Open Access Article
Open Access ArticleNanospace-confined preparation of uniform nitrogen-doped graphene quantum dots for highly selective fluorescence dual-function determination of Fe3+ and ascorbic acid†
Hongbo Xu,
Shenghai Zhou *,
Jinyu Liu and
Yajun Wei
*,
Jinyu Liu and
Yajun Wei
College of Chemistry and Chemical Engineering, Hebei Normal University for Nationalities, Chengde 067000, China. E-mail: zhoush10@mails.jlu.edu.cn; Tel: +86-314-2370567
First published on 31st January 2018
Abstract
N-Doped graphene quantum dots (N-GQDs) combine the advantages of N-doped carbon and quantum dot materials, displaying enhanced performance in electrocatalysis, drug delivery, sensing and so on. In this work, novel hydrotropic N-GQDs with controlled size are obtained for the first time via a nanospace-confined preparation strategy, in which HNO3 vapour serves as scissors for quickly cutting the N-doped carbon nanolayer in the confined nanospace of reusable mesoporous molecular sieves. The as-prepared N-GQDs exhibit a uniform lateral size of about 2.4 nm, high photostability and yellow fluorescence, which is strongly quenched upon addition of ferric ions due to the coordination between ferric ions and N/O-rich groups of the N-GQDs surface. Significantly, the fluorescence response to Fe3+ is linear in the 0.5 to 40 μM concentration range and the N-GQDs showed good selectivity and satisfying recovery for ferric ion detection in tap water. Noteworthily, the quenched fluorescence by Fe3+ can be recovered by adding ascorbic acid (AA), which efficiently destroyed the coordination between Fe3+ and N-GQDs. Based on this principle, the N-GQDs were used to successfully construct an AA sensor, exhibiting a wide linearity range (between 0.5 and 90 μM) with a low detection of limit (80 nM at S/N = 3) and better selectivity towards AA compared with other common physiological substances. Finally, the constructed fluorescence sensor was employed successfully for AA determination in fish blood with satisfactory recovery ranging from 95.3 to 106.2%. The results indicate that N-GQDs synthesized by the nanospace-confined strategy are promising in biosensor fabrication.
1. Introduction
Graphene quantum dots (GQDs) are a new kind of thin carbon quantum dot material. The lateral size of a graphene sheet is less than 100 nm.1 The unique structure and composition of GQDs endows them with favourable properties including low toxicity, good biocompatibility, large surface area and high photostability.2 As a result, GQDs have become an important material for various applications such as anticancer therapy, biomedical imaging, environmental monitoring, photovoltaic and light-emitting devices, etc.3–6 To further expand the application field of GQDs, a powerful method is to fabricate heteroatom-doped GQDs through doping heteroatoms into the carbon skeleton with the aim of altering the electronic characteristics of GQDs.7,8 Recently, some significant work demonstrated successful preparation of N-doped graphene quantum dots (N-GQDs) with enhanced performances for some given applications.9,10 For instance, Qu's group demonstrated the optoelectronic properties of GQDs were greatly altered by nitrogen incorporation.9 The N-GQDs prepared by an electrochemical synthesis method in their study exhibited an enhanced electrocatalytic performance for the oxygen reduction reaction compared with N-free counterparts. In another study, Chen's group reported an efficient fluorescent N-GQD probe for low-cost, simple and selective determination of ferric ions (Fe3+) in lake water samples.10 They found that the in-plane doping of the carbon skeleton with nitrogen promoted the coordination between GQDs and ferric ions, thus leading to success in the fluorescence determination of ferric ions.Currently, the N-GQDs are prepared by two main methods, including “bottom-up” and “top-down” routes.11,12 The bottom-up approach refers to growing small molecular precursors to big N-GQDs by utilizing the high-pressure and high-temperature method, thermal pyrolysis, stepwise organic synthesis, etc.6,13,14 Although these methods can produce successfully N-GQDs with well-defined sizes and shapes, this strategy is still in its infancy and has limitations to overcome such as low water solubility, strong aggregation between N-GQDs, complex synthetic procedure and special organic precursor.6 The top-down approach are the cleavage of bulk carbon materials into nanosized N-GQDs via acidic oxidation, electrochemical exfoliation, hydrothermal/solvothermal treatment, and so on.8,9,15,16 Compared with the bottom-up strategy, this method shows outstanding advantages like simple operation, abundant precursors, high water solubility, and easy surface functionalization for N-GQDs.6 However, the faults of time-consuming cutting process, relatively expensive cutting devices, sophisticated separation process, and the difficulty in size-controlled production caused by non-selective “top-down” cutting are non-negligible.17 Therefore, it is significant to prepare the N-GQDs with controlled size and good water-solubility via a top-down route that has the facile and fast separation process as well as low cost.
