Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal-free oxidative isocyanides insertion with aromatic aldehydes to aroylated N-heterocycles†
Yimiao He *,
Xuelian Wang,
Jun-An Xiao,
Jinying Pang,
Chunfang Gan,
Yanmin Huang and
Chusheng Huang*
*,
Xuelian Wang,
Jun-An Xiao,
Jinying Pang,
Chunfang Gan,
Yanmin Huang and
Chusheng Huang*
College of Chemistry and Materials Science, Guangxi Teachers Education University, Nanning 530001, P. R. China. E-mail: heyimiao@gxtc.edu.cn; huangcs@gxtc.edu.cn
First published on 15th January 2018
Abstract
A metal-free, mild and efficient protocol for the construction of aroylated N-heterocycles via radical cascade aroylation of phenyl or vinyl isocyanides with aromatic aldehydes has been developed. Both phenanthridine and isoquinoline derivatives are delivered quickly in moderate to good yields with high regioselectivity.
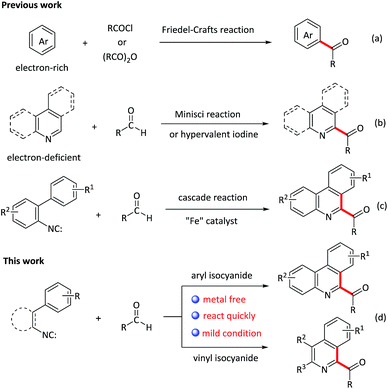
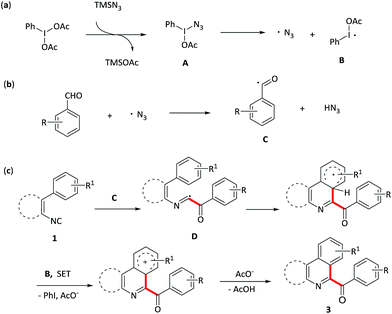
Acylation reactions are a powerful tool for the synthesis of carbonyl compounds, which are abundant in natural products, pharmaceuticals, and dye and agrichemical industries.1 Traditional acylation is Friedel–Crafts reaction (scheme 1a),2 which proceeds by reacting arenes with acyl halides in the presence of Lewis or Brønsted acids. However, this methodology also suffers from poor regioselectivity, the use of stoichiometric amounts of reactive acyl halide reagents as well as electron-rich arenes, which restrict its extensive application in organic synthesis. Recently, great progresses have been made for C–C bond formations directly from two different C–H bonds under oxidative conditions that is termed the cross dehydrogenative coupling (CDC reaction),3,4 representing the state-of-art in C–C bond-formation reactions. Such a coupling allows the use of simple nonfunctionalized substrates, thus making the synthesis shorter and more efficient. However, in contrast to the acylation of electron-rich aromatic compounds, very few methods are available for the acylation of electron-deficient heterocycles. Among these methods, the addition of nucleophilic acyl radicals to electron-deficient heterocyclic aromatic bases, that is, the Minisci reaction, is a commonly used approach (scheme 1b).5,6 Very recently, Antonchick et al. described a PhI(OCOCF3)2-mediated cross-dehydrogenative coupling of heterocycles with aldehydes to construct acylated N-heterocycles (scheme 1b).7 Compared with these previous works, the development of novel tandem reaction would be a better choice, as it allows to construct a series of acylated nitrogen-containing heterocycles in one pot.
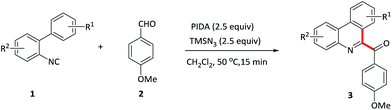
Isocyanides are the versatile building blocks in organic synthesis due to their special structural and reactive properties, and have been widely applied in the formation of N-heterocycles.8 Along with transition-metal catalyzed isocyanides insertion, isocyanides are used as radical receptors with subsequent homolytic aromatic substitution to construct substituted N-heterocycles recently have also been realized.9 For example, Studer and co-workers reported an efficient synthesis of 6-aroylated phenanthridines through base promoted homolytic aromatic substitution of 2-isocyanobiphenyls with aromatic aldehydes using FeCl3 as an initiator.10 However, this method also suffers from transition metal residues, harsh conditions and long time. Moreover, compared to the phenyl isocyanides, the addition of acyl radicals derived from aldehydes to vinyl isocyanides11 and finally receive 1-acylated isoquinolines, to our knowledge, has not been realised. Considering the importance of both N-heterocycles and acyl groups, herein, we elaborate a quick oxidative isocyanide insertion reaction with aromatic aldehydes through PIDA/TMSN3-mediated C–C bond formation, delivering an efficient approach to construction of 6-aroylated phenanthridine and 1-aroylated isoquinoline derivatives in one pot (Scheme 1d).
