Open Access Article
Open Access ArticleProinflammatory and osteolysis-inducing effects of 3D printing Ti6Al4V particles in vitro and in vivo
Cuidi Li†
 ab,
Chuan Jiang†c,
Mingzheng Pengb,
Tao Lib,
Zezheng Yangb,
Zhiyuan Liub,
Ning Lia,
Chengtao Wanga,
Kerong Dai*ab and
Jinwu Wang*ab
ab,
Chuan Jiang†c,
Mingzheng Pengb,
Tao Lib,
Zezheng Yangb,
Zhiyuan Liub,
Ning Lia,
Chengtao Wanga,
Kerong Dai*ab and
Jinwu Wang*ab
aSchool of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, 200030, China
bShanghai Key Laboratory of Orthopedic Implant, Department of Orthopedic Surgery, Shanghai Ninth People's Hospital Affiliated Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China. E-mail: jinwu_wang@163.com; Fax: +86-21-63139920
cDepartment of Orthopedics, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
First published on 9th January 2018
Abstract
Ti6Al4V printing particles have been recently used for fabricating orthopedic implants. Removing these particles completely from fabricated implants is challenging. Furthermore, recycled particles are commonly used in fabrication without additional analysis. Ti6Al4V wear particles derived from orthopedic implants are known to induce inflammatory responses and osteolysis. However, the biosafety of printing particles remains unknown. Here, we investigated the proinflammatory and osteolysis-inducing effects of commonly used original and recycled Ti6Al4V printing particles in vitro and in vivo. Our results indicated that although less serious effects were induced compared to wear particles, inflammatory responses and osteoclast-mediated bone resorption were induced by the original printing particles in a particle size-dependent manner. Recycled particles were found to more strongly stimulate bone resorption and inflammatory responses than the original particles; the in vivo effect was enhanced with an increase in particle concentration. Furthermore, the results of our in vitro experiments verified that the printing particles activate macrophages to secrete inflammatory cytokines and promote osteoclastogenesis, which is closely related to particle size and concentration. Taken together, our findings provide a valuable reference for the use of raw printing materials and examination of recycling procedures for implant fabrication.
1. Introduction
Titanium and Ti6Al4V titanium-alloy biomedical implants have been used widely since the early 1970s because of their high strength-to-weight ratio, good biocompatibility, and outstanding corrosion resistance.1 However, the application of these implants has been hampered by the high cost and poor workability involved in producing complex shapes by using traditional manufacturing processes. For fabrication of surgical implants, 3D printing techniques have recently emerged as a superior choice because they offer design flexibility, cost-saving and the ability to fabricate patient-specific shapes featuring extremely high size accuracy.2 The two most widely used 3D printing methods are the electron beam melting (EBM) and selective laser melting (SLM) techniques.3–6 Porous metal scaffolds fabricated using these techniques have been demonstrated to exhibit high biocompatibility.7–9 However, the printing particles that are retained deep inside large implants cannot be readily removed completely.10,11 Over the past several decades, 1–10 μm-sized wear particles generated from metal implants have been reported to elicit inflammatory reactions.12–14 Macrophages have been shown to be activated and to release proinflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and IL-6. Furthermore, osteoclasts could be activated through a complex sequence of these events, ultimately leading to osteolysis and implant failure.15,16The aforementioned inflammatory responses are widely recognized to be affected by particle size, chemistry, and charge.17,18 Because of the different mechanism, spherical particles with different particle sizes, 45–100 μm and 20–45 μm, were used for EBM and SLS techniques.19–21 Currently, little is known about the role played by 3D printing particles in these size ranges in inducing inflammation and osteolysis. Furthermore, the residual printing particles from the fabrication platform are commonly recycled and repetitively used after a simple sieving procedure. The biosafety of the recycled printing particles for implant fabrication deserves more attention. Therefore, in this study, we aimed to analyze the in vitro and in vivo inflammatory reaction and osteolysis induced by commonly used Ti6Al4V printing particles featuring distinct particle sizes and by one-time recycled particles, with pure ∼2 μm Ti particles being used as the positive-control wear particle model. Our results could serve as a useful reference when using printing particles or examining the feasibility of recycling procedures for implant fabrication.