Lately, the researchers have introduced the concept of nanoreactor to synthesize pristine GQDs in the confined nanospace of inorganic nanostructure materials. This ideal nanospace-confined synthesis strategy provides GQDs with controllable layer number and uniform lateral size.18,19 For instance, Lu's group synthesized the single-layered GQDs via hydrothermal carbonization of citrate guests in two-dimensional (2D) confined interlayer space of layered double hydroxides (LDHs) hosts.18 The success in the layer-controlled GQDs production is attributed to an obvious 2D nanospace confinement effect. Their work provides a real possibility to understand the intrinsic connection between the structure and property of GQDs, due to the controllable synthesis of GQDs. Besides, in our previous work, the size-controlled GQDs were obtained via using a HNO3 vapour as the acidic scissor to cleave the carbon nanolayer in the pore channel of mesoporous carbon/silica nanocomposite.19 The nanoscale pore of the carbon/silica material effectively limits the size of graphene quantum dots. More importantly, this nanoreactor-confined preparation for GQDs via top-down route not only endowed GQDs with good dispersibility, but also avoided time-consuming separation and purification processes. Inspired by the nanospace-confined synthesis strategy, the heteroatom-doped GQDs with controlled size and water-solubility may be obtained by using an appropriate nanoreactor and precursor. However, to the best of our knowledge, the controlled-synthesis of N-GQDs is not achieved via the above mentioned nanospace-confined synthesis strategy up to now. So, one of the purposes of the work is to develop a low-cost top-down method for the nanospace-confined preparation of N-GQDs with good water-solubility and controlled size.
Ferric ion, an inorganic metal ion, plays crucial role in the physiology of humans. Its abnormal amount will cause some serious diseases. For example, Fe3+ deficiency can result in anemia while Fe3+ with excess concentration in blood will cause kidney damage and hemochromatosis.20 On the other hand, as an important antioxidant in biological systems, ascorbic acid (AA) functions in some important biochemical reaction. For instance, AA can quickly react with free-radicals and thus effectively decrease free-radical damage that usually results in some serious diseases like Parkinson's disease and cancer.21 Hence, it is very essential to establish accurate and simple methods for the rapid determination of Fe3+ and AA. So far, various analytical methods for Fe3+ or AA have been reported, including fluorescence approach,22,23 colorimetric determination,24,25 electrochemical method26,27 and liquid chromatography,28 etc. Among the reported methods, fluorescent method provides obvious merits compared with other methods, because of its simplicity, convenience and sensitivity.29 As a result, various fluorescent sensing probes such as organic dyes, semiconductor quantum dots, metal nanoclusters, are utilized to selectively and sensitively determine ferric iron and AA. Compared with above mentioned fluorescent probes, graphene quantum dot has recently won more attention as a novel probe in analyte detection based on the following three obvious merits.11,22 Firstly, the accuracy and repeatability of determination results is ensured due to their excellent photostability. Secondly, the detection safety was guaranteed in view of their low toxicity and good biocompatibility. Thirdly, their good water-dispersibility also makes GQDs more feasible for real sample analysis.22 As a result, graphene quantum dots have a great potential in the sensitive determination of either Fe3+ or AA. However, the sensing applications of GQDs, especially N-GQDs, are still in their infancy. And very little work has been done for the dual-analyte determination of both Fe3+ and AA, especially using the N-GQDs with controlled size, good water-dispersibility and low-cost.
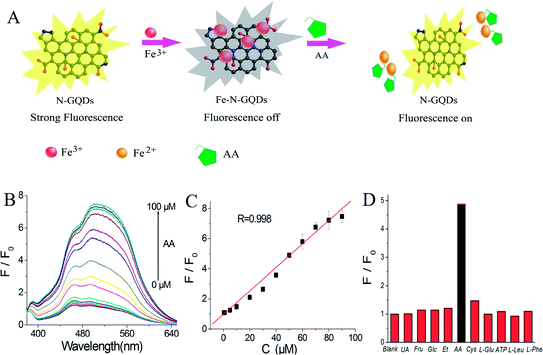
Herein, the nanospace-confined preparation of N-GQDs with controlled size was achieved for the first time via cutting N-containing carbon nanolayer with HNO3 vapour in the confined pore channel of renewable mesoporous silica molecular sieves. The as-prepared N-GQDs exhibit a uniform size, excellent water solubility and yellow fluorescence. Significantly, the fluorescence of N-GQDs is strongly and selectively quenched by ferric ion compared with environmentally relevant metal ions including Pb2+, Al3+, Hg2+, Cu2+, Ni2+, Co2+, Na+, K+, Fe2+, Cd2+, Ag+, Mn2+ and Zn2+, which may be due to the formation of the N-GQDs–Fe3+ coordination complex. Subsequently, the fluorescence of N-GQDs–Fe3+ system is selectively recovered via the addition of AA and increases linearly with its concentration. Based on the change of fluorescence signal, a sensitive and feasible analytical method is developed for the selective determination of both Fe3+ and AA in real samples with satisfactory results.