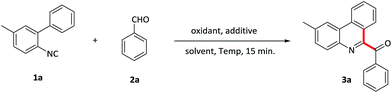
Initially, the reaction was carried out by using 2-isocyanobiphenyl 1a and benzaldehyde 2a as model substrates in benzene with PIFA as the oxidant and TMSN3 as the additive at room temperature (Table 1, entry 1). Pleasingly, the reaction of 1a with 4 equiv. of 2a in the presence of 2 equiv. of PIFA with TMSN3 to give the desired 3a in 23% yield. After screening various solvents, CH2Cl2 was found to be the most efficient and provided 3a in 27% yield (entries 2–4). Furthermore, replacing the additive TMSN3 with NaN3 didn't improve the reaction (entry 5). Subsequently, various oxidants were also evaluated and PIDA gave a better result, providing 3a in 31% yield (entries 6–8). Importantly, a further improvement was achieved upon elevating the temperature to 50 °C (entry 9). However, further increasing the temperature resulted in the yield reduction, maybe due to the aldehyde being oxidized to carboxylate under harsh conditions. We also investigated the relative ratio of reactants (entries 10–13), and found that the addition of the amounts of aldehydes to 6 equivalent amounts along with oxidants and additives to 2.5 equivalent amounts led to the optimum result and gave 3a in 74% yield (entry 12). The blank experiments demonstrated that both oxidants and additives were essential for the isocyanide insertion reactions (entries 14, 15). Consequently, the optimum reaction conditions were determined to be 2-isocyanobiphenyl 1a with aldehyde 2a (6 equiv.) in the presence of PIDA as well as TMSN3 (2.5 equiv.) at 50 °C under air condition. It was noteworthy that the reaction proceeded without any metal catalysts in only 15 minutes.
| Entry | Solvent | Oxidant | Additive | T [°C] | Yieldb [%] |
|---|---|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2a (0.8 mmol), oxidant (0.4 mmol), additive (0.4 mmol) in solvent (2 mL) for 15 min. PIDA = phenyliodine(III) diacetate, PIFA = phenyliodine(III) bis(tri-fluoroacetate).b Isolated yields.c Oxidant (0.5 mmol), additive (0.5 mmol).d 2a (1.0 mmol).e 2a (1.2 mmol).f 2a (1.4 mmol). | |||||
| 1 | Benzene | PIFA | TMSN3 | 25 | 23 |
| 2 | CH2Cl2 | PIFA | TMSN3 | 25 | 27 |
| 3 | DCE | PIFA | TMSN3 | 25 | 22 |
| 4 | CH3CN | PIFA | TMSN3 | 25 | n.d. |
| 5 | CH2Cl2 | PIFA | NaN3 | 25 | 25 |
| 6 | CH2Cl2 | PIDA | TMSN3 | 25 | 31 |
| 7 | CH2Cl2 | PhI(OPiv)2 | TMSN3 | 25 | n.d. |
| 8 | CH2Cl2 | TBHP | TMSN3 | 25 | n.d. |
| 9 | CH2Cl2 | PIDA | TMSN3 | 50 | 47 |
| 10c | CH2Cl2 | PIDA | TMSN3 | 50 | 52 |
| 11c,d | CH2Cl2 | PIDA | TMSN3 | 50 | 61 |
| 12c,e | CH2Cl2 | PIDA | TMSN3 | 50 | 74 |
| 13c,f | CH2Cl2 | PIDA | TMSN3 | 50 | 71 |
| 14e | CH2Cl2 | None | TMSN3 | 50 | n.d. |
| 15e | CH2Cl2 | PIDA | None | 50 | n.d. |
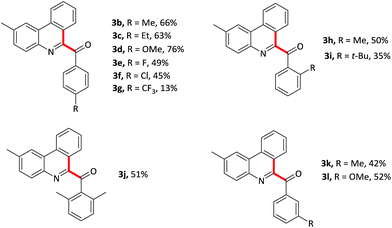
With optimized conditions in hand, various benzaldehydes 2a–l were first tested to react with 2-isocyanobiaryl 1a to form the corresponding 6-aroyl phenanthridine products 3a–l. As showed in Table 2, benzaldehydes bearing electron-rich groups (2b–d) such as methyl, ethyl and methoxy at the para-position underwent this oxidative isocyanide insertion process smoothly to afford the desired products in moderate to good yields (63–76%). Weakly electron-withdrawing groups, for example, fluoro (2e) and chloro (2f) substituents, gave lower yield. When the benzaldehyde moiety was substituted by strongly electron-withdrawing group such as CF3 (2g) at the para-position, the yield was sharply decreased and only 13% yield of 3g was obtained (2g was recovered in 82% yield). These results indicated that electronic effects exerted by the substituents on the arene ring of the benzaldehyde component had an important influence on this reaction. Moreover, lower yields were achieved for the ortho-substituents maybe due to the steric effect (3h–j). In addition, meta-substituted groups also gave the corresponding products 3k and 3l in moderate yield.
