DOI:
10.1039/C7RA13133J
(Paper)
RSC Adv., 2018,
8, 1934-1939
Eco-friendly polyethylene glycol (PEG-400): a green reaction medium for one-pot, four-component synthesis of novel asymmetrical bis-spirooxindole derivatives at room temperature†
Received
7th December 2017
, Accepted 3rd January 2018
First published on 9th January 2018
Abstract
PEG-400 has been used as a green and biodegradable polymeric solvent for the one-pot, two-step, multi-component synthesis of novel asymmetrical bis-spirooxindole derivatives by the reaction of N-alkyl isatin, isatin derivatives, alkylmalonates and C–H activated carbonyl compounds in the presence of K2CO3 at room temperature. Using this procedure, all the products were obtained in good to excellent yields.
Introduction
In recent years, the development of a high efficiency, high selectivity, green, safe, atom- and step-economical synthesis strategy has become of paramount importance in the field of organic chemistry. One-pot, multi-component reactions are a powerful tool in organic and medicinal chemistry because they can shorten the reaction time, simplify the separation step, reduce costs, and give a relatively higher total chemical yield compared to multistep synthesis.1,2
Solvents are widely used in organic synthesis and have been a cause of major concern due to their associated environmental hazards. Therefore, replacement of harmful organic solvents with an eco-friendly medium is one of the major focal points of green chemistry.3–10 Polyethylene glycol (PEG) and modified polyethylene glycol derivatives have become more popular alternate reaction media, due to their interesting properties like non-toxicity, bio-compatibility, and bio-degradability. Moreover, PEG is considered as a natural, inexpensive, safe, recyclable, degradable, non-flammable, facile, environmentally benign and abundantly available green solvent.11
Spirooxindoles are commonly occurring heterocyclic ring systems and are found in many natural products and pharmaceuticals.12,13 These compounds are known to display anti-tubercular,14 antifungal,15 anti-mycobacterial,16 anti-tumor,17,18 anti-malarial19 and anti-microbial20 activities. Until recently, there have been few reports on the synthesis of symmetric bis-spirooxindole derivatives.21–24 In continuation of our research interest in the synthesis of biologically important heterocyclic compounds,25–33 herein we report a synthetic route for the preparation of novel asymmetrical bis-spirooxindoles 6 via a one-pot four-component condensation reaction of N-alkyl isatin 1 (1 eq.), isatin derivatives 2 (1 eq.), alkyl malonates 4 (2 eq.) and C–H activated carbonyl compounds 5 (2 eq.) in the presence of K2CO3 in polyethylene glycol medium at room temperature (Scheme 1).
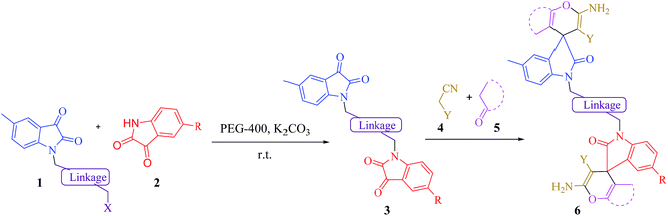 |
| | Scheme 1 The synthesis of novel asymmetrical bis-spirooxindoles 6 via the reaction between N-alkyl isatin 1, isatin derivatives 2, alkyl malonates 4 and carbonyl compounds 5 in the presence of K2CO3in PEG-400 at room temperature. | |
Results and discussion
At first, the N-alkyl isatins 1 were prepared from the reaction of 5-methyl isatin (7, 1 eq.) with dihalidederivatives (8, excess) in the presence of K2CO3 in PEG-400 at room temperature (Scheme 2).
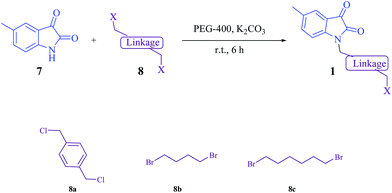 |
| | Scheme 2 Synthetic pathway for the synthesis of N-alkyl isatin derivatives. | |
Then, to optimize the reaction conditions for the synthesis of asymmetrical bis spirooxindole derivatives, the reaction of 1-(4-(chloromethyl)benzyl)-5-methylindoline-2,3-dione (1a), isatin (2a), malononitrile (4a) and 1,3-cyclohexanedione (5b) its behavior was studied in the presence of different catalytic systems, and the results are summarized in Table 1. As it is shown in Table 1, higher yield and shorter reaction time were obtained when the reaction was carried out in the presence of 1 mmol of K2CO3 in PEG-400 at room temperature (Table 1, entry 9).