2. Experimental section
2.1 Chemicals and instrumentation
In this work, all purchased reagents did not be further purified for experimental use. And, all the aqueous solutions were finished with ultrapure water before experiments. The morphology and microstructure of the materials were well-characterized by scanning electron microscopy (SEM, JEOL JSM 6700-F, Japan), X-ray diffraction (XRD, Siemens D5005, Germany), atomic force microscopic (AFM, Bruker, Multimode 8, America), transmission electron microscopy (TEM, JEOL, JEM-3010, Japan), Fourier transform infrared spectra (FT-IR, Nicolet Magna 5PC, America) X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, America), Raman spectra (Renishaw, inVia, England) and nitrogen adsorption (BEL, Sorpmax, Japan). The optical property of N-GQDs was investigated by UV-vis spectra (Shimadzu, UV-1800, Japan) and fluorescence spectra (Hitachi, F-7000, and Japan).2.2 Preparation of mesoporous silica molecular sieve (SBA-15)
The mesoporous silica molecular sieve template was prepared according to a reported method.30 Briefly, 4 g surfactant (P123: EO20PO70EO20), 105 ml water, 20 ml hydrochloric acid (37%) and 8.5 g tetraethoxysilane were mixed to form a homogeneous solution. Subsequently, the homogeneous solution was heated in an autoclave at 110 °C for 24 h. The precipitates in the autoclave were filtered and cleaned with water and ethanol sequentially. And then the product was heated for the removal of P123 surfactant at 550 °C for 6 h. Finally, the white SBA-15 material with opened pore was obtained.2.3 Synthesis of mesoporous nitrogen/carbon/silica composite (N/C/SBA-15)
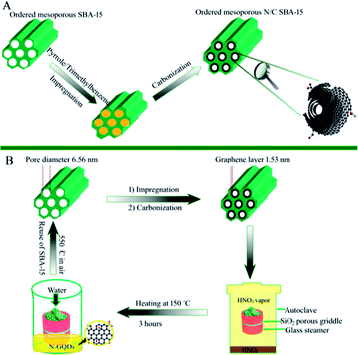
A key in the nanospace-confined preparation of N-GQDs is how to prepare ordered mesoporous N/C/SBA-15 composite containing both confined nanopore channel and N-doped graphene layer on the pore wall. Some significant works demonstrated the cladding of carbon layers on the pore wall of mesoporous silica molecular sieve resulted in the formation of carbon nanotube31 and mesoporous carbon with tube arrays.32 Inspired by those, the synthesis of N/C/SBA-15 composite with graphene layers and retained pore was achieved by the following processes, as shown in Fig. 1A. Briefly, sulphuric acid, pyrrole, ethanol and trimethylbenzene were stirred for the formation of a homogeneous solution in which the sulphuric acid and pyrrole were used as a catalyst and carbon nitrogen source, respectively. The calcined SBA-15 was then impregnated with the above solution at room temperature. The mixture was heated at 80 °C and then 160 °C each for 6 hours. Lastly, the resulting composite was carbonized at 800 °C for 2 h in nitrogen atmosphere, resulting in the formation of ordered mesoporous N/C/SBA-15 material.2.4 Nanospace-confined synthesis of N-GQDs
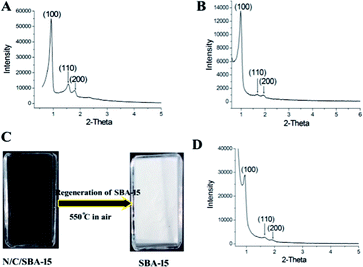
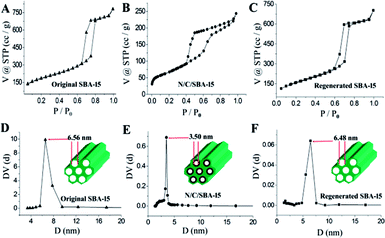