| a Reaction conditions: 1a (0.2 mmol), 2 (1.2 mmol), PIDA (0.5 mmol), TMSN3 (0.5 mmol) in CH2Cl2 (2 mL) at 50 °C for 15 min. |
|---|
 |
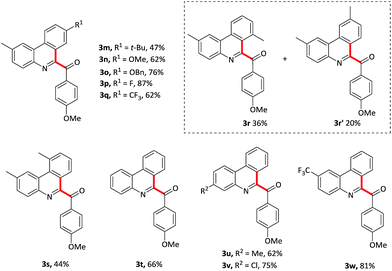
The isocyanobiaryl moiety was subsequently evaluated (Table 3). Various substituents on the arene ring without the isocyanide group at the 4-position were tolerant well, affording the desired products in moderate to good yield. For example, electron-donating groups such as t-Bu, OMe, OBn gave the product 3m–o in 47–76% yield. Electron-withdrawing F, CF3 substituents also formed the product 3p and 3q in 87% and 62% yield, respectively. To explore the regioselectivity, 3-methylisocyanidebiphenyl 1r was employed as substrate to proceed this oxidative isocyanide insertion process, and gave a mixture of two isomers 3r and 3r′, the ratio of which was 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Ortho-methyl derivative 1s gave the targeted product 3s in a slight lower yield maybe owing to the steric effect. In addition, the substituent effects on the aromatic ring attached to isocyanide moiety were also been explored. Not only the electron-rich group such as methyl substituent but also the electron-withdrawing groups such as chloro and trifluoromethyl substituents were compatible with this oxidative cyclization process, and formed the products 3u–w in 62–81% yield. Importantly, halogen substituent could be used as a coupling partner for further transformation.
1. Ortho-methyl derivative 1s gave the targeted product 3s in a slight lower yield maybe owing to the steric effect. In addition, the substituent effects on the aromatic ring attached to isocyanide moiety were also been explored. Not only the electron-rich group such as methyl substituent but also the electron-withdrawing groups such as chloro and trifluoromethyl substituents were compatible with this oxidative cyclization process, and formed the products 3u–w in 62–81% yield. Importantly, halogen substituent could be used as a coupling partner for further transformation.
| a Reaction conditions: 1 (0.2 mmol), 2 (1.2 mmol), PIDA (0.5 mmol), TMSN3 (0.5 mmol) in CH2Cl2 (2 mL) at 50 °C for 15 min. |
|---|
 |
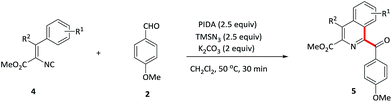
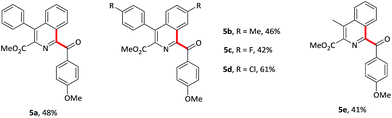
Vinyl isocyanides were also explored. Methyl-4-aryl-1-aroyl-isoquiniline-3-carboxylates 5a–d were prepared in moderate yields starting from the corresponding vinyl isocyanides and 4-methoxybenzaldehyde 2. Furthermore, 4-methyl substituent isoquinoline 5e was also formed in a slightly lower yield (41%).
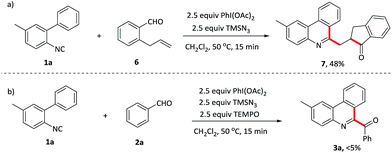
To investigate the mechanism for this oxidative isocyanide insertion reaction, several control experiments were conducted. Benzaldehyde 6 containing ortho terminal alkene was first reacted with 1a under the standard conditions (Scheme 2a). Phenanthridine derivatives 7, attached by 2,3-dihydro-1H-inden-1-one with a methylene linkage, was formed through acyl radical addition to the intramolecular C–C double bond, followed by the reaction with isocyanide 1a. It provided a novel and efficient approach for the synthesis of multi heterocyclic systems bearing a phenanthridine moiety. Furthermore, when TEMPO, a radical scavenge, was added into the system, the reaction was suppressed and the starting material 1a was recovered in 90% yield (Scheme 2b). These results indicated that the reaction probably involved a radical process (Table 4).