Table 1 Effects of reagent and solvent on the reaction of 1-(4-(chloromethyl)benzyl)-5-methylindoline-2,3-dione, isatin, malononitrile and 1,3-cyclohexanedion under different conditionsa
| Entry |
Reagent |
reagent (mmol) |
Solvent |
Temp. (°C) |
Time (h) |
Yield (%) |
| Isolated yields. |
| 1 |
— |
— |
PEG-400 |
r.t. |
48 |
— |
| 2 |
L-Proline |
1.0 |
PEG-400 |
r.t. |
48 |
— |
| 3 |
DABCO |
1.0 |
PEG-400 |
r.t. |
48 |
— |
| 4 |
Et3N |
1.0 |
PEG-400 |
r.t. |
48 |
— |
| 5 |
Cs2CO3 |
1.0 |
PEG-400 |
r.t. |
24 |
65 |
| 6 |
Na2CO3 |
1.0 |
PEG-400 |
r.t. |
24 |
30 |
| 7 |
NaHCO3 |
1.0 |
PEG-400 |
r.t. |
24 |
— |
| 8 |
CaCO3 |
1.0 |
PEG-400 |
r.t. |
24 |
25 |
| 9 |
K2CO3 |
1.0 |
PEG-400 |
r.t. |
7 |
95 |
| 10 |
K2CO3 |
0.5 |
PEG-400 |
r.t. |
15 |
70 |
| 11 |
K2CO3 |
0.8 |
PEG-400 |
r.t. |
15 |
87 |
| 12 |
K2CO3 |
1.5 |
PEG-400 |
r.t. |
7 |
94 |
| 13 |
K2CO3 |
1.0 |
MeCN |
r.t. |
48 |
Trace |
| 14 |
K2CO3 |
1.0 |
H2O |
r.t. |
48 |
— |
| 15 |
K2CO3 |
1.0 |
EtOH |
r.t. |
48 |
— |
| 16 |
K2CO3 |
1.0 |
MeOH |
r.t. |
24 |
— |
| 17 |
K2CO3 |
1.0 |
n-Propanol |
r.t. |
24 |
— |
| 18 |
K2CO3 |
1.0 |
tert-Butanol |
r.t. |
24 |
— |
| 19 |
K2CO3 |
1.0 |
DMSO |
r.t. |
13 |
80 |
| 20 |
K2CO3 |
1.0 |
DMF |
r.t. |
15 |
80 |
| 21 |
K2CO3 |
1.0 |
— |
80 |
48 |
— |
| 22 |
K2CO3 |
1.0 |
— |
100 |
48 |
— |
Subsequently, the scope and efficiency of the reagent were explored under the optimized reaction conditions for the condensation of different asymmetrical bis-isatins 3 with a broad range of structurally diverse carbonyl compounds and alkyl malonatesto furnish the related products. The structural diversity of reactants is summarized in Fig. 1 and the results are displayed in Table 2.
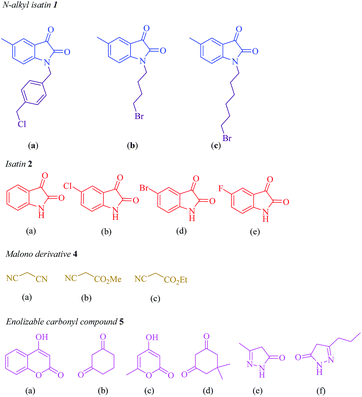 |
| | Fig. 1 Diversity elements employed for synthesis of asymmetrical bis-spirooxindoles. | |
Table 2 One-pot, four-component synthesis of asymmetrical bis-spirooxindole derivatives in the presence of K2CO3 in PEG-400 at room temperaturea
| Isolated yields. |
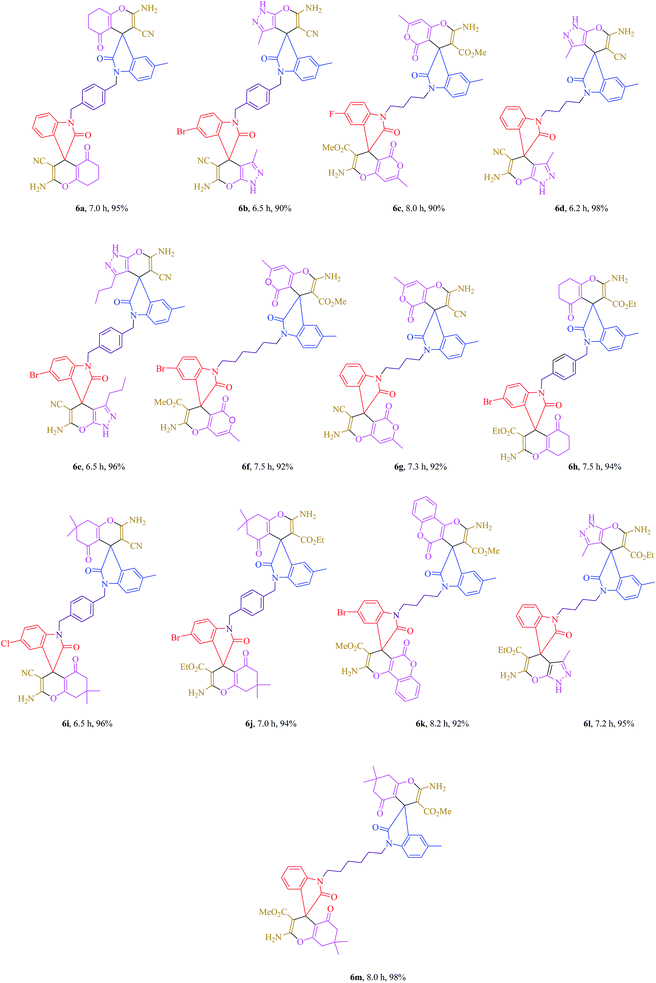 |
The synthetic pathway for the synthesis of titled compounds is consisting of two steps. At first, asymmetrical bis-isatin derivatives are obtained from the condensation reaction of N-alkyl isatin 1 and isatin derivatives 2. Then, the resulting products are treated with alkyl malonates 4 and carbonyl compounds 5 to afford the related asymmetrical bis-spirooxindole derivatives as the desired products.
