concentration of elements increased from blue to red and the elemental count levels, area%, are shown in the right side of the EPMA images. Fig. 4(a) and (b) show the TEM images for rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2, respectively. The corresponding electron diffraction patterns of rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2 are shown in Fig. 4(b) and (d), respectively. The rings in Fig. 4(b) correspond to Fe-doped TiO2 and the electron diffraction in Fig. 4(d) shows a typical octahedral geometry, which is in good correspondence with Ag and Fe-doped TiO2 polycrystalline nanopowders.42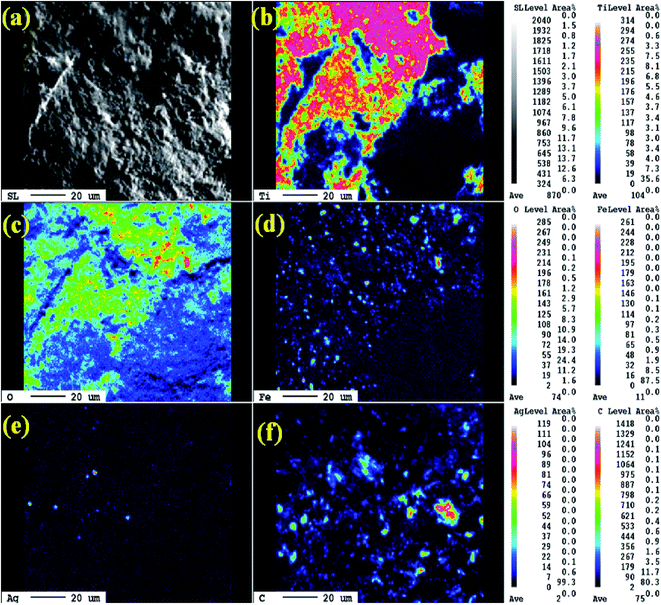 | ||
| Fig. 3 (a) SEM images of rGO/Ag/Fe-doped TiO2 and the EPMA elemental mapping of (b) Ti, (c) O, (d) Fe, (e) Ag, and (f) C. | ||
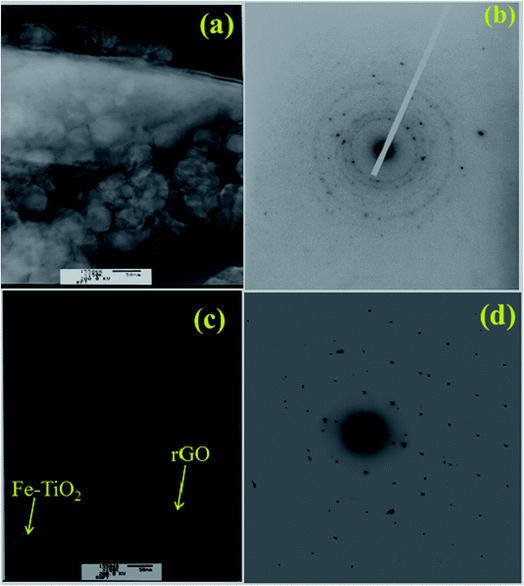 | ||
| Fig. 4 TEM characterization of (a) rGO/Fe-doped TiO2 and (c) rGO/Ag/Fe-doped TiO2. (b) Electron diffraction patterns of rGO/Fe-doped TiO2 and (d) rGO/Ag/Fe-doped TiO2. | ||
Fig. 5 shows the XRD patterns of commercially obtained graphite powder, and synthesized GO, rGO, rGO/Fe-doped TiO2, and rGO/Ag/Fe-doped TiO2 powders. In Fig. 5(a), graphite powder exhibits a sharp diffraction peak at 26.45°, corresponding to the inter-planar spacing of 0.33 nm and a somewhat less intense peak at 54.62°. These peaks can be attributed to the (002) and (004) hexagonal lattice planes of natural graphite, respectively. The diffraction pattern of GO in Fig. 5(b) shows a little broad peak at around 11.8°, corresponding to the (002) plane and the interlayer spacing of 0.75 nm, indicating the destruction of the graphite structure due to the oxidation and the structural conversion from graphite into GO.43 The XRD pattern of rGO is different from graphite and GO revealed a broader peak at 23.6° and 42.8°, corresponding to the reflection planes of (002) and (100) (Fig. 5(c)). The oxidation of graphite powder introduced numerous functional groups, which were bonded to the edges as well as both sides of the basal plane of the graphitic layer.44 For the synthesized rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2 nanocomposite, the XRD patterns shown in Fig. 5(d) and (e) display several sharp peaks at 25.3, 37.8, 48.0, 53.9, and 62.7°, which are due to the (101), (004), (200), (105), and (204) planes for the anatase phase of TiO2 (JCPDS 21-1272), and a less-intense peak at 27.5° with (110) plane corresponding to the rutile phases (JCPDS78-1508), respectively. However, the metallic Ag phase cannot be detected in the synthesized photocatalysts due to a low loading amount of Ag. No iron oxide peaks were observed in the XRD pattern. It is presumed that the iron ions were totally incorporated into the structures of TiO2 and replaced titanium ions or were located at interstitial sites. On the basis of the (200) diffraction peak of rGO/Fe-doped TiO2, the estimated lattice parameters for a, b and c are about 3.782, 3.782 and 9.515 Å, respectively.45
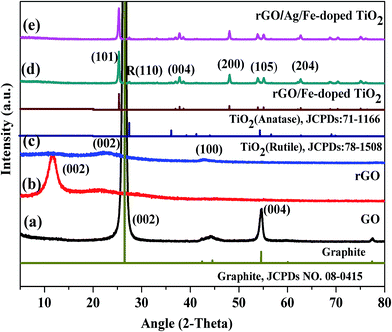 | ||
| Fig. 5 XRD patterns of (a) graphite, (b) GO, (c) rGO, (d) rGO/Fe-doped TiO2, and (e) rGO/Ag/Fe-doped TiO2. | ||
3.2. Optical characterization