Herein, the size-controlled N-GQDs were prepared by selecting the mesoporous N/C/SBA-15 with graphene layer as raw materials via a facile and low-cost steaming procedure. As shown in Fig. 1B, 0.15 g of N/C/SBA-15 power was added into a self-designed glass steamer with a porous SiO2 griddle in its middle. And then 2 ml liquid concentrated HNO3 were added into an autoclave, aiming at creating the acid vapour scissors. Subsequently, the glass steamer was transferred to above autoclave and heated for HNO3 vapour cleaving at 150 °C for 3 h. After reaction, the steamer was moved into a clean beaker and filtered via ultrapure water. Due to the confined effect of nanosized pore in N/C/SBA-15 and the in situ filtration of porous SiO2 griddle, the N-GQDs with controlled size dispersed well in the above filtrate that was defined as N-GQDs solution. After the filtration via glass steamer, residual N/C/SBA-15 composite was transferred to a ceramic crucible and then calcined at 550 °C for the rebirth of the mesoporous silica template. The SBA-15 template possesses still highly ordered mesoporous structure after six cycles, which was demonstrated by the following results and discussions, providing a low-cost in nanospace-confined preparation. And the N-GQDs can be easily separated from liquid concentrated HNO3 via the self-designed glass steamer, avoiding the tedious separation process compared to traditional solution routes. In addition, the vapour cutting approach only required about 2 ml HNO3 for the N-GQDs preparation, reducing the acid consumption. And the liquid HNO3 in autoclave can be recyclable use because it only provides HNO3 vapour in the N-GQDs preparation process, which makes the current vapour cutting method relatively eco-friendly.3. Results and discussions
3.1 Structure characterization of mesoporous SBA-15 template and N/C/SBA-15 composite
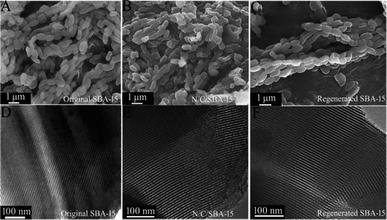 | ||
| Fig. 4 SEM (A) and TEM (D) images of original SBA-15, SEM (B) and TEM (E) images of N/C/SBA-15, SEM (C) and TEM (F) images of regenerated SBA-15. | ||
Reusability and recyclability of the mesoporous SBA-15 template are very important for the environment-friendly and low-cost preparation of N-GQDs. Besides above small-angle XRD and N2-adsorption characterizations for the regenerated SBA-15, scanning electron microscopy and transmission electron microscopy also were used for the structure investigation of regenerated SBA-15. From the SEM (Fig. 4C) and TEM (Fig. 4F) images, the regenerated SBA-15 still displays a rodlike morphology and an ordered mesopore arrangement, which agree very well with its small-angle XRD and adsorption result. And no distinguishable structural difference between original SBA-15 and generated SBA-15 demonstrate an exceptional recyclability of the parent SBA-15 template, thus endowing the unique top-down synthesis with low-cost and environmentally friendly.