As Table 2 indicates, a variety of isatin derivatives, alkyl malonates and carbonyl compounds were successfully applied in this process to afford the corresponding asymmetrical bis-spirooxindole derivatives as novel and potentially biologically important compounds in excellent yields.
Experimental
All chemicals were purchased from Merck or Fluka chemical companies. The 1H NMR (400 MHz) and 13C NMR (100 MHz) were run on a BrukerAvance400. Melting points were recorded on a Stuart Scientific Apparatus SMP3 (UK) in open capillary tubes. Elemental C, H and N analyses were performed using a Costech CHNS–O elemental analyzer.
General procedure for the synthesis of asymmetrical bisspirooxindolederivatives 6
K2CO3 (1 mmol) was added to a stirred mixture of N-alkylisatin 1 (1 mmol), isatin derivative 2 (1 mmol) in PEG-400 (3 mL) and the reaction mixture was stirred at room temperature to complete the formation of related asymmetrical bis-isatin 3 (monitored by TLC). Subsequently, alkyl malonates 4 (2 mmol) and cyclic ketone 5 (2 mmol) were added to this reaction mixture and reacted at room temperature for the appropriate amount of time (see Table 2). After completion of the reaction, 3 ml of water was added to the reaction mixture; the precipitate was filtrated and recrystallized from hot ethanol to afford the pure products.
2-Amino-1′-(4-((2-amino-3-cyano-5′-methyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indolin]-1′-yl)methyl)benzyl)-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile 6a. White powder, mp > 270 °C, 1H NMR (DMSO-d6,400 MHz) δ (ppm): 1.96 (t, J = 6.0 Hz, 4H), 2.23 (s, 3H), 2.25–2.31 (m, 4H), 2.70–2.73 (m, 4H), 4.88 (d, J = 10.0 Hz, 4H), 6.56 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 7.6 Hz, 1H), 6.93–6.99 (m, 3H), 7.12–7.16 (m, 3H), 7.34 (d, J = 8.8 Hz, 3H), 7.45 (s, 4H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 20.2, 21.1, 27.3, 31.2, 36.8, 43.6, 47.1, 47.2, 57.7, 57.9, 70.3, 109.1, 109.3, 112.2, 112.2, 117.9, 117.9, 123.0, 123.6, 124.2, 127.6, 128.7, 128.9, 131.9, 134.2, 134.2, 135.5, 135.6, 140.7, 143.0, 159.1, 159.2, 159.2, 166.8, 166.9, 177.2, 177.3, 195.6. Anal. calcd for C43H34N6O6: C, 70.67; H, 4.69; N, 11.50%. Found: C, 70.65; H, 4.72; N, 11.48%.