2. Materials and methods
2.1. Particle preparation and characterization
Ti particles, 99.99% pure, were provided by Johnson Matthey (Ward Hill, MA, USA). The original Ti6Al4V printing particles used here were acquired from three suppliers: EOS GmbH (Munich, Germany), Waston Medical Appliance Co., Ltd (Changzhou, China), and Arcam AB (Molndal, Sweden). Recycled particles that had undergone one-time recycling and sieving were supplied by Waston Medical Appliance Co., Ltd. These 5 types of particles are referred to in short as Ti, EOS, Waston, Arcam, and Waston recycled particles, respectively. Scanning electron microscopy (SEM, Zeiss LEO 1550) was used to analyze particle morphology; the number average particle sizes was analyzed according to the SEM graphs. Particle size distribution was separately characterized using dynamic laser light scattering (for Ti particles; Zetasizer Nano S, Malvern, Worcestershire, UK) and a laser diffraction particle size analyzer (for printing particles; Mastersizer 2000, Malvern, Worcestershire, UK).2.2. In vivo murine calvarial osteolysis model
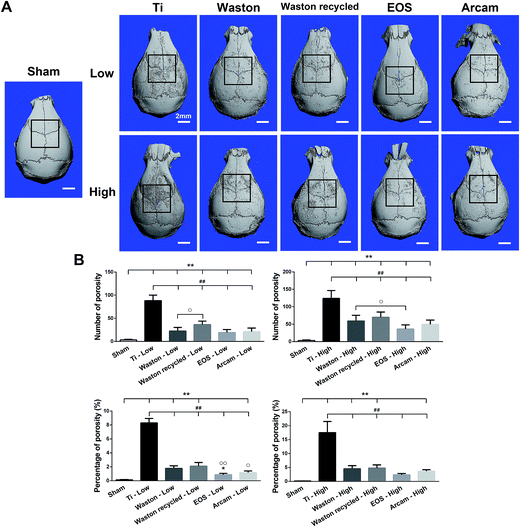
We established a murine calvarial osteolysis model as described previously22,23 to determine the effects of particles on osteolysis in vivo. In total, 54 healthy male C57BL/6 mice (8 weeks old, 20–23 g) were obtained from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All the animal experiments were performed in accordance with the guidelines and regulations for the care and use of laboratory animals of the National Institutes of Health. All procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine. Briefly, mice were anesthetized by intraperitoneal injection of 50 mg kg−1 pentobarbital, and then the periosteum was separated from the calvarium by using a surgical scalpel. Particles were dispersed in saline at a low concentration (10 mg) or high concentration (30 mg) and injected on the surfaces of the bilateral parietal bones. In the sham control, the incision was closed without further intervention. After 14 days, mice were sacrificed for further analysis.2.3. μCT imaging
Calvarium samples were analyzed by using a μCT system (μCT 80; SCANCO Medical AG, Bassersdorf, Switzerland) at an isometric resolution of 9 mm and X-ray energy settings of 70 kV, 114 μA, and 8 W; 3D reconstructed images were obtained using the manufacturer's software. The number of pores and the porosity in a square-shaped region of interest (3.5 mm × 3.5 mm) were analyzed using Image-Pro Plus 6.0 software.2.4. Histological staining and histomorphometric analysis
The collected calvaria were fixed with 4% paraformaldehyde (PFA) for 3 days, decalcified in 10% EDTA for 1 month, and embedded in paraffin. Cross-sections (5 mm) were cut in the coronal plane on a microtome. Afterwards, the sections were performed with hematoxylin/eosin (HE) and tartrate-resistant acid phosphatase (TRAP) staining and observed under an optical microscope (Leica DM4000B). The bone area and osteoclast number were calculated using Image-Pro Plus 6.0 software.2.5. Enzyme-linked immunosorbent assay (ELISA) for analyzing inflammation in vivo
As previously described,24,25 each of the harvested calvaria was carefully placed into one well of a 12-well plate and incubated at 37 °C and 5% CO2 in 1 mL of serum- and phenol-free Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) with 100 U mL−1 penicillin and 100 U mL−1 streptomycin. After 24 h of culture, the culture medium was collected for analysis of TNF-α and IL-6 levels using the respective ELISA kits purchased from R & D Systems (Minneapolis, MN, USA). Assays were performed according to the manufacturer's instructions.2.6. Proinflammatory assay in vitro
2.7. ELISA for in vitro analyses