| a Reaction conditions: 1 (0.2 mmol), 2 (1.2 mmol), PIDA (0.5 mmol), TMSN3 (0.5 mmol) and K2CO3 (0.4 mmol) in CH2Cl2 (2 mL) at 50 °C for 30 min. |
|---|
 |
Based on the above results and previous reports,7 a plausible mechanism pathway for PhI(OAc)2/N3-mediated synthesis of aroylated nitrogen-containing aromatic heterocycles starting from aryl or vinyl isocyanides with aromatic aldehydes is depicted in Scheme 3. Initial ligand exchange between PhI(OAc)2 and TMSN3 provides hypervalent iodide intermediate A, which next undergoes a thermal homolytic cleavage to generate an azide radical and radical B. Then, the azide radical abstracts an H atom from the aldehyde to release the acyl radical C, which attacks the isocyanide 1 to form the imidoyl radical D. Radical D subsequently experiences a homolytic aromatic substituent followed by an oxidative aromatization process and finally afford the targeted aroylated nitrogen-containing aromatic heterocycles 3.
In summary, we have developed a mild and efficient protocol for the construction of aroylated azaheterocyclic arenes via oxidative isocyanide insertion of isocyanides with benzaldehydes under metal-free conditions. Diversified phenanthridine and isoquinoline derivatives are assembled quickly in moderate to good yields with high regioselectivity. Interestingly, benzaldehyde bearing ortho-allyl substituent can also be applied in this reaction, allowing the installation of other heterocycle to phenanthridine by a methylene tether. Control experiments reveals that the reaction probably experiences a radical process. Further investigations on the chemistry of isocyanides and biological evaluation of the reductant products are underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21702034), Natural Science Foundation of Guangxi Province (2017GXNSFBA198089), Guangxi Colleges and Universities Basic Ability Promotion Project of Young Teachers (2017KY0405), Collaborative Innovation Center of Southwest Ethnic Medicine (Guangxi Normal University), Key Disciplines of Organic Chemistry Funding, Guangxi Teachers Education University (8941).Notes and references
-
(a) J. Slavík and L. Slavíková, Collect. Czech. Chem. Commun., 1996, 61, 1047 CrossRef
; (b) H. Surburg and J. Panten, Common Fragrance and Flavor Materials, Wiley-VCH, Weinheim, Germany, 5th edn, 2006 Search PubMed
; (c) M. A. J. Duncton, Med. Chem. Commun., 2011, 2, 1135 RSC
.
- G. A. Olah, Friedel–Crafts Chemistry, Wiley, New York, 1973 Search PubMed
.
- For recent reviews on CDC reactions, see:
(a) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed
; (b) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed
; (c) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed
; (d) F. Zhang and D. R. Spring, Chem. Soc. Rev., 2014, 43, 6906 RSC
; (e) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107 RSC
; (f) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC
; (g) Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247 CrossRef CAS PubMed
.
- For recent examples on C–C bond formations under metal-free conditions, see:
(a) A. P. Antonchick and L. Burgmann, Angew. Chem., Int. Ed., 2013, 52, 3267 CrossRef CAS PubMed
; (b) M. Ito, H. Kubo, I. Itani, K. Morimoto, T. Dohi and Y. Kita, J. Am. Chem. Soc., 2013, 135, 14078 CrossRef CAS PubMed
; (c) S. Y. Shang, D. Zhang-Negrerie, Y. F. Du and K. Zhao, Angew. Chem., Int. Ed., 2014, 53, 6216 CrossRef CAS PubMed
; (d) S. Manna and A. P. Antonchick, Angew. Chem., Int. Ed., 2014, 53, 7324 CrossRef CAS PubMed
; (e) R. Narayan, K. Matcha and A. P. Antonchick, Chem.–Eur. J., 2015, 21, 14678 CrossRef CAS PubMed
; (f) K. Morimoto, K. Sakamoto, T. Ohshika, T. Dohi and Y. Kita, Angew. Chem., Int. Ed., 2016, 55, 16 CrossRef PubMed
.