3.2 Characterization of N-GQDs
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
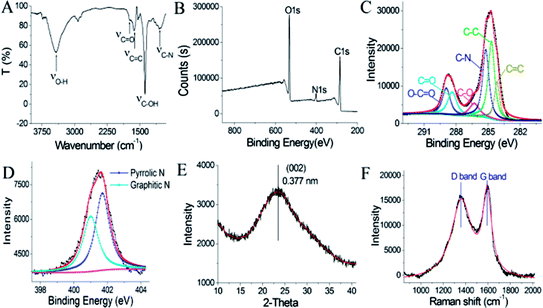
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in N-GQDs, respectively.37 The band at 3420 cm−1 is ascribed to the O–H bond, suggesting the presence of lots of hydroxyl groups on the N-GQDs surface.37 These O-rich groups lead to favourably hydrophilic property. And, their surface composition and elements were further confirmed by XPS characterization. Its full scan XPS spectrum (Fig. 6B) exhibits C1s (284 eV), N1s (401 eV) and O1s (532 eV) peak, indicating that nitrogen doping was effectively accomplished by top-down cutting method for graphene quantum dots.9 In detail, the nitrogen contents of N-GQDs are ca. 5.3 at% that approaches the previously published value.12 Also, C1s peak of N-GQDs (Fig. 6C) showed the six deconvoluted bands at 284.2 eV, 284.7 eV, 285.2 eV, 286.3 eV, 288.4 eV, 288.9 eV, which can be attributed to C
O in N-GQDs, respectively.37 The band at 3420 cm−1 is ascribed to the O–H bond, suggesting the presence of lots of hydroxyl groups on the N-GQDs surface.37 These O-rich groups lead to favourably hydrophilic property. And, their surface composition and elements were further confirmed by XPS characterization. Its full scan XPS spectrum (Fig. 6B) exhibits C1s (284 eV), N1s (401 eV) and O1s (532 eV) peak, indicating that nitrogen doping was effectively accomplished by top-down cutting method for graphene quantum dots.9 In detail, the nitrogen contents of N-GQDs are ca. 5.3 at% that approaches the previously published value.12 Also, C1s peak of N-GQDs (Fig. 6C) showed the six deconvoluted bands at 284.2 eV, 284.7 eV, 285.2 eV, 286.3 eV, 288.4 eV, 288.9 eV, which can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, C–N, C–O, C
C, C–C, C–N, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–C
O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristic peak, respectively.15 This assignment is well consistent with its the FT-IR result. Furthermore, N1s spectrum of the N-GQDs (Fig. 6D) exhibits the existence of N-containing groups like graphitic-N (∼401.7 eV) and pyrrolic-N (∼401 eV).9,38 The nitrogen/oxygen groups on the N-GQDs surface will also benefit a chelation of the N-GQDs with metal ions in aqueous solution, leading to the promising applications like fluorescence sensing of metallic ions, as discussed in the later part.
O characteristic peak, respectively.15 This assignment is well consistent with its the FT-IR result. Furthermore, N1s spectrum of the N-GQDs (Fig. 6D) exhibits the existence of N-containing groups like graphitic-N (∼401.7 eV) and pyrrolic-N (∼401 eV).9,38 The nitrogen/oxygen groups on the N-GQDs surface will also benefit a chelation of the N-GQDs with metal ions in aqueous solution, leading to the promising applications like fluorescence sensing of metallic ions, as discussed in the later part.
 | ||
| Fig. 6 FT-IR spectrum (A) and XPS survey scan (B) of N-GQDs. C1s (C) and N1s (D) XPS spectra of the N-GQDs. XRD (E) and Raman spectra (F) of N-GQDs. | ||
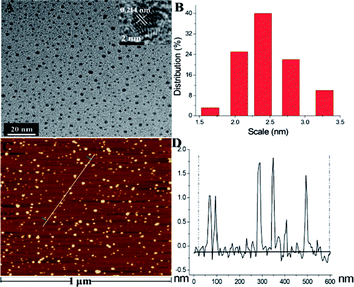
The structure of as-prepared N-GQDs was further investigated via X-ray diffraction and Raman spectroscopy. As shown in Fig. 6E, XRD pattern of the as-prepared N-GQDs displays a broad (002) diffraction band at 23.6° corresponded to a bilayer or few-layer graphene structure. The corresponding interlayer spacing is about 0.377 nm, which is 0.037 nm larger in comparison with 0.34 nm of graphite because of the existence of N/O-richgroups.39 Furthermore, the Raman spectrum of the N-GQDs (Fig. 6F) exhibits two obvious characteristic bands of carbon materials, namely G band at 1590 cm−1 and D band at 1359 cm−1, respectively.16 On the other hand, the intensity ratio ID/IG of 0.92 suggested the formation of defects and a concomitant introduction of nitrogen into in-plane of graphene.16 These investigations combined with FT-IR and XPS results indicated N-GQDs containing rich nitrogen/oxygen groups were successfully accomplished by this developed nanospace-confined strategy.