RAW264.7 cells (5 × 104 per well) were plated in 24-well plates in triplicate and incubated for 2 h. After the cells were attached to the plates, the medium was replaced with the dispersion liquid containing 10 mg mL−1 Ti, Waston, Waston recycled, EOS, or Arcam particles. After culturing for 1 or 3 days, the culture media were collected and the TNF-α and IL-6 concentrations were determined using the ELISA kit from R & D Systems (see Section 2.5). As the control, medium was collected from cells cultured in parallel in the absence of particles. Each experiment was performed three times in triplicate.2.8. Osteoclastogenesis assay in vitro
2.9. Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used to analyze the data obtained from at least three independent experiments. Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.3. Results and discussion
3.1. Characterization of different particles
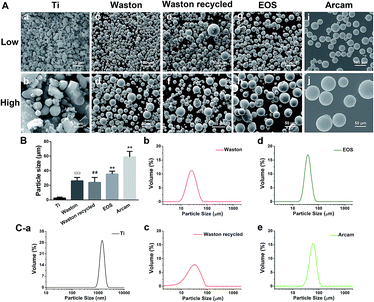
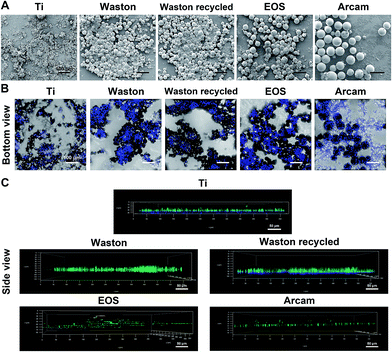
Morphology and particle-size distributions of different particles are shown in Fig. 1. The SEM results (Fig. 1A) revealed that Ti particles featured irregular shapes as expected for a typical wear particle model, whereas the particles used for 3D printing systems featured a regular spherical shape with a smooth surface. The results also showed clear differences in particle sizes; according to our calculations (Fig. 1B and Table 1), Ti particles (2.62 ± 1.25 μm) showed a typical size around 2 μm. Arcam particles presented the largest particle size (58.93 ± 7.59 μm) among the printing particles, EOS particles featured a relatively intermediate size (35.44 ± 3.96 μm), whereas Waston (26.15 ± 4.59 μm) and Waston recycled particles (23.97 ± 6.78 μm) presented the smallest size. Moreover, the Waston recycled particles contained a higher number of smaller and larger particles than the original particles, which might be due to the lasing and recycling process. We further analyzed the size distribution of the particles by using the laser diffraction method (Fig. 1C). The Ti, EOS, and Arcam particles exhibited a relatively uniform size distribution. Among all of the examined particles, Ti particles presented the smallest mean volume particle size and Arcam particles presented the largest size. The Waston particles (Fig. 1C-b) and Waston recycled particles (Fig. 1C-c) featured smaller mean volume particle sizes than other printing particles, but their size distribution, particularly that of Waston recycled particles, was wider than that of EOS and Arcam particles, which supported our imaging results.| Particles | Mean size (μm) | Surface area (cm2 mg−1) |
|---|---|---|
| Ti | 2.62 ± 1.25 | 5.20 |
| Waston | 26.15 ± 4.59 | 0.52 |
| Waston recycled | 23.97 ± 6.78 | 0.56 |
| EOS | 35.44 ± 3.96 | 0.38 |
| Arcam | 58.93 ± 7.59 | 0.23 |
3.2. Effects of particles on osteoclast-mediated bone resorption in vivo
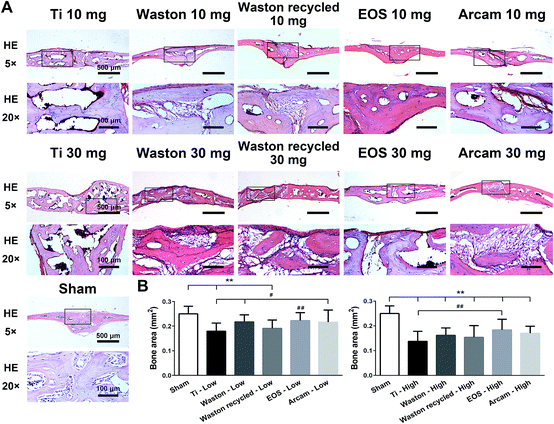
3.2.2.1. Histological staining analysis. To further verify the osteolysis induced by the particles, we performed HE staining (Fig. 3). The results confirmed the aforementioned phenomenon observed using μCT imaging, with regard to the different extents of bone destruction induced by the particles. When the printing particles were implanted at a low concentration, osteolysis was induced weakly inside the bone tissue; by contrast, at a high concentration, all particles triggered serious osteolysis inside the bone tissue as compared to sham treatment (bone area = 0.2501 ± 0.0308 mm2) (P < 0.01). The bone area measured for the Ti group (0.1375 ± 0.0406 mm2) was significantly smaller than that measured for the other particle groups (P < 0.01), whereas the area was considerably larger for the EOS group (0.1837 ± 0.0432 mm2); the Waston recycled group (0.1537 ± 0.0477 mm2) showed the second smallest bone area, followed by the Waston group (0.1621 ± 0.0299 mm2).