Optical properties of the prepared samples were tested using UV-vis diffuse reflectance spectroscopy (DRS) (Fig. 6(a)). For undoped TiO2, the tangent line intercepts the x-axis at the wavelength of 402 nm and it corresponds to the bandgap energy of 3.08 eV. The red-shifts in the absorption edges were revealed for Fe-doped-, rGO/Fe-doped- and rGO/Ag/Fe-doped TiO2. When TiO2 is doped with Fe, the absorption edge spreads into the visible region, and the absorption edge corresponds to the electron transfer from the valence band (VB) to the conduction band (CB). Since Fe3+ in the 3d orbital is half filled, as Fe3+ is doped into TiO2, the empty Eg state is near the bottom of the conduction band, while the occupied t2g state of Fe is located at the top of the valence band.46 There are multiple electronic transitions in Fe-doped TiO2, and there are multiple energy levels between the VB and CB. Fe-doped-, rGO/Fe-doped- and rGO/Ag/Fe-doped TiO2, showed broad absorption bands and d–d transitions of Fe3+ from the DRS spectra based on a deconvolution of the data (Fig. 6(b)–(d)). For Fe-doped TiO2, a strong transition observed near 4.8 eV corresponds to the charge transfer excitations of the 3d electrons of Fe3+ to the TiO2 CB and the broad absorption band from 400–700 nm assigned to the d–d transitions of Fe3+ (2T2g → 2A2g, 2T1g) or to the charge transfer transition between interacting iron ions via the conduction band (Fe3+ + Fe3+ → Fe4+ + Fe2+).11,47,48 For rGO/Ag/Fe-doped TiO2, there is a broad range absorption in the visible region, mainly due to the local surface plasmonic resonance (LSPR) effect of Ag nanoparticles. This effect could enhance the solar-energy-conversion efficiency by increasing light absorption to longer wavelength and motivating photogenerated-carriers in the semiconductor by transferring the plasmonic energy from the Ag0 to the TiO2 semiconductor.49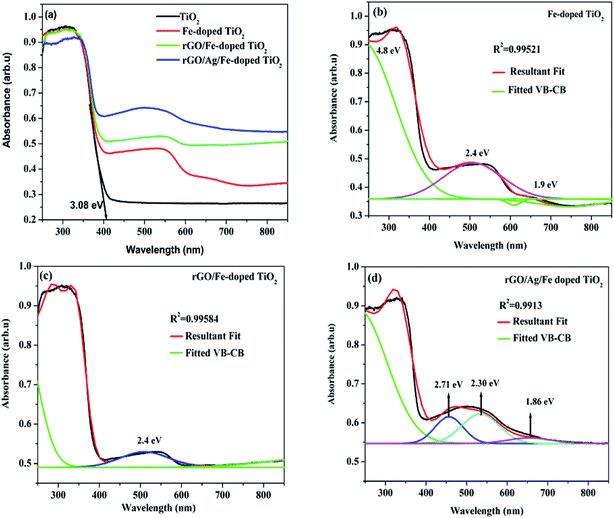 | ||
| Fig. 6 (a) Absorption spectra of TiO2, Fe-doped TiO2, rGO/Fe-doped TiO2, and rGO/Ag/Fe-doped TiO2. Deconvolution spectrum for (b) Fe-doped TiO2, (c) rGO/Fe-doped TiO2, and (d) rGO/Ag/Fe-doped TiO2. | ||
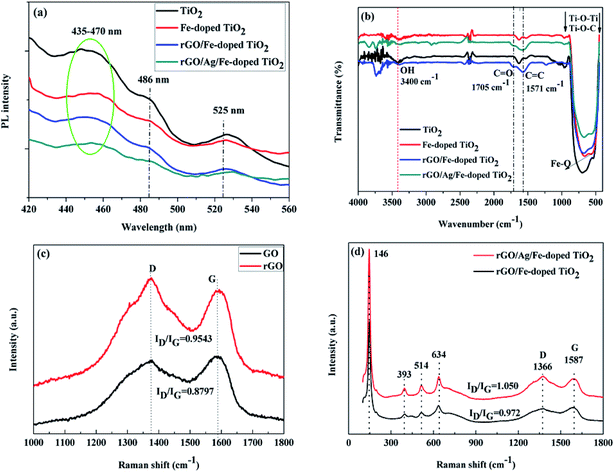
To further figure out the effect of rGO and Ag on the electron–hole separation for rGO/Ag/Fe-doped TiO2, photoluminescence spectroscopy (PL) was employed, particularly to characterize the recombination probability for photocatalysts. Fig. 7(a) shows that there is a broad emission band at around 435–470 nm, which could be assigned to the charge transfer transition of oxygen vacancy trapped electrons in TiO2.50 The excitonic PL signal at around 486 nm is related to the surface oxygen vacancies or defects in the specimen, and the PL signal located at the 525 nm band may originate from the F+ center on the surface of the TiO2.51,52 Notably, the luminescence intensities of the rGO/Ag/Fe-doped TiO2 were lower compared to other photocatalysts, which confirmed the lower electron–hole recombination probability for the rGO/Ag/Fe-doped TiO2 compared with others. This revealed that rGO and Ag nanoparticles slow down the electron–hole pair recombination in the photocatalyst, which may increase the photocatalytic activity.