3.3 Optical properties of the N-GQDs
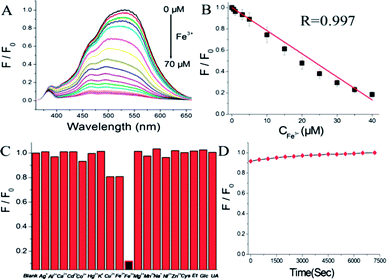
To examine the selectivity of the N-GQDs for Fe3+ detection, the environmentally relevant metal ions including Pb2+, Al3+, Ni2+, Hg2+, Cu2+, Co2+, Fe2+, Na+, K+, Cd2+, Ag+, Zn2+ and Mn2+ were examined under the same concentration of 50 μM. Besides, Ca2+ of 130 μM and Mg2+ of 370 μM were employed as interferential ions too, due to their high content in reality. Also, some biomolecules such as glucose (Glc, 4 mM), ethanol (Et, 10 mM), cysteine (Cys, 100 μM) and uric acid (UA, 100 μM) in the blood plasma41 were investigated for the interferential study (see Fig. 7C). It was found that the fluorescence intensity of N-GQDs decrease remarkably about 88% in the presence of 50 μM Fe3+ while other metal ions and those biomolecules exhibited negligible fluorescence fluctuations at normal physiological concentration. These experimental results suggested N-GQDs possess higher selectivity towards ferric ion in aqueous solution. Its high selectivity may be due to the specific coordination of ferric ions with N/O functional groups on the N-GQDs, which may form a Fe3+–N-GQDs complex, and thus result in a strong fluorescence quenching.10 Furthermore, the photostability of N-GQDs was investigated and exhibited in Fig. 7D. Significantly, fluorescence intensity of the N-GQDs increased merely 9% after a continuous irradiation of two hours with a Xe lamp (150 W), demonstrating a relatively high photostability of this quantum dot.
The analytical application of N-GQDs was carried out by employing the N-GQDs as fluorescence probes for the detection of ferric ion in tap water samples. The detection results were displayed in Table S1.† Significantly, the recoveries of three samples are 93.1–107.3% and corresponding RSD is 3.2–5.7%, demonstrating the applicability and reliability of Fe3+ detection by the N-GQDs fluorescent sensor.
Selectivity of fluorescence sensor is another important indicator for evaluating its sensing performance. Therefore, in this work, the influence of biologically relevant reactive species such as uric acid, fructose, glucose, ethanol, cysteine, L-glutamic acid (L-Glu), adenosine triphosphate (ATP), L-phenylalanine (L-Phe), L-Leucine (L-Leu) was studied at the same experimental condition. As indicated in Fig. 8D, these relevant interfering compounds did not cause any obvious interference, clearly demonstrating that the N-GQDs-based sensing system possesses high selectivity towards AA.
To indicate the reliability of this developed sensor in AA detection, this sensor was employed to detection of AA in blood plasma of fish. Table S2† exhibits a satisfactory recovery (95.3–106.2%) and a low RSD (1.5–4.8%), suggesting the reliability of this fluorescence sensor in ascorbic acid determination. Furthermore, a comparison of the performances of this N-GQDs sensor with other relevant sensors was presented in Table 1. It could be observed that this developed fluorescence sensor showed wider linear range than those of Au nanoclusters,46 CdS QDs,47 CQDs/Au nanoclusters nanohybrid48 fluorescence sensors and some colorimetry AA sensors.25,43 Meanwhile, it also exhibited lower detection limit compared with GQDs,2 CdTe QDs@silica nanobeads,45 silver nanoparticles23 fluorescence sensors and some electrochemistry AA sensors.27,44
| Method | Technique in detail | Detection limit (μM) | Linear range (μM) | Ref. |
|---|---|---|---|---|
| Colorimetry | Mesoporous silica-coated gold nanorods | 0.049 | 0.1–2.5 | 25 |
| Colorimetry | Fe3O4/nitrogen-doped carbon hybrid nanofibers | 0.04 | 0–50 | 43 |
| Electrochemistry | Iridium oxide nanofibers modified glassy carbon electrode | 0.4 | 1–1000 | 44 |
| Electrochemistry | Mesoporous silica/polyaniline/gold nanoparticles modified carbon paste electrode | 0.97 | 5–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
27 |
| Fluorescence | GQDs | 0.32 | 1.11–300 | 2 |
| Fluorescence | CdTe QDs@silica nanobeads | 1.25 | 3.33–400 | 45 |
| Fluorescence | Au nanoclusters | 0.022 | 0.1–10 | 46 |
| Fluorescence | Silver nanoparticles | 0.1 | 4.1–100 | 23 |
| Fluorescence | CdS QDs | 0.002 | 0.06–0.3 | 47 |
| Fluorescence | CQDs/Au nanoclusters nanohybrid | 0.105 | 0.15–15 | 48 |
| Fluorescence | N-GQDs | 0.08 | 0.5–90 | This work |
4. Conclusions
To sum up, a novel N-GQD with controlled size have been firstly achieved by steaming N-doped graphene layers in the confined nanospace of ordered mesoporous SBA-15 with an acid vapour. The present nanospace-confined synthesis of N-GQDs is low-cost and eco-friendly based on the reusability of SBA-15 template and liquid HNO3 as well as avoidable time-consuming separation in terms of a self-designed glass steamer. The obtained N-GQDs are well-dispersed and exhibit yellow coloured fluorescence. And their fluorescence could be selectively quenched by ferric ion and subsequently restored with the addition of AA, exhibiting an on–off–on fluorescence response. As an application example, the as-prepared N-GQDs with high photostability and low toxicity were employed as fluorescence probes to determine both Fe3+ and AA with important biological role, exhibiting high selectivity, wide linear range and satisfactory recovery. Furthermore, the application of the N-doped graphene quantum dots with controllable preparation, low-cost and facile separation may be broadened in anticancer therapy, bioimaging, energy storage, drug delivery and so on.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the project of Hebei Province Natural and Scientific Foundation (B2017101022, B2017101018), the project of Chengde Science and Technology Bureau (201606A070, 201606A069), and the young foundation project of Hebei Province Educational Bureau (QN2017408).Notes and references
- Z. P. Zhang, J. Zhang, N. Chen and L. T. Qu, Energy Environ. Sci., 2012, 5, 8869–8890 CAS.