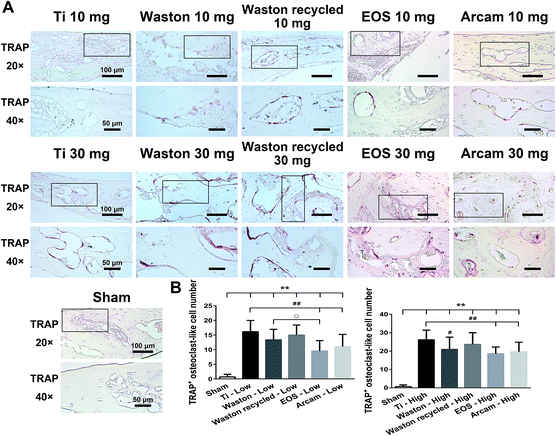
3.2.2.2. Histomorphometric analysis. Osteoclast formation is known to be responsible for both physiological and pathological bone resorption.28 Therefore, we identified the osteoclasts present in bone tissue by TRAP staining (Fig. 4). Although bone loss was induced weakly, TRAP-positive osteoclasts were sensitively induced by all the printing particles while implanted at low concentration. After implantation of particles at high concentration, abundant osteoclasts were observed along the eroded bone surface inside the calvarial bone. The trend observed in the particle-induced TRAP-positive multinucleated osteoclasts was consistent with bone-loss results.
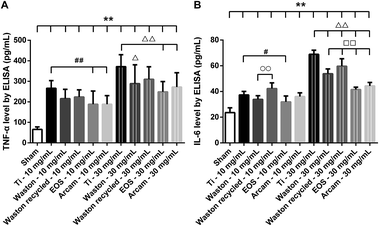
The tested particles also induced higher levels of IL-6 as compared to the level in the sham group (P < 0.01). When implanted at a low concentration, all particles induced IL-6 at low levels, and levels induced by printing particles did not differ markedly from those induced by positive-control Ti particles. However, at a high concentration, the levels of IL-6 induced by the printing particles were significantly lower than that induced by the Ti particles (P < 0.01). Among the original printing particles, Waston particles most strongly stimulated IL-6 production (P < 0.01). Additionally, Waston recycled particles resulted in a considerable increase in IL-6 production relative to that induced by the original particles (P < 0.01).
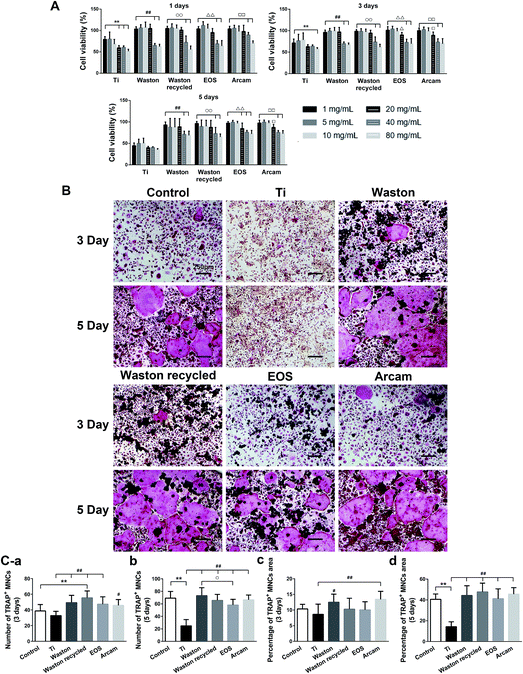
3.3. Proinflammatory assay in vitro
3.4. Osteoclastogenesis assay in vitro
4. Discussion
Clinical use of 3D-printed titanium-alloy orthopedic implants has been gradually increasing. The printing particles retained inside the implants are hard to be removed completely. To the best of our knowledge, no previous study has investigated the biological activity of printing particles sized between 20 and 100 μm at cell and tissue level, including the inflammatory responses and osteolysis elicited by these particles. In addition, particles are usually recycled and used after a simple sieving process. Whether differences of biocompatibility exist between the recycled particles and original particles remains unknown. The chemical composition, size, charge, and surface area of these particles are believed to primarily determine the intensity of the foreign body macrophage response and the extent of osteolysis.17,18,29 With particles possessing uniform composition and spherical morphology, we examined how particle size, particle concentration, and recycling of the particles affected inflammation/osteolysis-stimulating and osteoclastogenesis in vitro and in vivo with the classic wear particle model (Ti particles) used as the positive control.First, our physical characterizations verified the specific differences of particle sizes used for EBM and SLM as reported previously.19–21 Although the particles were used with the same printing technique, Waston particles were found to feature smaller mean particle sizes than EOS particles. Moreover, although the composition was unchanged (data not shown), Waston recycled particles displayed a wider particle size distribution as compared to the original particles.