 | ||
| Fig. 7 (a) PL emission spectra (b) FTIR spectra (c and d) Raman spectra of TiO2, Fe-doped TiO2, rGO/Fe-doped TiO2, and rGO/Ag/Fe-doped TiO2. | ||
The molecular structures of the TiO2, Fe-doped TiO2, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2 composites were characterized by FTIR spectroscopy (Fig. 7(b)). The peak around 3400 cm−1 is due to physically adsorbed water molecules (OH) on the specimens.53 As Fe was doped into TiO2, a new characteristic peak located at 575 cm−1 was attributed to the stretching vibration of the Fe–O bond.46 After rGO connected with Fe-doped TiO2-based specimens, several functional groups could be seen, such as the peak located at 1571 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic bonding, and the lowering of the absorption peak at around 1705 cm−1 assigned to the C
C aromatic bonding, and the lowering of the absorption peak at around 1705 cm−1 assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching accredited to rGO. Typically, Ti–O–Ti and Ti–O–C bonds can be seen at low frequency bands around 450 to 900 cm−1, and their shifting towards a higher wavenumber, compared with Fe-doped TiO2, indicates the chemical interaction of Fe-doped TiO2 with rGO. Based on the FTIR spectra, the intensity of the absorption bands was somewhat decreased for the rGO/Ag/Fe-doped TiO2 composite compared with the others. This indicates that the composite was covalently implanted over rGO sheets; the rGO sheets may shield the transmittance of infrared rays. A Raman study was carried out to further explain the chemical, structural, and vibrational bands of the TiO2-based composite. Fig. 7(c) shows the Raman spectra of GO and rGO using 532 nm laser irradiation. There are two sharp peaks located at 1341 and 1587 cm−1, which can be attributed to the disordered carbon (D-band) and graphitic carbon (G-band), respectively. The G-band with 1587 cm−1 has E2g symmetry and it is due to the in-plane bond-stretching motion of pairs of C sp2 atoms. The D-band with 1341 cm−1 is a breathing mode of A1g symmetry, and the intensity of the D-band is strictly connected to the presence of the six-fold aromatic ring.54 The ratios of D- and G-band intensities (ID/IG) for GO and rGO were 0.8797 and 0.9543, respectively. The ID/IG ratio of rGO is greater compared to that of GO, indicating that the reduction process changed the structure of GO, and there was an increase in the number of smaller sp2 domains for rGO.55 Fig. 7(d) shows the Raman spectra for rGO/Ag/Fe-doped TiO2 and rGO/Ag/TiO2 composites, which revealed almost the same pattern, except for the ratio of D- and G-band intensities (ID/IG). This exhibited specific vibration modes centered at 146 (Eg), 393 (B1g), 514 (B1g + A1g) and 634 cm−1 (Eg), indicating the presence of the anatase phase of TiO2, which is consistent with the XRD results. Moreover, after loading Ag nanoparticles, the intensity of the Raman peaks of anatase TiO2 as well as the intensity ratio of the D- and G-band (ID/IG = 1.050) significantly increased, as compared to that without Ag-loading (ID/IG = 0.972). This could be related to the LSPR effect of Ag nanoparticles, which is in agreement with the DRS and PL studies for the rGO/Ag/Fe-doped TiO2 composite. An increase in the intensities of the Raman peaks was observed on anatase TiO2 and rGO, which indicated that Ag nanoparticles were deposited on the surface of TiO2 and well connected to the rGO layers. Overall, the addition of rGO and Ag nanoparticles increased the charge conduction and light absorption.
O stretching accredited to rGO. Typically, Ti–O–Ti and Ti–O–C bonds can be seen at low frequency bands around 450 to 900 cm−1, and their shifting towards a higher wavenumber, compared with Fe-doped TiO2, indicates the chemical interaction of Fe-doped TiO2 with rGO. Based on the FTIR spectra, the intensity of the absorption bands was somewhat decreased for the rGO/Ag/Fe-doped TiO2 composite compared with the others. This indicates that the composite was covalently implanted over rGO sheets; the rGO sheets may shield the transmittance of infrared rays. A Raman study was carried out to further explain the chemical, structural, and vibrational bands of the TiO2-based composite. Fig. 7(c) shows the Raman spectra of GO and rGO using 532 nm laser irradiation. There are two sharp peaks located at 1341 and 1587 cm−1, which can be attributed to the disordered carbon (D-band) and graphitic carbon (G-band), respectively. The G-band with 1587 cm−1 has E2g symmetry and it is due to the in-plane bond-stretching motion of pairs of C sp2 atoms. The D-band with 1341 cm−1 is a breathing mode of A1g symmetry, and the intensity of the D-band is strictly connected to the presence of the six-fold aromatic ring.54 The ratios of D- and G-band intensities (ID/IG) for GO and rGO were 0.8797 and 0.9543, respectively. The ID/IG ratio of rGO is greater compared to that of GO, indicating that the reduction process changed the structure of GO, and there was an increase in the number of smaller sp2 domains for rGO.55 Fig. 7(d) shows the Raman spectra for rGO/Ag/Fe-doped TiO2 and rGO/Ag/TiO2 composites, which revealed almost the same pattern, except for the ratio of D- and G-band intensities (ID/IG). This exhibited specific vibration modes centered at 146 (Eg), 393 (B1g), 514 (B1g + A1g) and 634 cm−1 (Eg), indicating the presence of the anatase phase of TiO2, which is consistent with the XRD results. Moreover, after loading Ag nanoparticles, the intensity of the Raman peaks of anatase TiO2 as well as the intensity ratio of the D- and G-band (ID/IG = 1.050) significantly increased, as compared to that without Ag-loading (ID/IG = 0.972). This could be related to the LSPR effect of Ag nanoparticles, which is in agreement with the DRS and PL studies for the rGO/Ag/Fe-doped TiO2 composite. An increase in the intensities of the Raman peaks was observed on anatase TiO2 and rGO, which indicated that Ag nanoparticles were deposited on the surface of TiO2 and well connected to the rGO layers. Overall, the addition of rGO and Ag nanoparticles increased the charge conduction and light absorption.