- H. Liu, W. D. Na, Z. P. Liu, X. Q. Chen and X. G. Su, Biosens. Bioelectron., 2017, 92, 229–233 CrossRef CAS PubMed.
- D. Iannazzo, I. Ziccarelli and A. Pistone, J. Mater. Chem. B, 2017, 5, 6471–6489 RSC.
- C. Cheng, S. Li, A. Thomas, N. A. Kotov and R. Haag, Chem. Rev., 2017, 117, 1826–1914 CrossRef CAS PubMed.
- S. Benitez-Martinez and M. Valcarcel, TrAC, Trends Anal. Chem., 2015, 72, 93–113 CrossRef CAS.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S. J. Jeon, T. W. Kang, J. M. Ju, M. J. Kim, J. H. Park, F. Raza, J. Han, H. R. Lee and J. H. Kim, Adv. Funct. Mater., 2016, 26, 8211–8219 CrossRef CAS.
- B. J. Moon, D. Jang, Y. Yi, H. Lee, S. J. Kim, Y. Oh, S. H. Lee, M. Park, S. Lee and S. Bae, Nano Energy, 2017, 34, 36–46 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS PubMed.
- J. Ju and W. Chen, Biosens. Bioelectron., 2014, 58, 219–225 CrossRef CAS PubMed.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- C. Zhu, S. Yang, G. Wang, R. Mo, P. He, J. Sun, Z. Di, N. Yuan, J. Ding, G. Ding and X. Xie, J. Mater. Chem. C, 2015, 3, 8810–8816 RSC.
- Y. Y. Yin, Q. Liu, D. Jiang, X. J. Du, J. Qian, H. P. Mao and K. Wang, Carbon, 2016, 96, 1157–1165 CrossRef CAS.
- Q. Li, S. Zhang, L. Dai and L. S. Li, J. Am. Chem. Soc., 2012, 134, 18932–18935 CrossRef CAS PubMed.
- C. Hu, Y. Liu, Y. Yang, J. Cui, Z. Huang, Y. Wang, L. Yang, H. Wang, Y. Xiao and J. H. Rong, J. Mater. Chem. B, 2013, 1, 39–42 RSC.
- Y. Liu and P. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 3362–3369 CAS.
- M. Bacon, S. J. Bradley and T. Nann, Part. Part. Syst. Charact., 2014, 31, 415–428 CrossRef CAS.
- L. Q. Song, J. J. Shi, J. Lu and C. Lu, Chem. Sci., 2015, 6, 4846–4850 RSC.
- H. B. Xu, S. H. Zhou, L. L. Xiao, S. Z. Li, T. Song, Y. Wang and Q. H. Yuan, Carbon, 2015, 87, 215–225 CrossRef CAS.
- S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195–7227 RSC.
- J. S. A. Devi, S. Salini, A. H. Anulekshmi, G. L. Praveen and G. Sony, Sens. Actuators, B, 2017, 246, 943–951 CrossRef.
- S. H. Zhou, H. B. Xu, W. Gan and Q. H. Yuan, RSC Adv., 2016, 6, 110775–110788 RSC.
- H. W. Park, S. M. Alam, S. H. Lee, M. M. Karim, S. M. Wabaidur, M. Kang and J. H. Choi, Luminescence, 2009, 24, 367–371 CAS.