To study the biological activity of the printing particles in vivo, an immunocompetent C57BL/6 murine model that produces foreign-body responses similar to those observed in human patients was used.30 Results of histological staining and 3D reconstructed μCT images verified that the particle size and concentration affected bone destruction on both the surface and inside of the calvarial bone. The severity of the effects observed in the groups showed the following trend: Sham < EOS < Arcam < Waston < Waston recycled < Ti. Printing particles that around 20 μm or 60 μm might exert strong osteolysis-inducing effects. Recycled Waston particles caused more serious osteolysis than the original particles, which might be owing to the recycled particles including a higher number of small and large particles when compared to the original particles, as was clearly observed in the SEM images. Analysis of TRAP-positive osteoclast formation in the bone destruction area revealed a similar trend of particle size- and concentration-dependent effects and confirmed our hypothesis that the osteolysis-induction effect of the printing particles is closely related to osteoclast activation.
Inflammatory responses have been implicated as a major contributor to increased osteoclastic activity and accelerated osteolysis.15,16,31,32 Titanium-alloy wear particles (0.5–10 μm) are widely recognized to play a critical role in osteolysis by inducing inflammatory responses.33–35 Recently, large particles at the macro level (size of >100 μm) have been observed to lead to a maintenance of foreign-body reaction and inflammatory responses.36–38 Thus, we hypothesis that the inflammatory response might be an underlying reason for the size-dependent osteoclast-activating and osteolysis-inducing effects of printing particles observed here.
Moreover, an interesting phenomenon was also observed here: serious osteolysis inside the bone tissue was observed in the histological analysis, whereas the bone loss induced on the surface by the printing particles appeared to be comparatively weak according to the imaging results. This phenomenon further supports our inflammatory response hypothesis that the particles activate macrophages as wear particles to secrete proinflammatory cytokines, including IL-1, IL-6, and TNF-α, which indirectly stimulate osteoclastic bone resorption and promote the recruitment of mononuclear cells into these osteolytic lesions.15,16,39–43
Given these findings, we measured the concentration of the proinflammatory cytokines, IL-6 and TNF-α, in the medium of cultured calvaria. The levels of both cytokines were increased drastically after implantation of the particles at either a low or high concentration. Furthermore, the severity of the effect measured here showed a similar trend as that observed in the osteolysis results, which implies that osteolysis is related to the inflammatory response and the secretion of the proinflammatory cytokines, TNF-α and IL-6.
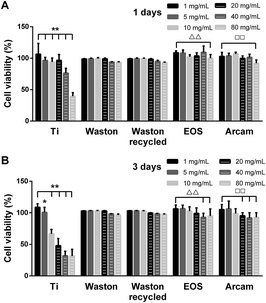
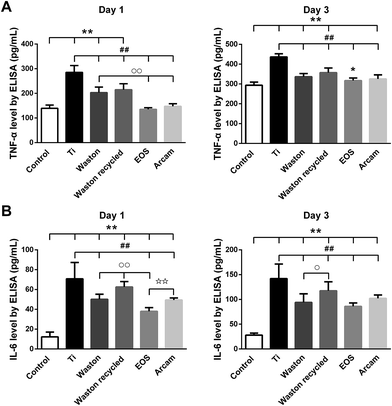
To further study how the particles triggered inflammation and osteoclastogenesis in vitro, the model cells for monocytes and macrophages, BMMs and RAW264.7 cells, respectively, were used. Being different from Ti group which showed cytotoxicity, the printing particles were found to be nontoxic to the RAW264.7 cells. In addition, much more cells were observed around and on the original and recycled Waston particles than other printing particles in the SEM and CLSM results (Fig. 7). Furthermore, the levels of the cytokines TNF-α and IL-6 increased with time and the effect showed a size-dependence similar to that observed in the osteolysis assay in vivo. Thus, we hypothesized that the size-dependent induction effects of the particles might be partly relevant to their role in cell accumulation and attachment. It is known that wear particles swiftly adsorb a layer of host proteins immediately after implantation and exposure to physiologic fluids.44 Upon binding to the proteins adsorbed on biomaterial surfaces, macrophages that are recruited to the implant site could be activated, resulting in release of inflammatory cytokines such as TNF-α, IL-6, and IL-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44–46 and accelerated osteolysis.39–43,47,48 Among the printing particles, Waston original and recycled particles, with a smaller particle size and larger specific surface area per unit weight (Table 1), contacted with more cells and thereby probably led to the more pro-inflammation cytokines induced. However, the high level of inflammatory cytokines stimulated by Arcam particles suggests that the biological activity of particles with a size of approximately 60 μm might be a result of other mechanisms that need further study.