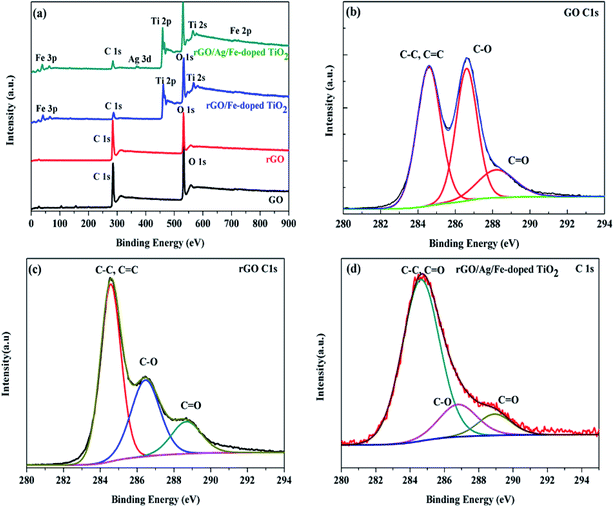
X-ray photoelectron spectroscopy (XPS) measurements were performed for comparison with the difference in the chemical state of carbon among GO, rGO, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2. Fig. 8(a) reveals the presence of C 1s, O 1s, Fe 2p, Ti 2p, and Ag 3d peaks in these materials. In Fig. 8(b) and (c), the XPS spectra of the GO and rGO for C 1s indicates that there are three peaks corresponding to the sp2 carbon at 284.6 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C, sp3 carbon at 286.6 eV for (C–O), and 288.4 eV for (C
C/C–C, sp3 carbon at 286.6 eV for (C–O), and 288.4 eV for (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). After the reduction process, the peak intensity (C–O) for all the oxygen species decreased dramatically, suggesting the effective removal of the oxygen-containing groups in GO. In addition, a significant increase in the C
O). After the reduction process, the peak intensity (C–O) for all the oxygen species decreased dramatically, suggesting the effective removal of the oxygen-containing groups in GO. In addition, a significant increase in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C peak indicated the restoration of the sp2 carbon network.56 Fig. 8(d) shows that in the XPS spectrum of C 1s of the rGO/Ag/Fe-doped TiO2, the intensity of the peaks related to the oxygen functionalities became weaker than that of GO. The reduction in the peak intensities of the oxygen-functionalities declares the presence of residual oxygen-containing groups on rGO. It was observed that in the rGO/Ag/Fe-doped TiO2 composite, the C/O ratio was enhanced, which indicated that the rGO sheets can serve as a conductive channel between the metal oxide nanoparticles, and they are favorable for the photocatalytic process.57 The main broad peak was located at 284.6 eV and other less intense peaks were located at 286.6 and 288.4 eV, respectively. The presence of oxygenated weaker peaks indicates the deoxygenation of GO and formation of rGO. The spectrum in Fig. S1(a)† shows the Ti 2p-related peaks for rGO/Ag/Fe-doped TiO2, where the spin–orbit splitting of the Ti 2p peak leads to the doublets Ti 2p3/2 and Ti 2p1/2, which are located at 458.2 eV and 464.0 eV, respectively. The splitting of the 2p doublet is 5.8 eV, confirming the state of Ti4+ in the rGO/Ag/Fe-doped TiO2. Fig. S1(b)† shows the XPS spectra of Ag 3d5/2 and Ag 3d3/2 and the splitting of the 3d doublet is 6.1 eV, indicating the metallic nature of silver.58,59 Fig. S1(c)† shows the O 1s spectral peak at 531.3 eV for the surface O–H group. In Fig. S2,† XPS peaks were seen at 457.67 eV and 463.36 eV for Ti 2p3/2 and Ti 2p1/2, respectively, for the TiO2 specimen. In Fig. S3,† for the Fe3+ doped TiO2, two peaks located at 458.31 eV and 464.03 eV slightly shifted toward higher binding energy compared to TiO2, which confirmed the presence of the Fe3+ ions in Fe-doped TiO2. The Fe 2p XPS peaks located at 709.16 eV and 723.78 eV are attributed to the binding energies of the Fe 2p3/2 and Fe 2p1/2, also confirming the presence of the Fe3+ dopant in the TiO2 lattice. From Fig. 8(a) showing the survey spectrum of rGO/Ag/Fe-doped TiO2, the Ti 2p3/2 and Ti 2p1/2 peaks are located at 459.14 eV and 464.62 eV, and slightly shift towards higher binding energy compared to those of Fe-doped TiO2, which implies the interactions of Ti with the oxygen centers of rGO.
C/C–C peak indicated the restoration of the sp2 carbon network.56 Fig. 8(d) shows that in the XPS spectrum of C 1s of the rGO/Ag/Fe-doped TiO2, the intensity of the peaks related to the oxygen functionalities became weaker than that of GO. The reduction in the peak intensities of the oxygen-functionalities declares the presence of residual oxygen-containing groups on rGO. It was observed that in the rGO/Ag/Fe-doped TiO2 composite, the C/O ratio was enhanced, which indicated that the rGO sheets can serve as a conductive channel between the metal oxide nanoparticles, and they are favorable for the photocatalytic process.57 The main broad peak was located at 284.6 eV and other less intense peaks were located at 286.6 and 288.4 eV, respectively. The presence of oxygenated weaker peaks indicates the deoxygenation of GO and formation of rGO. The spectrum in Fig. S1(a)† shows the Ti 2p-related peaks for rGO/Ag/Fe-doped TiO2, where the spin–orbit splitting of the Ti 2p peak leads to the doublets Ti 2p3/2 and Ti 2p1/2, which are located at 458.2 eV and 464.0 eV, respectively. The splitting of the 2p doublet is 5.8 eV, confirming the state of Ti4+ in the rGO/Ag/Fe-doped TiO2. Fig. S1(b)† shows the XPS spectra of Ag 3d5/2 and Ag 3d3/2 and the splitting of the 3d doublet is 6.1 eV, indicating the metallic nature of silver.58,59 Fig. S1(c)† shows the O 1s spectral peak at 531.3 eV for the surface O–H group. In Fig. S2,† XPS peaks were seen at 457.67 eV and 463.36 eV for Ti 2p3/2 and Ti 2p1/2, respectively, for the TiO2 specimen. In Fig. S3,† for the Fe3+ doped TiO2, two peaks located at 458.31 eV and 464.03 eV slightly shifted toward higher binding energy compared to TiO2, which confirmed the presence of the Fe3+ ions in Fe-doped TiO2. The Fe 2p XPS peaks located at 709.16 eV and 723.78 eV are attributed to the binding energies of the Fe 2p3/2 and Fe 2p1/2, also confirming the presence of the Fe3+ dopant in the TiO2 lattice. From Fig. 8(a) showing the survey spectrum of rGO/Ag/Fe-doped TiO2, the Ti 2p3/2 and Ti 2p1/2 peaks are located at 459.14 eV and 464.62 eV, and slightly shift towards higher binding energy compared to those of Fe-doped TiO2, which implies the interactions of Ti with the oxygen centers of rGO.