6′-Amino-1-(4-((6′-amino-5′-cyano-3′,5-dimethyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-1-yl)methyl)benzyl)-5-bromo-3′-methyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile 6b. Cream powder, mp > 270 °C, 1H NMR (DMSO-d6,400 MHz) δ (ppm): 0.98 (s, 3H), 1.05 (s, 3H), 2.35 (s, 3H), 4.71 (d, J = 15.6 Hz, 2H), 4.97 (d, J = 15.6 Hz, 2H), 6.65–6.69 (m, 2H), 6.85–7.06 (m, 6H), 7.42 (s, 1H), 7.59 (s, 4H), 7.97 (s, 1H), 12.43 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 21.1, 27.7, 28.0, 32.5, 43.6, 47.1, 50.4, 57.8, 109.2, 128.3, 130.3, 132.5, 133.3, 138.1, 139.0, 139.2, 140.1, 140.2, 142.0, 143.0, 146.3, 147.6, 148.3, 149.7, 149.9, 156.5, 157.1, 159.5, 171.2, 173.0, 173.8. Anal. calcd for C39H29BrN10O4: C, 59.93; H, 3.74; Br, 10.22; N, 17.92%. Found: C, 59.96; H, 3.72; N, 17.90%.
Methyl 2′-amino-1-(4-(2′-amino-3′-(methoxycarbonyl)-5,7′-dimethyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-1-yl)butyl)-5-fluoro-7′-methyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-3′-carboxylate 6c. Cream powder, mp > 270 °C, 1H NMR (DMSO-d6,400 MHz) δ (ppm): 1.49–1.51 (m, 2H), 1.64–1.72 (m, 2H), 2.21 (s, 3H), 2.98 (s, 3H), 3.02 (s, 3H), 3.23 (s, 3H), 3.37 (s, 3H), 3.55–3.64 (m, 2H), 3.71–3.74 (m, 2H), 6.32 (s, 2H), 6.80–6.93 (m, 2H), 6.99–7.00 (m, 1H), 7.11–7.19 (m, 2H), 8.03 (s, 2H), 8.08 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 27.5, 27.7, 28.0, 32.5, 43.5, 47.2, 50.3, 56.9, 110.0, 110.1, 117.7, 121.0, 121.5, 125.5, 127.7, 128.4, 129.3, 130.6, 137.9, 157.6, 159.6, 163.1, 163.9, 165.6, 167.9, 178.6, 195.7. Anal. calcd for C41H35FN4O12: C, 61.96; H, 4.44; F, 2.39; N, 7.05%. Found: C, 61.94; H, 4.42; N, 7.08%.
6′-Amino-1-(4-(6′-amino-5′-cyano-3′,5-dimethyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-1-yl)butyl)-3′-methyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile 6d. Pink powder, mp > 270 °C, 1H NMR (DMSO-d6,400 MHz) δ (ppm): 1.31–1.42 (m, 2H), 1.57–1.65 (m, 2H), 2.05 (s, 3H), 3.08 (s, 6H), 3.60–3.68 (m, 4H), 6.90–6.94 (m, 1H), 6.99 (d, J = 8.4 Hz, 2H), 7.42 (dd, J = 8.4, 2.0 Hz, 4H), 7.49–7.52 (m, 2H), 8.06 (s, 4H), 12.03 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.1, 14.4, 25.0, 44.5, 47.3, 59.3, 62.4, 121.2, 123.4, 125.4, 125.7, 127.0, 127.7, 127.9, 128.3, 130.3, 134.5, 134.7, 135.6, 139.3, 147.4, 152.6, 155.4, 157.5, 158.5, 159.08, 159.3. Anal. calcd for C35H30N10O4: C, 64.21; H, 4.62; N, 21.39%. Found: C, 64.22; H, 4.60; N, 21.41%.