44–46 and accelerated osteolysis.39–43,47,48 Among the printing particles, Waston original and recycled particles, with a smaller particle size and larger specific surface area per unit weight (Table 1), contacted with more cells and thereby probably led to the more pro-inflammation cytokines induced. However, the high level of inflammatory cytokines stimulated by Arcam particles suggests that the biological activity of particles with a size of approximately 60 μm might be a result of other mechanisms that need further study.
Previously, we found that printing particles did not directly induce osteoclast maturation from precursors in the absence of RANKL (data not shown), which is consistent with a previous study.49 In this study, the promotion effect of particles on osteoclastogenesis was analyzed in the presence of M-CSF and RANKL. Although no statistically significant difference was observed in the effects of the printing particles, all of the particles at a non-cytotoxic concentration were found to effectively stimulate BMMs differentiation and promote the formation of multinucleated osteoclasts; moreover, the trend here was similar to that observed in vivo. These results indicate that bone loss induced by printing particles is also closely related to promotion of osteoclastogenesis.
However, some limitations exist in our current study. First, even though size-dependent effects of printing particles on osteolysis and the inflammatory reaction were clearly verified in this study, the underlying molecular mechanisms remain unclear and need to be investigated in future studies. Second, several typical printing particles from main supplier companies were chosen as study objects here. With the increased popularity of the 3D printing industry, more companies have been established in the last 3 years. Comprehensive investigations including distinct printing particle samples from other suppliers should be designed to better characterize the biological activity of printing particles. Despite these limitations, this study would still be valuable for improving the use of printing raw materials in the application of clinical implant fabrication.
5. Conclusion
In this study, we analyzed for the first time the in vitro and in vivo inflammatory reaction and osteolysis induced by commonly used Ti6Al4V printing particles and by one-time recycled particles; differences in particle size were observed not only between particles used for EBM and SLM techniques, but also between those from different companies based on the same technique. Recycled particles were found to possess a wider size distribution than the original particles. Although the effect was less serious than that observed with classic Ti particles, the printing particles stimulated macrophages to secrete proinflammatory cytokines and promoted BMMs differentiation into multinucleated osteoclasts in vitro, and triggered a proinflammatory reaction and osteolysis in vivo. At both low and high concentrations, particles sized approximately 20 μm and 60 μm strongly triggered proinflammatory reactions, osteoclastogenesis, and osteolysis. And recycled particles may have an even stronger effect than the original particles. The results of our findings might could serve as a valuable reference when using printing particles or examining the feasibility of recycling procedures for implant fabrication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This investigation was supported by the National High Technology Research and Development Program of China (863 Program: 2015AA020308), Key Developing Disciplines Program of Shanghai Health System (2015ZB04904), Natural Science Foundation of Guangdong Province (2017A03031854), National Natural Science Foundation of China (81572156), and the Key Diseases and Technological Program of the Shanghai Health System (2013ZYJB0501).Notes and references
- S. L. Sing, J. An, W. Y. Yeong and F. E. Wiria, J. Orthop. Res., 2016, 34, 369–385 CrossRef CAS PubMed.
- A. Sidambe, Materials, 2014, 7, 8168 CrossRef PubMed.
- P. Fischer, V. Romano, H. P. Weber, N. P. Karapatis, E. Boillat and R. Glardon, Acta Mater., 2003, 51, 1651–1662 CrossRef CAS.
- J. M. Williams, A. Adewunmi, R. M. Schek, C. L. Flanagan, P. H. Krebsbach, S. E. Feinberg, S. J. Hollister and S. Das, Biomaterials, 2005, 26, 4817–4827 CrossRef CAS PubMed.
- P. Heinl, L. Müller, C. Körner, R. F. Singer and F. A. Müller, Acta Biomater., 2008, 4, 1536–1544 CrossRef CAS PubMed.
- J. Parthasarathy, B. Starly, S. Raman and A. Christensen, J. Mech. Behav. Biomed. Mater., 2010, 3, 249–259 CrossRef PubMed.
- G. Li, L. Wang, W. Pan, F. Yang, W. Jiang, X. Wu, X. Kong, K. Dai and Y. Hao, Sci. Rep., 2016, 6, 34072 CrossRef CAS PubMed.