 | ||
| Fig. 8 (a) XPS survey scan of GO, rGO, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2. (b) C 1s of GO, (c) C 1s of rGO, and (d) C 1s of rGO/Ag/Fe-doped TiO2 (inset shows the Ti 2p spectrum). | ||
3.3. Photocatalytic activity and mechanism
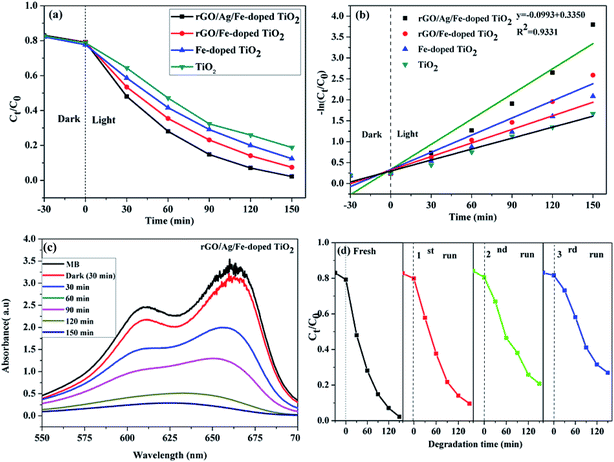
The photocatalytic performance was tested in the photodegradation of MB (20 ppm) under irradiation with a 35 W Xe arc lamp, in aqueous solution and ambient conditions. The photodegradation efficiencies were 95.33, 88.79, 82.40, and 74.59% for rGO/Ag/Fe-doped TiO2, rGO/Fe-doped TiO2, Fe-doped TiO2, and pure TiO2, respectively, within 150 min of irradiation (Fig. 9(a)). It was assumed that the degradation of the MB solution under visible light obeyed the pseudo-first order reaction kinetics as follows:
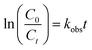 | (1) |
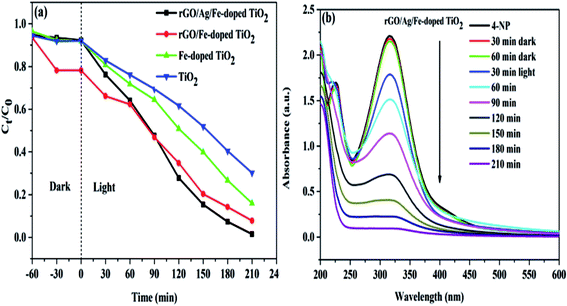 | ||
| Fig. 10 (a) Photodegradation of 4-NP solution for four different photocatalysts. (b) Time-dependent UV-visible absorption spectra of the 4-NP solution in the presence of rGO/Ag/Fe-doped TiO2. | ||
Based on the experimental data discussed above, we propose a possible photocatalysis mechanism in Fig. 11. Generally, the photogenerated electrons in the conduction band are consumed by the surface adsorbed oxygen molecules to produce superoxide anion (O2−˙) radicals, while generated holes in the valence band are scavenged by surface hydroxyl groups to generate hydroxyl radicals (OH˙). Subsequently, these radicals can be used for the degradation of the pollutant. UV-vis spectra revealed that the doping of Fe3+ ions in the TiO2 lattice modified its original band structure. The visible-light response for the Fe-doped TiO2 specimen is due to narrowing of the band gap, which is attributed to the following: (1) the d–d transition between Fe3+ ions and conduction band electrons; (2) charge transfer transitions between interacting Fe3+ ions to create electronic states (Fe4+ and Fe2+) that are spread across the band gap of TiO2. These different electronic states act as electron- and hole-trapping sites. They decrease the electron–hole pair recombination rate and enhance photocatalytic activity. Fe3+ can act as a hole trapper (Fe3+ + h+ → Fe4+) because the energy level of Fe3+/Fe4+ lies above the valence band. The trapped holes in Fe4+ can migrate to the surface adsorbed hydroxyl ions to produce hydroxyl radicals (Fe4+ + OH− → Fe3+ + OH˙). Fe3+ ions can also serve as trapping sites for both photogenerated electrons and the electrons relaxed from the conduction band. They form Fe2+ ions via the reduction reaction (Fe3+ + e− → Fe2+). These Fe2+ ions are oxidized to Fe3+ ions by transferring the electrons to the adsorbed O2 molecules on the catalyst surface (Fe2+ + O2ads → Fe3+ + O2−˙).60 The O2−˙ ions can easily trap photogenerated holes, and hydroxyl radicals and hydroxyl ions are produced (O2−˙ + h+ → O−˙; O−˙ + H2Oads → + OH˙ + OH−).48 Because the Fe2+/Fe3+ energy level is close to that of Ti3+/Ti4+, it is possible that the trapped electrons in Fe2+ are transferred to Ti4+, and anion radicals are generated upon reacting with adsorbed oxygen (Fe2+ + Ti4+ → Ti3+ + Fe3+; Ti3+ + O2 → O2−˙ + Ti4+).61 However, as the Fe3+ doping-level exceeds a certain amount due to a decrease in the distance between trapping sites, Fe3+ ions may also act as the recombination centers of the photogenerated electrons and holes, which is unfavorable to photocatalytic reaction. To overcome this problem, metal Ag0 particles are deposited on the catalyst surface and can act as electron traps. Since the Schottky barrier is formed between the Ag NPs and Fe-doped TiO2 interface, the electrons migrate from Fe-doped TiO2 to the Ag NPs.50 Simultaneously, the LSPR effect is induced under visible light irradiation and produces a strong local electronic field that enhances the energy of trapped electrons. The Fermi level of rGO is below the potential of the conduction band of TiO2, and it is speculated that rGO may act as an electronic acceptor that could accept electrons from the valence band. Thus, both Ag and rGO could serve as acceptors for the photogenerated electrons from the valence band of Fe-doped TiO2.
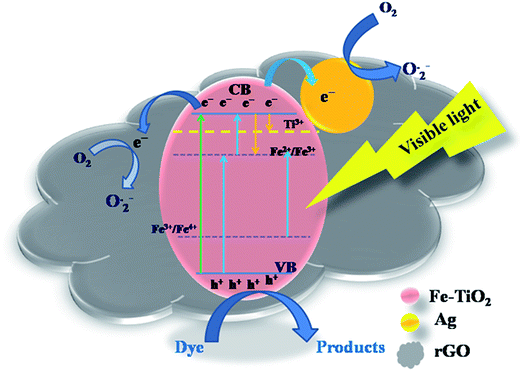 | ||
| Fig. 11 Schematic of the mechanism of charge separation for the rGO/Ag/Fe-doped TiO2 under the 35 W Xe arc lamp. | ||
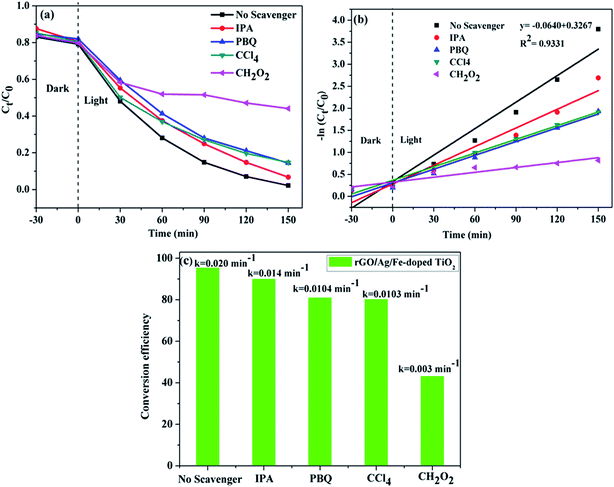
To further understand the photocatalytic mechanism, it is important to detect the main oxidative species of the photocatalytic process for explaining the photocatalytic mechanism. The main oxidative species in the photocatalytic process were detected through the radicals-trapping experiment using IPA as the hydroxyl radical scavenger and CH2O2 as a hole scavenger. Tetrachloromethane (CCl4) and para-benzoquinone (PBQ) were used as the electron and superoxide radical scavengers.61,65 Fig. 12(a) reveals the photocatalytic degradation of MB addition with various scavengers for the rGO/Ag/Fe-doped TiO2 composite. Fig. 12(c) shows that in the presence of CH2O2, a scavenger of holes, the degradation conversion efficiency is only 43%, indicating that the photogenerated holes (h+) accumulated in the valence band of Fe-doped TiO2; they are one of the main reactive species for the degradation of MB that could directly oxidize the pollutants. On the contrary, the degradation conversion efficiency is close to 90% in the presence of IPA, a scavenger of hydroxyl radicals (OH˙), indicating that the hydroxyl radicals are not the main reactive species for MB degradation. In the presence of PBQ and CCl4 for scavengers of photogenerated electrons (e−) and superoxide radicals (O2−˙), they revealed the minor reactive species for MB degradation. It was concluded that the most important radicals to degrade MB solution are ranked as follows: h+ > O2−˙ > e− > OH˙. The first order kinetic plots for MB degradation for different scavengers are shown in Fig. 12(b). Fig. 12(c) addresses the values of rate constants. Table 1 lists the comparison of composite photocatalysts and their photocatalytic performances.
| Material | Pollutant & catalyst loading | Light source | Degradation (%) | Degradation time (min)/temperature | Stability performance (no. of recycle tests) | Ref. |
|---|---|---|---|---|---|---|
| Graphene/Fe–TiO2 nanowire | MB (10 ppm) & 100 mg | 400 W Xe lamp | 99.5 | 80 min/room temp. | 5 cycles | 62 |
| Fe3O4@rGO@TiO2 | MB (10 ppm) & 0.1 to 1.5 mg L−1 | 300 W UV-vis lamp | 99 | 140/room temp. | 6 cycles | 63 |
| TiO2–Ag/GR | MB (1 ppm–5 ppm) & 1 mg L−1 −5 mg L−1 | HP Hg lamp | 100 | 160/pH-6/room temp. | 5 cycles | 64 |
| TiO2@C/Ag | RB and MO (5 ppm) & 0.03 g | 150 W Xe lamp | 91 and MO not mentioned | 360 min/room temperature | 3 cycles | 59 |
| rGO-Fe3O4–TiO2 | MB (1 mg L−1) & 0.5 mg mL−1 | 125 W HPMV lamp | 100% under UV light & 91% in visible light | 5 min/room temp. | 3 cycles | 7 |
| Ag–Cu2O/rGO composite | MO (32 mg L−1) & 10 mg | 400 W metal halide lamp | 95% | 60 min/room temp. | 3 cycles | 56 |
| TiO2-rGO composite | MB (10 ppm) & 1 mg mL−1 | LED torches (∼0.1 mW mm−2) | 98.72 | 300 min/room temp. | — | 60 |
| Fe doped TiO2 | MB (7.5 ppm) & 100 mg L−1 | UV light | >95 | 60 min/room temp. | 6 cycles | 11 |
| rGO/Ag/Fe doped TiO2 | MB (20 ppm) & 10 mg | 35 W Xe arc lamp | 95.33 | 150 min/room temp. | 4 cycles | This work |
3.4. Photoelectrochemical properties
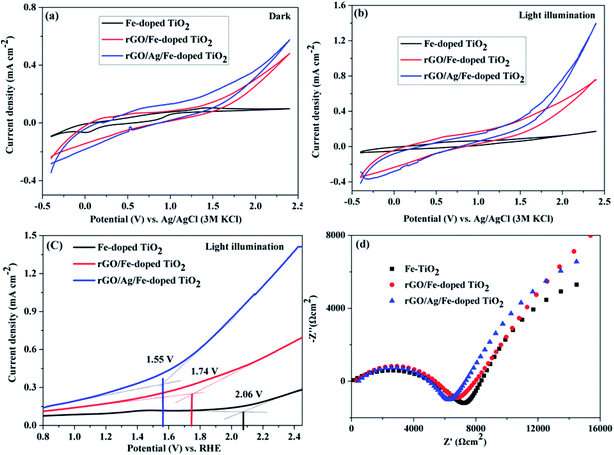
The photoelectrochemical properties of Fe-doped TiO2, rGO/Fe-doped TiO2 and rGO/Ag/Fe-doped TiO2 were studied in 1 M H2SO4 electrolyte. Fig. 13(a) and (b) show cyclic voltammograms (under dark conditions and under 35 W light irradiation in 1 M H2SO4 solutions). The current densities were observed at the anodic vertex of 2.4 V for dark and light irradiation; rGO/Ag/Fe-doped TiO2 has the highest current density of 1.39 mA cm−2 under the light. Table S1† shows the current densities of all materials under dark and light conditions. Fig. 13(c) shows linear sweep voltammetry curves for the photocatalysts with light irradiation and EIS spectra, respectively. The oxygen evolution reaction (OER) begins as an onset potential of 2.06 V for Fe-doped TiO2 (η = 0.83 V), 1.74 V for rGO/Fe-doped TiO2 (η = 0.51 V), and 1.55 V for rGO/Ag/Fe-doped TiO2 (η = 0.32 V) with respect to the reversible hydrogen electrode (RHE), where η is the overpotential. The onset potential of OER for rGO/Ag/Fe-doped TiO2 (1.55 V vs. RHE) is a little higher than for the in-house synthesized as well as commercially obtained IrO2 (onset potential 1.43 V vs. RHE).66 The rGO/Ag/Fe-doped TiO2 has a low overpotential compared to Fe-doped TiO2. This is one of the best attributes of our photocatalysts and further investigation is needed to improve the electrochemical activity of metal oxide based photocatalysts for OER activity. Fig. 13(d) reveals the Nyquist plot; the electron transfer resistance (Ret) for rGO/Ag/Fe-doped TiO2 is about 6 kΩ cm−2, for rGO/Fe-doped TiO2 it is about 7 kΩ cm−2, and for Fe-doped TiO2 it is about 8 kΩ cm−2. Fig. S5† shows the corresponding equivalent circuit. The results suggest that the rGO/Ag/Fe-doped TiO2 is a good photocatalyst for maintaining OER activity.4. Conclusion