6′-Amino-1-(4-((6′-amino-5′-cyano-5-methyl-2-oxo-3′-propyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-1-yl)methyl)benzyl)-5-bromo-2-oxo-3′-propyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile 6e. White powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.86–0.92 (m, 3H), 1.02–1.08 (m, 3H), 1.91–1.94 (m, 2H), 2.19–2.25 (m, 2H), 2.69 (s, 3H), 3.01–3.06 (m, 4H), 4.67–4.79 (m, 2H), 4.88–4.99 (m, 2H), 6.65–6.68 (m, 3H), 6.82–6.85 (m, 2H), 6.94 (d, J = 6.8 Hz, 1H), 7.02–7.06 (m, 4H), 7.59 (s, 2H), 7.91 (s, 2H), 12.21 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.1, 14.4, 25.0, 27.1, 28.3, 32.1, 45.0, 46.6, 51.1, 59.0, 62.4, 76.6, 113.9, 114.0, 119.6, 119.7, 124.9, 125.6, 126.1, 129.6, 130.7, 130.9, 133.9, 136.2, 136.2, 137.5, 137.6, 142.6, 144.9, 161.1, 161.2, 161.2, 168.8, 168.9, 179.2, 179.3. Anal. calcd for C43H37BrN10O4: C, 61.65; H, 4.45; Br, 9.54; N, 16.72%. Found: C, 61.67; H, 4.43; N, 16.71%.
Methyl 2′-amino-1-(6-(2′-amino-3′-(methoxycarbonyl)-5,7′-dimethyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-1-yl)hexyl)-5-bromo-7′-methyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-3′-carboxylate 6f. White powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 1.02–1.08 (m, 2H), 1.46–1.49 (m, 2H), 1.59–1.63 (m, 4H), 2.10 (s, 3H), 2.20 (s, 6H), 3.36 (s, 6H), 3.59–3.72 (m, 2H), 6.31 (s, 2H), 6.79 (d, J = 8.4 Hz, 3H), 6.97 (d, J = 7.2 Hz, 2H), 7.60 (d, J = 7.6 Hz, 1H), 8.02 (s, 4H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.0, 19.6, 21.0, 26.7, 27.4, 46.5, 50.9, 59.3, 76.0, 114.2, 125.0, 128.1, 129.3, 129.6, 130.4, 133.3, 135.4, 137.7, 137.8, 138.9, 139.9, 140.2, 142.5, 146.4, 146.7, 147.4, 149.1, 149.4, 159.5, 164.7, 167.4, 170.3, 171.1, 171.1, 174.6. Anal. calcd for C43H39BrN4O12: C, 58.44; H, 4.45; Br, 9.04; N, 6.34%. Found: C, 58.42; H, 4.44; N, 6.36%.
2′-Amino-1-(4-(2′-amino-3′-cyano-5,7′-dimethyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-1-yl)butyl)-7′-methyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[4,3-b]pyran]-3′-carbonitrile 6g. Cream powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 1.03–1.08 (m, 4H), 2.10 (s, 3H), 2.23 (s, 3H), 2.24 (s, 3H), 3.59–3.65 (m, 2H), 3.74–3.80 (m, 2H), 6.34 (s, 2H), 6.86–6.91 (m, 1H), 6.97–7.02 (m, 4H), 7.14–7.20 (m, 2H), 8.09 (s, 4H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.2, 27.2, 28.2, 32.1, 44.4, 46.7, 51.0, 59.1, 76.7, 113.3, 114.3, 114.5, 118.3, 120.2, 122.4, 122.7, 124.2, 124.8, 124.9, 125.3, 127.3, 131.1, 131.7, 132.6, 136.3, 144.3, 149.6, 152.4, 155.5, 155.5, 156.0, 156.3, 169.3. Anal. calcd for C39H30N6O8: C, 65.91; H, 4.25; N, 11.83%. Found: C, 65.89; H, 4.26; N, 11.85%.
Ethyl 2-amino-1′-(4-((2-amino-3-(ethoxycarbonyl)-5′-methyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indolin]-1′-yl)methyl)benzyl)-5′-bromo-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate 6h. Pink powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.38 (q, J = 7.2 Hz, 3H), 0.59 (q, J = 7.2 Hz, 3H), 1.92 (t, J = 6.0 Hz, 4H), 2.10 (s, 3H), 2.20–2.30 (m, 4H), 2.69 (t, J = 5.8 Hz, 4H), 3.41–3.49 (m, 2H), 3.76–3.83 (m, 2H), 4.69 (d, J = 16.0 Hz, 2H), 4.97 (dd, J = 15.8, 2.6 Hz, 2H), 6.64 (dd, J = 8.0, 2.4 Hz, 4H), 7.17 (d, J = 1.6 Hz, 1H), 7.25 (d, J = 8.4 Hz, 4H), 7.56 (s, 4H), 8.02 (s, 1H). Sample solubility was too low for 13C-NMR even after heating. Anal. calcd for C47H43BrN4O10: C, 62.46; H, 4.80; Br, 8.84; N, 6.20%. Found: C, 62.47; H, 4.78; N, 6.22%.