- For reviews on Minisci reaction, see:
(a) F. Minisci, A. Citterio and C. Giordano, Acc. Chem. Res., 1983, 16, 27 CrossRef CAS
; (b) F. Minisci, F. Fontana and E. Vismara, J. Heterocycl. Chem., 1990, 27, 79 CrossRef CAS
; (c) C. Punta and F. Minisci, Trends Heterocycl. Chem., 2008, 13, 1 CAS
; (d) M. A. J. Duncton, Med. Chem. Commun., 2011, 2, 1135 RSC
.
-
(a) W. Pfleiderer, Tetrahedron Lett., 1984, 25, 1031 CrossRef CAS
; (b) F. Minisci, F. Recupero, A. Cecchetto, C. Punta, C. Gambarotti, F. Fontana and G. F. Pedulli, J. Heterocycl. Chem., 2003, 40, 325 CrossRef CAS
; (c) M. A. J. Duncton, M. A. Estiarte, R. J. Johnson, M. Cox, D. J. R. O'Mahony, W. T. Edwards and M. G. Kelly, J. Org. Chem., 2009, 74, 6354 CrossRef CAS PubMed
; (d) J. M. Pruet, J. D. Robertus and E. V. Anslyn, Tetrahedron Lett., 2010, 51, 2539 CrossRef CAS PubMed
; (e) C. A. Correia, L. Yang and C.-J. Li, Org. Lett., 2011, 13, 4581 CrossRef CAS PubMed
.
- K. Matcha and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 2082 CrossRef CAS PubMed
.
-
(a) I. Ugi, Isonitrile Chemistry, Academic Press, New York, 1971 Search PubMed
; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef
; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed
; (d) A. V. Lygin and A. Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed
; (e) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed
; (f) V. G. Nenajdenko, Isocyanide Chemistry, Wiley-VCH, Weinheim, Germany, 2012 Search PubMed
.
-
(a) B. Zhang, C. Muck-Lichtenfeld, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2013, 52, 10792 CrossRef CAS PubMed
; (b) Q. Wang, X. Dong, T. Xiao and L. Zhou, Org. Lett., 2013, 15, 4846 CrossRef CAS PubMed
; (c) X. Sun and S. Yu, Org. Lett., 2014, 16, 2938 CrossRef CAS PubMed
; (d) M. Tobisu, K. Koh, T. Furukawa and N. Chatani, Angew. Chem., Int. Ed., 2012, 51, 11363 CrossRef CAS PubMed
; (e) B. Zhang, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 250 CrossRef CAS PubMed
; (f) J. Liu, C. Fan, H. Yin, C. Qin, G. Zhang, X. Zhang, H. Yi and A. Lei, Chem. Commun., 2014, 50, 2145 RSC
; (g) X. Li, M. Fang, P. Hu, G. Hong, Y. Tang and X. Xu, Adv. Synth. Catal., 2014, 356, 2103 CrossRef CAS
; (h) C. Pan, J. Han, H. Zhang and C. Zhu, J. Org. Chem., 2014, 79, 5374 CrossRef CAS PubMed
; (i) H. Y. Tu, Y. R. Liu, J. J. Chu, B. L. Hu and X. G. Zhang, J. Org. Chem., 2014, 79, 9907 CrossRef CAS PubMed
; (j) Z. Xu, C. Yan and Z.-Q. Liu, Org. Lett., 2014, 16, 5670 CrossRef CAS PubMed
; (k) Z. Li, F. Fan, J. Yang and Z.-Q. Liu, Org. Lett., 2014, 16, 3396 CrossRef CAS PubMed
; (l) L. Wang, H. Zhu, S. Guo, J. Cheng and J.-T. Yu, Chem. Commun., 2014, 50, 10864 RSC
; (m) P. Xiao, J. Rong, C. Ni, J. Guo, X. Li, D. Chen and J. Hu, Org. Lett., 2016, 18, 5912 CrossRef CAS PubMed
.
- D. Leifert, C. G. Daniliuc and A. Studer, Org. Lett., 2013, 15, 6286 CrossRef CAS PubMed
.
-
(a) Y. Cheng, X. Yuan, H. Jiang, R. Wang, J. Ma, Y. Zhang and S. Yu, Adv. Synth. Catal., 2014, 356, 2859 CrossRef CAS
; (b) B. Zhang and A. Studer, Org. Biomol. Chem., 2014, 12, 9895 RSC
; (c) H. Jiang, Y. Cheng, R. Wang, Y. Zhang and S. Yu, Chem. Commun., 2014, 50, 6164 RSC
; (d) Z. Xiong, J. Wang, Y. Wang, S. Luo and Q. Zhu, Org. Chem. Front., 2017, 4, 1768 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12755c |
| This journal is © The Royal Society of Chemistry 2018 |