- J. J. Li, X. F. Wang, D. Q. Huo, C. J. Hou, H. B. Fa, M. Yang and L. Zhang, Sens. Actuators, B, 2017, 242, 1265–1271 CrossRef CAS.
- G. Q. Wang, Z. P. Chen and L. X. Chen, Nanoscale, 2011, 3, 1756–1759 RSC.
- H. Obata and C. M. G. van den Berg, Anal. Chem., 2001, 73, 2522–2528 CrossRef CAS PubMed.
- S. C. Hsu, H. T. Cheng, P. X. Wu, C. J. Weng, K. S. Santiago and J. M. Yeh, Electrochim. Acta, 2017, 238, 246–256 CrossRef CAS.
- M. G. Gioia, P. Andreatta, S. Boschetti and R. Gatti, J. Pharm. Biomed. Anal., 2008, 48, 331–339 CrossRef CAS PubMed.
- L. L. Feng, Y. X. Wu, D. L. Zhang, X. X. Hu, J. Zhang, P. Wang, Z. L. Song, X. B. Zhang and W. H. Tan, Anal. Chem., 2017, 89, 4077–4084 CrossRef CAS PubMed.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- S. S. Kim, D. K. Lee, J. Shah and T. J. Pinnavaia, Chem. Commun., 2003, 12, 1436–1437 RSC.
- A. H. Lu, W. C. Li, W. Schmidt, W. Kiefer and F. Schuth, Carbon, 2004, 42, 2939–2948 CrossRef CAS.
- S. Jarnbhrunkar, M. H. Yu, J. Yang, J. Zhang, A. Shrotri, L. Endo-Munoz, J. Moreau, G. Q. Lu and C. Z. Yu, J. Am. Chem. Soc., 2013, 135, 8444–8447 CrossRef PubMed.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Q. Shi, L. E. Deng, Y. B. Hou and L. T. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- Y. Dong, H. Pang, S. Ren, C. Chen, Y. Chi and T. Yu, Carbon, 2013, 64, 245–251 CrossRef CAS.
- P. Guo, F. Xiao, Q. Liu, H. Liu, Y. Guo, J. R. Gong, S. Wang and Y. Liu, Sci. Rep., 2013, 3, 3499 CrossRef PubMed.
- L. B. Tang, R. B. Ji, X. M. Li, K. S. Teng and S. P. Lau, J. Mater. Chem. C, 2013, 1, 4908–4915 RSC.
- Y. Dong, C. Chen, X. Zheng, L. Gao, Z. Cui, H. Yang, C. Guo, Y. Chi and C. M. Li, J. Mater. Chem., 2012, 22, 8764–8766 RSC.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS PubMed.
- W. Wang, L. L. Zhang, S. F. Tong, X. Li and W. B. Song, Biosens. Bioelectron., 2009, 25, 708–714 CrossRef CAS PubMed.
- J. J. Liu, Z. T. Chen, D. S. Tang, Y. B. Wang, L. T. Kang and J. N. Yao, Sens. Actuators, B, 2015, 212, 214–219 CrossRef CAS.
- Y. Z. Jiang, N. Song, C. Wang, N. Pinna and X. F. Lu, J. Mater. Chem. B, 2017, 5, 5499–5505 RSC.
- S. J. Kim, Y. L. Kim, A. Yu, J. Lee, S. C. Lee, C. Lee, M. H. Kim and Y. Lee, Sens. Actuators, B, 2014, 196, 480–488 CrossRef CAS.
- Q. Ma, Y. Li, Z. H. Lin, G. C. Tang and X. G. Su, Nanoscale, 2013, 5, 9726–9731 RSC.
- H. J. Meng, D. Q. Yang, Y. F. Tu and J. L. Yan, Talanta, 2017, 165, 346–350 CrossRef CAS PubMed.
- M. Ganiga and J. Cyriac, Anal. Bioanal. Chem., 2016, 408, 3699–3706 CrossRef CAS PubMed.
- W. J. Niu, D. Shan, R. H. Zhu, S. Y. Deng, S. Cosnier and X. J. Zhang, Carbon, 2016, 96, 1034–1042 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis absorption spectrum of the N-GQDs dispersed in water and photograph of the N-GQDs solutions taken under illumination of UV light (Fig. S1a); fluorescence emission spectra of the N-GQDs aqueous solution at different excitation wavelengths from 300 to 400 nm (Fig. S1b); determination of Fe3+ in tap water samples (Table S1); determination of AA in fish blood samples (Table S2). See DOI: 10.1039/c7ra13001e |
| This journal is © The Royal Society of Chemistry 2018 |