In summary, 1 wt% of Ag nanoparticles loaded Fe-doped TiO2 on rGO (rGO/Ag/Fe-doped TiO2) was successfully prepared and photocatalytic MB degradation was examined under a 35 W Xe arc lamp. The rGO/Ag/Fe-doped TiO2 revealed the highest MB solution degraded performance for which photocatalytic conversion efficiency reached 95.33% in 150 min, and the rate of degradation constant k was 0.020 min−1. The DRS study used trap levels and band gap tuning in TiO2 to investigate the photocatalytic activity in near-visible wavelength conditions. The photocatalytic mechanism was evaluated by using different kinds of radical scavengers. The photoelectrochemical studies of OER were carried out, and the overpotential η was 0.32 V for rGO/Ag/Fe-doped TiO2 and the corresponding current density was 1.39 mA cm−2 under the light illumination. Overall, the studies suggest that the rGO/Ag/Fe-doped TiO2 has a good photocatalytic activity towards MB degradation and maintains OER activity in an acid electrolyte.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Ministry of Science and Technology of Taiwan for financially supporting this research under contract number: MOST 106-2113-M-259-001.References
- Y. Z. Chen, N. Li, Y. Zhang and L. D. Zhang, J. Colloid Interface Sci., 2014, 422, 9–15 CrossRef CAS PubMed.
- M. L. Rache, Appl. Catal., B, 2014, 146, 192–200 CrossRef CAS.
- R. K. Upadhyay, N. Soin and S. S. Roy, RSC Adv., 2014, 4, 3823–3851 RSC.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Bioresour. Technol., 2001, 77, 247–255 CrossRef CAS PubMed.
- B. Prasad, C. Ghosh, A. Chakraborty, N. Bandyopadhyay and R. Ray, Desalination, 2011, 274, 105–112 CrossRef CAS.
- P. Benjwal and K. K. Kar, J. Environ. Chem. Eng., 2015, 3, 2076–2083 CrossRef CAS.
- P. Benjwal, M. Kumar, P. Chamoli and K. K. Kar, RSC Adv., 2015, 5, 73249–73260 RSC.
- J. M. Wu, Y. Chen, L. Pan, P. Wang, Y. Cui, D. Kong, L. Wang, X. Zhang and J. J. Zou, Appl. Catal., B, 2018, 221, 187–195 CrossRef CAS.
- L. Pan, S. Wang, J. Xie, L. Wang, X. Zhang and J. J. Zou, Nano Energy, 2016, 28, 296–303 CrossRef CAS.
- L. Ma, H. Han, L. Pan, M. Tahir, L. Wang, X. Zhang and J. J. Zou, RSC Adv., 2016, 6, 63984–63990 RSC.
- H. Khan and I. K. Swati, Ind. Eng. Chem. Res., 2016, 55, 6619–6633 CrossRef CAS.
- T. Sun, J. Fan, E. Liu, L. Liu, Y. Wang, H. Dai, Y. O. Yang, W. Hou, X. Hu and Z. Jiang, Powder Technol., 2012, 228, 210–221 CrossRef CAS.
- V. C. Papadimitriou, V. G. Stefanopoulos, M. N. Romanias, P. Papagiannakopoulos, K. Sambani, V. Tudose and G. Kiridas, Thin Solid Films, 2011, 520, 1195–1201 CrossRef CAS.
- V. D. Binas, K. Sambani, T. Maggos, A. Katsanaki and G. Kiriakidis, Appl. Catal., B, 2012, 113–4, 79–86 CrossRef.
- T. B. Nguyen, M. J. Hwang and K. S. Ryu, Appl. Surf. Sci., 2012, 258, 7299–7305 CrossRef CAS.
- X. F. Wang, T. Li, R. Yu, H. Yu and J. Yu, J. Mater. Chem. A, 2016, 4, 8682–8689 CAS.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- Y. P. Zhang and C. X. Pan, J. Mater. Sci., 2011, 46, 2622–2626 CrossRef CAS.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 2392–2415 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska and L. Niu, Langmuir, 2009, 25, 12030–12033 CrossRef CAS PubMed.
- X. Y. Zhang, H. P. Li, X. L. Cui and Y. J. Lin, Mater. Chem., 2010, 20, 2801–2806 RSC.
- T. Xu, L. Zhang, H. Cheng and Y. Zhu, Appl. Catal., B, 2011, 101, 382–387 CrossRef CAS.
- Y. Zhang, Z. R. Tang, X. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS PubMed.
- Z. W. Xie, Z. Yu, W. F. Fan, G. C. Peng and M. Qu, RSC Adv., 2015, 5, 90041–90048 RSC.
- C. Huang, C. Li and G. Shi, Energy Environ. Sci., 2012, 5, 8848–8868 CAS.
- H. Yu, J. Tian, F. Chen, P. Wang and X. Wang, Sci. Rep., 2015, 5, 13083 CrossRef CAS PubMed.
- Y. Liu, RSC Adv., 2014, 4, 36040–36045 RSC.
- D. Chen, L. Zou, S. X. Li and F. Zheng, Sci. Rep., 2016, 6, 20335 CrossRef CAS PubMed.
- W. Fan, Q. Lai, Q. Zhang and Y. Wang, J. Phys. Chem. C, 2011, 115, 10694–10701 CAS.
- L. Wei, S. Chen, Y. Yang, Y. Dong, W. Song and R. Fan, RSC Adv., 2016, 6, 100866–100875 RSC.
- J. M. Hu, H. M. Meng, J. Q. Zhang and C. N. Cao, Corros. Sci., 2002, 44, 1655–1668 CrossRef CAS.
- M. K. Datta, K. Kadakia, O. I. Velikokhatnyi, P. H. Jampani, S. J. Chung, J. A. poston, A. Manivanan and P. N. Kumta, J. Mater. Chem. A, 2013, 1, 4026–4037 CAS.
- P. P. Patel, M. K. Datta, O. I. Velikokhatnyi, R. Kuruba, K. Damodaran, P. Jampani, B. Gattu, P. M. Shanthi, S. S. Damle and P. N. Kumta, Sci. Rep., 2016, 6, 28367 CrossRef CAS PubMed.
- B. B. Erick, C. C. Chen, Y. T. Hung, M. S. Al Ahmad and Y. P. Fu, Integr. Ferroelectr., 2016, 168, 1–9 CrossRef.
- A. Morais, C. Longo, J. R. Araujo, M. Barroso, J. R. Durrant and A. Flavia Nogueira, Phys. Chem. Chem. Phys., 2016, 18, 2608–2616 RSC.
- F. Chen, F. Yan, Q. Chen, Y. Wang, L. Han, Z. Chen and S. Fang, Dalton Trans., 2014, 43, 13537–13544 RSC.
- J. Qang, S. Dengm, Z. Liu and Z. Liu, Natl. Sci. Rev., 2015, 2, 22–39 CrossRef.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- M. A. Al-Mamun, Y. Kusumoto and M. Shariful Islam, J. Mater. Chem., 2012, 22, 5460–5469 RSC.
- Z. Xiong, L. L. Zhang, J. Ma and X. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC.
- S. Stankovich, D. A. Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- J. Zhang, X. Chen, Y. Shen, Y. Li, Z. Hu and J. Chu, Phys. Chem. Chem. Phys., 2011, 13, 13096–13105 RSC.
- J. Guo, Z. Gan, Z. Lu, J. Liu, J. Xi, Y. Wan, L. Le, H. Liu, J. Shi and R. Xiong, J. Appl. Phys., 2013, 114, 104903 CrossRef.
- C. Adan, A. Bahamonde, M. F. Fernandez and A. M. Arias, Appl. Catal., B, 2007, 72, 11–17 CrossRef CAS.
- J. Zhu, F. Chen, J. Zhang, H. Chen and M. Anpo, J. Photochem. Photobiol., A, 2006, 180, 196–204 CrossRef CAS.
- X. Fan, J. Fan, X. Hu, E. Liu, L. Kang, C. Tang, Y. Ma, H. Wu and Y. Li, Ceram. Int., 2014, 40, 15907–15917 CrossRef CAS.
- Y. Zhou, Y. Wang, X. O. Yang, L. Liu and W. Zhu, Semicond. Sci. Technol., 2017, 32, 035009 CrossRef.
- S. G. Babu, R. Vinoth, D. P. Kumar, M. V. Shankar, H. L. Chou, K. Vinodgopal and B. Neppolian, Nanoscale, 2015, 7, 7849–7857 RSC.
- Y. Lei, L. D. Zhang, G. W. Meng, G. H. Li, X. Y. Zhang, C. H. Liang, W. Chen and S. X. Wang, Appl. Phys. Lett., 2001, 78, 8 CrossRef.
- N. S. Alim, H. O. Lintang and L. Yuliati, Mater. Sci. Eng., 2016, 107, 012001 Search PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 95–107 CrossRef.
- H. Feng, R. Cheng, X. Zhao, X. Duan and J. Li, Nat. Commun., 2013, 4, 1539–1545 CrossRef PubMed.
- L. Xu, F. Zhang, X. Song, Z. Yin and Y. Bu, J. Mater. Chem. A, 2015, 3, 5923–5933 CAS.
- M. Zong, Y. Huang, Y. Zhao, X. Sun, C. Qu, D. Luo and J. Zheng, RSC Adv., 2013, 3, 23638–23648 RSC.
- Y. Qu and X. Duan, Chem. Soc. Rev., 2013, 42, 2568–2580 RSC.
- Z. Peng, S. Changlu, Z. Zhenyi, Z. Mingyi, M. Jingbo, G. Zengcai, S. Yangyang and L. Yichun, J. Mater. Chem., 2011, 21, 17746–17753 RSC.
- U. Sima, A. Shiju, T. Frank, P. Shobhit, C. Valerian, P. K. Shukla, J. R. Caroline, C. Sudip, A. Rajeev, P. Jurgen, D. Benjamin and S. Anchal, RSC Adv., 2014, 4, 59890–59901 RSC.
- X. Liu, Y. Su, Q. Zhao, C. Du and Z. Liu, Sci. Rep., 2016, 6, 28689 CrossRef CAS PubMed.
- W. Li, X. Liu and H. Li, J. Mater. Chem. A, 2015, 3, 15214–15224 CAS.
- X. L. Yang, W. Chen, J. F. Huang, Y. Zhou, Y. Zhu and C. Z. Li, Sci. Rep., 2015, 5, 10632 CrossRef PubMed.
- L. Xiao, L. Youji, C. Feitai, X. Peng and L. Ming, RSC Adv., 2017, 7, 25314–25324 RSC.
- D. Chena, K. Wanga, W. Honga, R. Zong, W. Yaob and Y. Zhu, Appl. Catal., B, 2015, 166–167, 366–373 CrossRef.
- K. S. Kadakia, P. Jampani, O. I. Velikokhatnyi, M. K. Datta, S. J. Chung, J. A. Poston, A. Manivannan and P. N. Kumta, J. Electrochem. Soc., 2014, 161, F868–F875 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13418e |
| This journal is © The Royal Society of Chemistry 2018 |