- P. H. Warnke, T. Douglas, P. Wollny, E. Sherry, M. Steiner, S. Galonska, S. T. Becker, I. N. Springer, J. Wiltfang and S. Sivananthan, Tissue Eng., Part C, 2008, 15, 115–124 CrossRef PubMed.
- A. Palmquist, A. Snis, L. Emanuelsson, M. Browne and P. Thomsen, J. Biomater. Appl., 2011, 27, 1003–1016 CrossRef PubMed.
- H. Hasib, O. L. A. Harrysson and H. A. West, JOM, 2015, 67, 639–646 CrossRef CAS.
- C. M. Cheah, C. K. Chua, K. F. Leong and S. W. Chua, Int. J. Adv. Des. Manuf. Technol., 2003, 21, 291–301 CrossRef.
- L. D. Dorr, K. R. Hilton, Z. Wan, G. D. Markovich and R. Bloebaum, Clin. Orthop. Relat. Res., 1996, 333, 108–117 Search PubMed.
- L. Saldaña and N. Vilaboa, Acta Biomater., 2010, 6, 1649–1660 CrossRef PubMed.
- T. Akisue, T. W. Bauer, C. F. Farver and Y. Mochida, J. Biomed. Mater. Res., 2002, 59, 507–515 CrossRef CAS PubMed.
- C. Nich, J. Langlois, A. Marchadier, C. Vidal, M. Cohen-Solal, H. Petite and M. Hamadouche, Arthritis Res. Ther., 2011, 13, R100 CAS.
- T.-h. Lin, Z. Yao, T. Sato, M. Keeney, C. Li, J. Pajarinen, F. Yang, K. Egashira and S. B. Goodman, Acta Biomater., 2014, 10, 3747–3755 CrossRef PubMed.
- M. L. Wang, P. F. Sharkey and R. S. Tuan, J. Arthroplasty, 2004, 19, 1028–1038 CrossRef PubMed.
- H. Gelb, H. Ralph Schumacher, J. Cuckler and D. G. Baker, J. Orthop. Res., 1994, 12, 83–92 CrossRef CAS PubMed.
- L. E. Murr, S. A. Quinones, S. M. Gaytan, M. I. Lopez, A. Rodela, E. Y. Martinez, D. H. Hernandez, E. Martinez, F. Medina and R. B. Wicker, J. Mech. Behav. Biomed. Mater., 2009, 2, 20–32 CrossRef CAS PubMed.
- H. Gong, K. Rafi, H. Gu, G. D. Janaki Ram, T. Starr and B. Stucker, Mater. Des., 2015, 86, 545–554 CrossRef CAS.
- H. Gong, K. Rafi, H. Gu, T. Starr and B. Stucker, Addit. Manuf., 2014, 1–4, 87–98 CrossRef.
- C. Jiang, J. Shang, Z. Li, A. Qin, Z. Ouyang, X. Qu, H. Li, B. Tian, W. Wang, C. Wu, J. Wang and M. Dai, J. Cell. Physiol., 2016, 231, 142–151 CrossRef CAS PubMed.
- C. Jiang, F. Xiao, X. Gu, Z. Zhai, X. Liu, W. Wang, T. Tang, Y. Wang, Z. Zhu, K. Dai, A. Qin and J. Wang, Biochimie, 2015, 111, 107–118 CrossRef CAS PubMed.
- Z. Zhai, X. Qu, H. Li, K. Yang, P. Wan, L. Tan, Z. Ouyang, X. Liu, B. Tian, F. Xiao, W. Wang, C. Jiang, T. Tang, Q. Fan, A. Qin and K. Dai, Biomaterials, 2014, 35, 6299–6310 CrossRef CAS PubMed.
- H. Shao, J. Shen, M. Wang, J. Cui, Y. Wang, S. Zhu, W. Zhang, H. Yang, Y. Xu and D. Geng, Biomaterials, 2015, 60, 92–99 CrossRef CAS PubMed.
- T. Koga, M. Inui, K. Inoue, S. Kim, A. Suematsu, E. Kobayashi, T. Iwata, H. Ohnishi, T. Matozaki, T. Kodama, T. Taniguchi, H. Takayanagi and T. Takai, Nature, 2004, 428, 758–763 CrossRef CAS PubMed.
- J.-P. Hu, K. Nishishita, E. Sakai, H. Yoshida, Y. Kato, T. Tsukuba and K. Okamoto, Eur. J. Pharmacol., 2008, 580, 70–79 CrossRef CAS PubMed.
- B. Tian, T. Jiang, Z. Shao, Z. Zhai, H. Li, Q. Fan, X. Liu, Z. Ouyang, T. Tang, Q. Jiang, M. Zheng, K. Dai, A. Qin, Y. Yu and Z. Zhu, Biomaterials, 2014, 35, 8937–8950 CrossRef CAS PubMed.