2-Amino-1′-(4-((2-amino-3-cyano-5′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indolin]-1′-yl)methyl)benzyl)-5′-chloro-7,7-dimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile 6i. Cream powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.57 (d, J = 2.4 Hz, 1H), 0.59 (d, J = 2.4 Hz, 1H), 0.99 (s, 3H), 1.04 (s, 3H), 1.23 (s, 3H), 1.25 (s, 3H), 2.90 (s, 2H), 4.66 (d, J = 16.0 Hz, 2H), 4.96 (d, J = 15.6 Hz, 2H), 6.52 (d, J = 7.6 Hz, 3H), 6.75 (s, 1H), 6.85 (d, J = 7.6 Hz, 4H), 7.57 (s, 4H), 7.95 (s, 1H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 10.4, 11.3, 20.2, 21.2, 27.3, 31.2, 36.7, 43.5, 47.1, 47.2, 57.7, 57.9, 70.3, 109.1, 109.3, 112.2, 112.3, 117.9, 117.9, 123.0, 123.6, 124.2, 127.5, 128.8, 128.9, 132.0, 134.2, 134.2, 135.4, 135.6, 140.6, 143.0, 159.2, 159.2, 159.2, 166.8, 166.9, 177.2, 177.3, 192.5, 192.6. Anal. calcd for C47H41ClN6O6: C, 68.73; H, 5.03; Cl, 4.32; N, 10.23%. Found: C, 68.74; H, 5.05; N, 10.21%.
Ethyl 2-amino-1′-(4-((2-amino-3-(ethoxycarbonyl)-5′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indolin]-1′-yl)methyl)benzyl)-5′-bromo-7,7-dimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate 6j. White powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.55 (t, J = 6.4 Hz, 6H), 0.98 (s, 6H), 1.05 (s, 6H), 2.04–2.11 (m, 2H), 2.21 (d, J = 16.0 Hz, 2H), 2.62–2.69 (m, 2H), 2.91 (s, 3H), 3.05 (d, J = 9.6 Hz, 2H), 3.72–3.78 (m, 4H), 4.71 (d, J = 16.4 Hz, 1H), 4.76 (d, J = 16.0 Hz, 1H), 4.90 (d, J = 15.6 Hz, 1H), 4.97 (d, J = 15.6 Hz, 1H), 6.68 (d, J = 3.6 Hz, 2H), 6.86 (d, J = 7.2.0 Hz, 4H), 6.94 (d, J = 6.0 Hz, 2H), 7.05 (s, 1H), 7.59 (s, 4H), 7.95 (d, J = 4.4 Hz, 1H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 13.9, 21.0, 26.2, 27.1, 31.2, 42.8, 46.8, 50.9, 51.1, 53.4, 62.4, 62.5, 76.9, 79.1, 122.9, 123.5, 124.1, 127.5, 128.7, 128.9, 132.0, 134.2, 134.2, 135.4, 135.6, 140.7, 143.0, 159.1, 159.2, 159.2, 166.8, 166.9, 177.2, 177.3, 195.7. Anal. calcd for C51H51BrN4O10: C, 63.82; H, 5.36; Br, 8.32; N, 5.84%. Found: C, 63.81; H, 5.38; N, 5.82%.
Methyl 2′-amino-1-(4-(2′-amino-3′-(methoxycarbonyl)-5-methyl-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[3,2-c]chromen]-1-yl)butyl)-5-bromo-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[3,2-c]chromene]-3′-carboxylate 6k. White powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.67–0.74 (m, 4H), 1.85 (s, 3H), 3.04 (s, 3H), 3.27 (s, 3H), 3.66–3.72 (m, 2H), 3.75–3.82 (m, 2H), 6.86–6.89 (m, 2H), 7.04–7.08 (m, 3H), 7.10 (d, J = 7.2 Hz, 2H), 7.19 (t, J = 7.0 Hz, 1H), 7.47 (d, J = 6.8 Hz, 1H), 7.55 (t, J = 7.6 Hz, 2H), 7.77 (t, J = 7.8 Hz, 2H), 8.07 (d, J = 8.0 Hz, 1H), 8.21 (s, 4H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.1, 20.1, 27.4, 44.3, 46.8, 50.9, 53.7, 76.7, 113.2, 125.7, 128.0, 129.3, 129.5, 130.6, 133.3, 135.4, 137.7, 137.7, 139.0, 140.9, 141.2, 143.7, 147.2, 147.8, 148.4, 149.1, 149.4, 159.4, 164.6, 167.4, 169.5, 170.1, 170.1, 173.4. Anal. calcd for C47H35BrN4O12: C, 60.85; H, 3.80; Br, 8.61; N, 6.04%. Found: C, 60.87; H, 3.82; N, 6.01%.
Ethyl 6′-amino-1-(4-(6′-amino-5′-(ethoxycarbonyl)-3′,5-dimethyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-1-yl)butyl)-3′-methyl-2-oxo-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate 6l. Cream powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.64–0.70 (m, 6H), 0.96 (s, 6H), 1.83 (s, 3H), 2.02–2.06 (m, 2H), 2.59–2.61 (m, 2H), 3.34–3.48 (m, 4H), 3.58–3.64 (m, 2H), 3.72–3.78 (m, 2H), 6.85 (td, J = 7.4, 2.4 Hz, 2H), 6.91–6.95 (m, 3H), 7.11 (d, J = 8.0 Hz, 1H), 7.14 (d, J = 7.6 Hz, 1H), 7.94 (s, 4H), 12.43 (s, 2H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.2, 27.1, 27.2, 28.2, 28.3, 31.2, 32.1, 44.3, 45.2, 46.7, 50.9, 59.1, 76.7, 101.6, 121.3, 124.2, 126.2, 130.7, 131.9, 133.0, 133.0, 135.7, 140.0, 140.4, 141.0, 141.3, 142.8, 152.5, 161.4, 164.2, 167.5, 167.5. Anal. calcd for C39H40N8O8: C, 62.56; H, 5.38; N, 14.96%. Found: C, 62.57; H, 5.40; N, 14.94%.
Methyl 2-amino-1′-(6-(2-amino-3-(methoxycarbonyl)-5′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indolin]-1′-yl)hexyl)-7,7-dimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate 6m. White powder, mp > 270 °C, 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 0.70 (s, 3H), 0.93 (s, 6H), 1.01 (s, 6H), 1.45–1.52 (m, 2H), 1.61–1.69 (m, 2H), 1.98 (d, J = 15.6 Hz, 2H), 2.11 (d, J = 15.6 Hz, 2H), 2.15 (d, J = 16.0 Hz, 4H), 2.91 (s, 6H), 3.10–3.21 (m, 4H), 3.62 (t, J = 7.0 Hz, 4H), 6.82–6.91 (m, 3H), 7.13 (t, J = 7.6 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.88 (s, 4H). 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 14.4, 24.8, 25.0, 27.1, 28.3, 32.1, 42.8, 44.6, 46.8, 50.9, 51.1, 53.4, 62.4, 123.0, 126.5, 128.6, 129.2, 134.3, 135.0, 135.4, 136.1, 136.3, 137.6, 138.9, 142.3, 143.3, 144.5, 145.6, 145.9, 152.5, 153.1, 155.5, 167.2, 169.2, 169.9, 202.5. Anal. calcd for C47H52N4O10: C, 67.77; H, 6.29; N, 6.73%. Found: C, 67.75; H, 6.31; N, 6.72%.
Conclusions
In conclusion, we have reported a highly efficient method for the synthesis of asymmetrical bis-spirooxindole derivatives via a one-pot, two-step, four-component condensation reaction using K2CO3 in PEG-400 as a green and biodegradable polymeric solvent at room temperature.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors thank Persian Gulf University Research Councils for the financial support of this work.
References
- M. Zhang, Q. Fu, G. Gao, H. He, Y. Zhang, Y. Wu and Z. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 6175–6182 CrossRef CAS.
- C. M. R. Volla, L. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390–2431 CrossRef CAS PubMed.
- S. Chandrasekhar, C. Narsihmulu, S. Shameem Sultana and N. Ramakrishna Reddy, Org. Lett., 2002, 4, 4399–4401 CrossRef CAS PubMed.
- Y. L. Gu and F. Jerome, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC.
- P. Liu, J. W. Hao, L. P. Mo and Z. H. Zhang, RSC Adv., 2015, 5, 48675–48704 RSC.
- Q. Zhang, K. D. O. De Oliveira Vigier, S. Royer and F. Jeró
![[m with combining circumflex]](https://www.rsc.org/images/entities/char_006d_0302.gif) e, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
e, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC. - M. B. Gawande and P. S. Branco, Green Chem., 2011, 13, 3355–3359 RSC.
- B. L. Li, P. H. Li, X. N. Fang, C. X. Li, J. L. Sun, L. P. Mo and Z. H. Zhang, Tetrahedron, 2013, 69, 7011–7018 CrossRef CAS.
- H. C. Hu, Y. H. Liu, B. L. Li, Z. S. Cui and Z. H. Zhang, RSC Adv., 2015, 5, 7720–7728 RSC.
- P. Liu, J. Hao and Z. Zhang, Chin. J. Chem., 2016, 34, 637–645 CrossRef CAS.
- M. V. Reddy, J. S. Kim, K. T. Lim and Y. T. Jeong, Tetrahedron Lett., 2014, 55, 6459–6462 CrossRef.
- C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed.
- K. Ding, Y. P. Lu and N. Coleska, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS PubMed.
- P. Prasanna, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 5653–5661 CrossRef CAS PubMed.
- A. Thangamani, Eur. J. Med. Chem., 2010, 45, 6120–6126 CrossRef CAS PubMed.
- S. U. Maheswari, K. Balamurugan, S. Perumal, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2010, 20, 7278–7282 CrossRef CAS PubMed.
- K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y.-S. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S.-M. Wang, J. Am. Chem. Soc., 2005, 127, 10130–10131 CrossRef CAS PubMed.
- K. Ding, Y.-P. Lu, Z. Nikolovska-Coleska, G.-P. Wang, S. Qiu, S. Shangary, W. Gao, D.-G. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S.-M. Wang, J. Med. Chem., 2006, 49, 3432–3435 CrossRef CAS PubMed.
- B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S.-H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, B. Reto, V. Dartois, T. T. Diagana and T. H. Keller, J. Med. Chem., 2010, 53, 5155–5164 CrossRef CAS PubMed.
- A. Nandakumar, P. Thirumurugan, P. T. Perumal, P. Vembu, M. N. Ponnuswamy and P. Ramesh, Bioorg. Med. Chem. Lett., 2010, 20, 4252–4258 CrossRef CAS PubMed.
- S. A. Padvi, Y. A. Tayade, Y. B. Wagh and D. S. Dalal, Chin. Chem. Lett., 2016, 27, 714–720 CrossRef CAS.
- A. R. Karimi, R. D. Abadi and Z. Dalirnasab, Res. Chem. Intermed., 2015, 41, 7427–7435 CrossRef CAS.
- G. Khanna, K. Aggarwal and J. M. Khurana, Synth. Commun., 2016, 46, 1880–1886 CrossRef CAS.
- E. Safari, A. Maryamabadi and A. Hasaninejad, RSC Adv., 2017, 7, 39502–39511 RSC.
- M. Beyrati and A. Hasaninejad, Tetrahedron Lett., 2017, 58, 1947–1951 CrossRef CAS.
- A. Maryamabadi, A. Hasaninejad, N. Nowrouzi and G. Mohebbi, Bioorg. Med. Chem., 2017, 25, 2057–2064 CrossRef CAS PubMed.
- M. Beyrati, M. Forutan, A. Hasaninejad, E. Rakovský, S. Babaei, A. Maryamabadi and G. Mohebbi, Tetrahedron, 2017, 73, 5144–5152 CrossRef CAS.
- A. Maryamabadi, A. Hasaninejad, N. Nowrouzi, G. Mohebbi and B. Asghari, Bioorg. Med. Chem., 2016, 24, 1408–1417 CrossRef CAS PubMed.
- M. Beyrati and A. Hasaninejad, Org. Prep. Proced. Int., 2016, 48, 393–400 CrossRef CAS.
- A. Hasaninejad, N. Golzar, M. Beyrati, A. Zare and M. M. Doroodmand, J. Mol. Catal. A: Chem., 2013, 372, 137–150 CrossRef CAS.
- A. Hasaninejad and S. Firoozi, Mol. Diversity, 2013, 17, 499–513 CrossRef CAS PubMed.
- A. Hasaninejad, A. Zare, M. Shekouhy and J. Ameri Rad, Green Chem., 2011, 13, 958–964 RSC.
- A. Hasaninejad, M. Shekouhy, A. Zare, S. M. S. Hoseini Ghattali and N. Golzar, J. Iran. Chem. Soc., 2011, 8, 411–423 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13133j |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article * and
Maryam Beyrati
* and
Maryam Beyrati

![[m with combining circumflex]](https://www.rsc.org/images/entities/char_006d_0302.gif) e, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
e, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.