- A. Sabokbar, R. Pandey and N. A. Athanasou, J. Mater. Sci.: Mater. Med., 2003, 14, 731–738 CrossRef CAS PubMed.
- M. Kolb, P. Bonniaud, T. Galt, P. J. Sime, M. M. Kelly, P. J. Margetts and J. Gauldie, Am. J. Respir. Cell Mol. Biol., 2002, 27, 141–150 CrossRef CAS PubMed.
- J. J. Jacobs, K. A. Roebuck, M. Archibeck, N. J. Hallab and T. T. Glant, Clin. Orthop. Relat. Res., 2001, 393, 71–77 CrossRef.
- T. W. Bauer, Clin. Orthop. Relat. Res., 2002, 405, 138–143 CrossRef.
- G. Vallés, P. González-Melendi, J. L. González-Carrasco, L. Saldaña, E. Sánchez-Sabaté, L. Munuera and N. Vilaboa, Biomaterials, 2006, 27, 5199–5211 CrossRef PubMed.
- J. H. Dumbleton, M. T. Manley and A. A. Edidin, J. Arthroplasty, 2002, 17, 649–661 CrossRef PubMed.
- C. A. Holding, D. M. Findlay, R. Stamenkov, S. D. Neale, H. Lucas, A. S. S. K. Dharmapatni, S. A. Callary, K. R. Shrestha, G. J. Atkins, D. W. Howie and D. R. Haynes, Biomaterials, 2006, 27, 5212–5219 CrossRef CAS PubMed.
- S. P. Nichols, A. Koh, W. L. Storm, J. H. Shin and M. H. Schoenfisch, Chem. Rev., 2013, 113, 2528–2549 CrossRef CAS PubMed.
- K. L. Helton, B. D. Ratner and N. A. Wisniewski, J. Diabetes Sci. Technol., 2011, 5, 632–646 CrossRef PubMed.
- T. Lange, A. F. Schilling, F. Peters, J. Mujas, D. Wicklein and M. Amling, Biomaterials, 2011, 32, 4067–4075 CrossRef CAS PubMed.
- Y. Hirashima, N. Ishiguro, S. Kondo and H. Iwata, J. Biomed. Mater. Res., 2001, 56, 177–183 CrossRef CAS PubMed.
- S. D. Neale, Y. Fujikawa, A. Sabokbar, R. Gundle, D. W. Murray, S. E. Graves, D. W. Howie and N. A. Athanasou, J. Bone Jt. Surg., Br. Vol., 2000, 82, 892–900 CrossRef CAS.
- C. Jiang, Y. Zou, X. Liu, J. Shang, M. Cheng and M. Dai, J. Rare Earths, 2013, 31, 420–427 CrossRef CAS.
- K. D. Merkel, J. M. Erdmann, K. P. McHugh, Y. Abu-Amer, F. P. Ross and S. L. Teitelbaum, Am. J. Pathol., 1999, 154, 203–210 CrossRef CAS PubMed.
- A. Sabokbar, R. Pandey and N. A. Athanasou, J. Mater. Sci.: Mater. Med., 2003, 14, 731–738 CrossRef CAS PubMed.
- T. W. Bauer and J. Schils, Skeletal Radiology, 1999, 28, 423–432 CrossRef CAS PubMed.
- T. D. Zaveri, N. V. Dolgova, J. S. Lewis, K. Hamaker, M. J. Clare-Salzler and B. G. Keselowsky, Biomaterials, 2017, 115, 128–140 CrossRef CAS PubMed.
- K. D. Merkel, J. M. Erdmann, K. P. McHugh, Y. Abu-Amer, F. P. Ross and S. L. Teitelbaum, Am. J. Pathol., 1999, 154, 203–210 CrossRef CAS PubMed.
- J. Yadav, L. Samelko, P. Gilvar, K. McAllister and N. J. Hallab, Open Orthop. J., 2013, 7, 605–613 CrossRef PubMed.
- N. J. Hallab and J. J. Jacobs, Front. Neuroendocrinol., 2017, 8, 5 Search PubMed.
- Y.-Y. Kong, H. Yoshida, I. Sarosi, H.-L. Tan, E. Timms, C. Capparelli, S. Morony, A. J. Oliveira-dos-Santos, G. Van, A. Itie, W. Khoo, A. Wakeham, C. R. Dunstan, D. L. Lacey, T. W. Mak, W. J. Boyle and J. M. Penninger, Nature, 1999, 397, 315–323 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |