Open Access Article
Open Access ArticleFacile complexation reactions for the selective spectrofluorimetric determination of albendazole in oral dosage forms and spiked human plasma
Ahmed A. Hamada,
Ramadan Alia,
Hassan Refat H. Alib,
Dalia M. Nagyc and
Sayed M. Derayea *c
*c
aDepartment of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Al-Azhar University, Assiut Branch, Assiut 71524, Egypt
bDepartment of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Assiut University, Assiut, Egypt
cDepartment of Analytical Chemistry, Faculty of Pharmacy, Minia University, Minia 61519, Egypt. E-mail: sayed_derayea@mu.edu.eg
First published on 31st January 2018
Abstract
Two simple, sensitive, and rapid spectrofluorimetric methods were developed and validated for the determination of albendazole. The first method (method I) was based on the quenching effect of albendazole on the native fluorescence of erythrosine B. The fluorescence intensity was measured at 554 nm after extraction at 527 nm. In the second method (method II) the drug was reacted with lanthanum(III) ions to form a metal complex, which was measured at 340 nm after excitation at 295 nm. The suitable pH was 3.4 (Teorell–Stenhagen buffer) and pH 5.5 (phosphate buffer solution), for method I and II, respectively. The influence of experimental factors on the fluorescence intensity of the reaction products was investigated and optimized. The linear concentration ranges were 0.2–3.5 and 0.06–0.90 μg mL−1, with detection limits of 0.049 and 0.019 μg mL−1 for method I and II, respectively. ICH guidelines were followed for validation of the developed procedures, and the results were acceptable. The Gibb's free energy change of the reactions was −24.6 and −27.5 kJ mol−1 for method I and II, respectively. These negative values indicated the high feasibility of these reactions at ambient temperature. The proposed procedures were applied successfully for the determination of albendazole in commercial dosage forms and spiked human plasma. The results showed high precision, accuracy and recovery of the reported methods without any significant interference from pharmaceutical excipients or plasma components.
1. Introduction
Albendazole (ABZ, Fig. 1) is an anthelmintic drug belonging to the benzimidazole group which is commonly applied in the treatment of infection caused by a wide range of worms such as pin worms, round worms, hookworms and whipworms.1 ABZ is still an effective treatment for hydrated cysts, echinococcosis, and neurocysticercosis. ABZ as a benzimidazole drug produces its effect through specific binding to tubulin, the microtubule subunit protein. This binding will interrupt the structure and function of the microtubule and hence interfere with the microtubule-mediated transport of secretory vesicles in absorptive tissues of the worm.2 Due to the great clinical importance of ABZ, several analytical methods have been reported for its determination either in pharmaceutical dosage forms or in biological fluids. The reported analytical methods include: spectrophotometry,3–9 spectrofluorimetry,10–13 high-performance liquid chromatography (HPLC)14–17 HPTLC,18 thermogravimetry19 and electrochemical methods.20,21Most of the reported spectrophotometric methods have low sensitivity. In addition, some of these methods were indirect and involved time consuming, multi steps and multi-reagents reactions. This could affect the accuracy and precision of these methods.6 In spite of the suitable sensitivity of the ion-pair complex based methods,7,8 they suffer from the extraction step which involve the use of hazardous organic solvents and the possibility of emulsion formation or incomplete extraction. HPLC consume large volumes of highly pure organic solvent and labor sample pretreatment steps. HPLC, capillary electrophoresis and electrochemical methods require relatively expensive and sophisticated instruments. On the other hand, the spectrofluorimetry apply a relatively simple procedure and easy to handle instrument. The reported spectrofluorimetric methods were not applied in the analysis of human plasma samples.10–13 In addition chloroform was used which is a hazardous organic solvent10 in one method while the charge transfer based method have low sensitivity (LOD = 2.0 μg mL−1).12
Erythrosine B (EB, Fig. 1) as an acidic dye, is a food colorant that has been used for the spectrofluorimetric determination of some basic compounds such as fluoroquinolones22 imipramine23 and proteins.24 As ABZ has a basic centre (secondary amino group), it can form an ion pair associate with the dye in weakly acidic solution. The complex formation process was accompanied by a significant quenching of the native fluorescence of EB. The mechanism of the fluorescence quenching was investigated using Stern–Volmer equation.
| F0/F = 1 + KSV[Q] |
Another feature of ABZ molecule is the presence of the benzimidazole moiety which impart a native fluorescence for the drug. However, this fluorescence activity is very weak in aqueous solutions. Fortunately, the secondary amino and carbonyl groups of ABZ, give it the ability to form a stable complex with many metal ions. Lanthanide complexes have been used as fluorescent label for a wide variety of analytical techniques. It was found that, the weak native fluorescence of the drug was greatly enhanced by its metal complex reaction with lanthanum(III) ions.
In the present study, EB dye and La(III) ion were utilized for the development of two spectrofluorimetric methods for the determination of ABZ based on complex formation reactions. The fluorescence quenching in the first method and fluorescence intensity in the second method were directly proportional to the drug concentration. The mole ratios of both reactions were investigated, and the thermodynamic parameters were calculated. Both methods were applied for the determination of the cited drug in oral dosage forms and spiked human plasma.
The simplicity and low detection limits are among the advantages of the proposed methods over the previously reported methods. In addition the applied procedures were less time consuming and utilized: green solvents (water and methanol for method I and II, respectively), inexpensive reagents and relatively low price instrument. Furthermore, the developed methods could determine the cited drug with good recoveries without any interference from frequently encountered excipients or plasma components. Therefore, the proposed methods are suitable for the determination of the ABZ in its oral dosage forms in quality control laboratories.
2. Experimental
2.1. Instrumentation
All spectrofluorimetric measurements were carried out on SCINCO FluoroMate spectrofluorimeter (FS-2, Korea), equipped with 150 W Xe-arc lamp, PMT detector and 1 cm matched quartz cells. Super-mixer (GEMMY industrial CORD, Taiwan, R.O.C), pH-meter, model AD11P (Adwa, Romania) and bath sonicator (SONICOR SC-101TH) were also used. All weighing were performed on an electronic single pan balance (Precisa XB 220A, Switzerland). Distilled water was prepared by water distiller (TYUMEN-MIDI-A0-25 MO, Russia).2.2. Chemicals and reagents
All chemicals were of analytical grade and reagent solutions were prepared fresh daily. Albendazole powder was kindly supplied by the Egyptian International Pharmaceutical Industries Company (EIPICO, Cairo, Egypt) and was used without further purification. Solution of 5.1 × 10−4 mol L−1 lanthanum ion was freshly prepared by dissolving 12.51 mg lanthanum(III) chloride (Panreac Chemicals, Lyon, France) in 100 mL distilled water. Erythrosine B (Market Harborough Leicestershire, UK) solution was prepared in distilled water as 0.015% g w/v (1.7 × 10−3 M). Cetyl pyridinium chloride (CPC), polyethylene glycol (PEG), sodium dodecyl sulfate (SDS) and Tween-80 were obtained from El-Nasr Chemical Company (Cairo, Egypt). The authors got the permission for using plasma sample of human volunteers from Minia University Hospital, Minia, Egypt, according to institutional guidelines. In all cases, informed written consent was obtained from all participants. Human plasma samples were kept frozen at 20 °C until assay after gentle thawing.2.3. Pharmaceutical formulations
The following pharmaceutical preparations were analyzed which were present in the local market; Alzental tablets (Egyptian International Pharmaceutical Industries Company, EIPICO, Cairo, Egypt) labeled to contain 200 mg of albendazole per tablet. Alzental suspension was labeled to contain 20 mg of albendazole per 1 mL.2.4. Preparation of standard solution
An accurately weighed amount (10 mg) of ABZ was dissolved in 100 mL methanol. This gave a stock standard drug solution (0.1 mg mL−1) which was further diluted with the same solvent to obtain working standard drug solution containing concentrations covering the required range.2.5. General analytical procedure
2.6. Construction of calibration curves
Different aliquots of the standard drug solutions (2.0–35 μg mL−1 for method I or 0.7–9.0 μg mL−1 of method I) were transferred into a series of 10 mL calibrated volumetric flasks and the general analytical procedure was applied as previously mentioned. The calibration graph was obtained by plotting the corrected fluorescence intensity versus the final concentration of ABZ, and the corresponding regression equation was estimated.2.7. Samples preparation of for analysis
2.8. Determination of the reaction stoichiometry
Job's method of continuous variation26 was used to find out the molar ratios between the drug and the reagent. Equimolar solutions of both ABZ and the reagents (5.1 × 10−4 mol L−1 in case of lanthanum and 1.7 × 10−4 mol L−1 in case of erythrosine B) were prepared. Series of 1.0 mL solutions were made up comprising different complimentary proportions of the reagent and drug (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, 0.1
1.0, 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9, …, 0.9
0.9, …, 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1.0
0.1, 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) in 10 mL volumetric flasks. One milliliter of the buffer solution (Toerell–Stenhagen buffer, pH 3.4 and phosphate buffer solution, pH 5.5) was added to each flask and the general analytical procedure was followed as described previously.
0) in 10 mL volumetric flasks. One milliliter of the buffer solution (Toerell–Stenhagen buffer, pH 3.4 and phosphate buffer solution, pH 5.5) was added to each flask and the general analytical procedure was followed as described previously.
3. Results and discussion
The ability of ABZ to form complex with different substances was utilized for the development of two sensitive spectrofluorimetric methods. The basic nitrogen of the drug could form an ion pair complex with erythrosine, while both the secondary amino group and its neighbor carbonyl group give ABZ the capability to form metal complex.3.1. Fluorescence spectra
Erythrosine B is an acidic dye which has a native fluorescence appears at 554 nm after excitation at 527 nm. When the solution of ABZ was added to dye solution a significant reduction in the fluorescence intensity of the dye was observed as a result of the ion-pair complex formation. This represent the basis for the first spectrofluorimetric method. However, ABZ itself has a very weak native fluorescence in aqueous solution which could be greatly improved upon the addition of lanthanum ions. The stability of the complex was improved in the presence of phosphate buffer (pH 5.5). Based on this fact, another spectrofluorimetric method was constructed. The excitation and emission spectra of the studied reagents in the presence and absence of ABZ are shown in Fig. 2.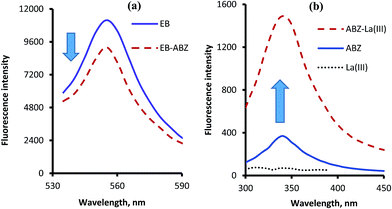 | ||
| Fig. 2 Fluorescence emission spectra of the complexes of ABZ (1.0 μg mL−1) with erythrosine B (a) after excitation at 527 nm and with lanthanum(III) ions (b) after excitation at 295 nm. | ||
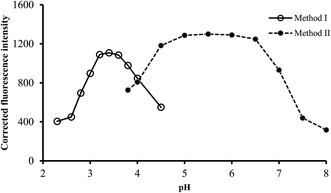
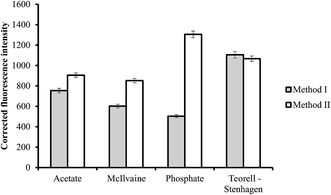
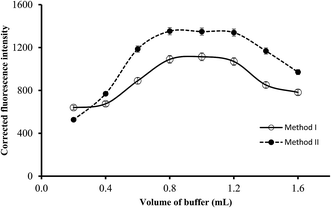
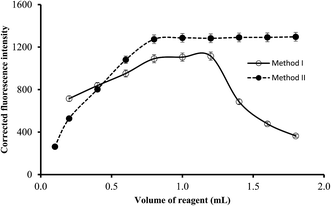
3.2. Optimization of reaction conditions
The best reaction conditions were attained by changing each experimental variable individually and monitoring its effect on the fluorescence intensity. The investigated variables were buffer type, pH and volume, reagents concentration (La(III) or erythrosine B), diluting solvents and reaction time.3.3. Determination of the reactions stoichiometry
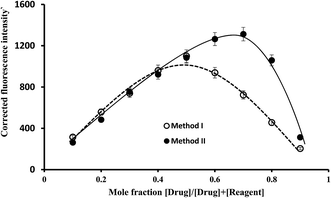
The ratio between ABZ and the reagent was estimated by applying Job's method of continuous-variation. A series of solutions containing various molar ratios of the drug and the reagents (EB or La(III)) were prepared keeping the total moles in all solutions constant. The fluorescence intensities of at the specific wavelengths for each solution was measured. In method I, the observed fluorescence intensity of each mixture at various mole fractions was subtracted from that of the solutions containing the same concentration of the dye alone. While, in method II, the fluorescence intensity of the blank (containing the La(III) ions alone) was subtracted from that of the solution experiment. The difference in the fluorescence intensities was plotted against the mole fraction of the drug, Fig. 7. Curves were obtained in which the position of the maxima indicated that the molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for EB
1 for EB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ABZ and 1
ABZ and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for La(III)
2 for La(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ABZ. Based on the estimated molar ratios, the chemical structure of the formed complexes were presented in Fig. 8.
ABZ. Based on the estimated molar ratios, the chemical structure of the formed complexes were presented in Fig. 8.
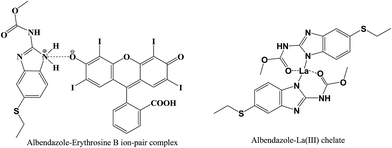 | ||
| Fig. 8 The suggested chemical structure of the complexes of ABZ with either erythrosine B or lanthanum(III) ions. | ||
In weakly acidic medium (pH 3.4), EB will dissociate to form a mono-valent anion in which the ionization of the hydroxyl group is predominant than carboxylic group of the phenyl ring. This is due to the presence of two strong electron withdrawing groups (iodine atoms) on the xanthene ring adjacent to the hydroxyl. The negatively charged mono-valent anion of EB will interact with the drug cation through both electrostatic interaction and hydrophobic forces to form the ion pair complex.
The mechanism of metal complex formation between ABZ and La(III) was similar to the previously reported complex between ferric(III) and ABZ.28 Accordingly, in the proposed chemical structure of La(III)–ABZ chelate, the drug molecule acts as a neutral bidentate ligand through the carbonyl group of the side chain and NH of five-member ring with octahedral fashion toward La(III) ions.
Furthermore, the quenching mechanism of ABZ on the native fluorescence of EB was studied by applying Stern–Volmer method. Stern–Volmer plot was constructed by plotting the fluorescence intensities in the absence (F0) and presence (F) of the quencher (the drug) against the quencher concentration [Q]. The obtained straight line was fitted to the Stern–Volmer equation.29
| F0/F = 1 + KSV[Q] |
The intercept of the straight line is approximately 1 and the slope (2.45 × 104) is called Stern–Volmer constant (KSV) which in turn is the product of the natural radiation lifetime (the lifetime in the absence of quencher), τ0, and the quenching rate constant, Kq, (KSV = Kqτ0). This equation provides an important way for the estimation of the quenching rate constant. Since the reported fluorescence lifetime of erythrosine B is 89 ps,30 hence, the calculated quenching rate constant value is 2.49 × 1014 L mol−1 s−1. This value is significantly higher than the largest reported Kq for collisional quenching (2 × 1010 L mol−1 s−1).31 Consequently, the assumed quenching mechanism for the interaction between ABZ and erythrosine B is considered a single static process.
3.4. The rate constants and free energy changes of the reactions
The reaction rate constant (K) for the reaction between ABZ and EB could be calculated using following equation.32
log((F0 − F)/F) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K + n K + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[D] log[D]
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the drug–dye ion pair complex.
1) in the drug–dye ion pair complex.
| Parameter | ABZ–EB | ABZ–La(III) |
|---|---|---|
| Equation no. | (1) | (2) |
| Slope | 0.9623 | 2.04 × 10−9 |
| Intercept | 4.3166 | 1.32 × 10−4 |
| Correlation coefficient, r2 | 0.9965 | 0.9996 |
| Reaction rate constant, K | 2.07 × 104 | 6.48 × 104 |
| Gibbs free energy, (ΔG°, kJ mol−1) | −24.63 | −27.45 |
For the reaction between lanthanum and the drug, the binding constant (K) was calculated using the modified Benesi–Hildebrand equation:33
| 1/ΔF = 1/ΔFmax + 1/K[D]ΔFmax | (2) |
| ΔF = F − F0 and ΔFmax = Fmax − F0 |
Gibb's free energy (ΔG°) of the reactions can be calculated from the obtained reaction rate constants using the formula: ΔG° = −2.303RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K, where, R is the gas constant (8.314 J K−1 mol−1), T is the absolute temperature in Kelvin and K is the rate constant. The estimated values for ΔG° were −24.6 and −27.5 kJ mol−1 for EB–ABZ and La–ABZ reactions, respectively. Since the values of the obtained free energy change are largely negative, it is expected that both reactions have high spontaneity and feasibility at room temperature.
K, where, R is the gas constant (8.314 J K−1 mol−1), T is the absolute temperature in Kelvin and K is the rate constant. The estimated values for ΔG° were −24.6 and −27.5 kJ mol−1 for EB–ABZ and La–ABZ reactions, respectively. Since the values of the obtained free energy change are largely negative, it is expected that both reactions have high spontaneity and feasibility at room temperature.
3.5. Validation of the developed method
To confirm the feasibility of the developed procedure in routine analysis of ABZ in real samples, the procedure was fully validated according to guidelines of the International Conference on Harmonization (ICH).34 The studied parameters included; linearity and range, accuracy and precision, sensitivity, selectivity and robustness.| Parameters | Method I | Method II |
|---|---|---|
| Linear range (μg mL−1) | 0.2–3.5 | 0.06–0.90 |
| Intercept (a) | 247.2 | 57.07 |
| Standard deviation of intercept | 12.63 | 7.47 |
| Slop (b) | 850.7 | 1252 |
| Standard deviation of slope | 5.35 | 7.88 |
| Correlation coefficient (r) | 0.9998 | 0.9998 |
| Determination coefficient (r2) | 0.9996 | 0.9996 |
| Limit of detection, LOD (μg mL−1) | 0.049 | 0.019 |
| Limit of quantitation LOQ (μg mL−1) | 0.15 | 0.059 |
| Drug | Taken (μg mL−1) | Founda (μg mL−1) | Recovery ± % SDa | RSDb% | Erb% |
|---|---|---|---|---|---|
| a The value is the mean of three replicates measurement, SD is the standard deviation.b RSD is the relative standard deviation and Er% is the relative error percentage. | |||||
| Method I | 0.5 | 0.502 | 100.34 ± 0.39 | 0.39 | 0.34 |
| 1.5 | 1.506 | 100.39 ± 0.393 | 0.391 | 0.39 | |
| 2.5 | 2.493 | 99.73 ± 0.587 | 0.589 | −0.27 | |
| Method II | 0.1 | 0.1003 | 100.32 ± 0.96 | 0.96 | 0.32 |
| 0.5 | 0.5001 | 100.02 ± 0.88 | 0.88 | 0.02 | |
| 0.7 | 0.704 | 100.53 ± 0.92 | 0.91 | 0.53 | |
| Drug | Conc. (μg mL−1) | Intra-day precisions | Inter-day precisions | ||
|---|---|---|---|---|---|
| % recoverya ± SD | % RSD | % recoverya ± SD | % RSD | ||
| a The value is the mean of 3 determinations, SD is the standard deviation, and RSD is relative standard deviation. | |||||
| Method I | 0.5 | 101.86 ± 0.55 | 0.54 | 101.31 ± 0.59 | 0.58 |
| 1.5 | 100.89 ± 0.64 | 0.63 | 100.22 ± 0.38 | 0.37 | |
| 2.5 | 100.14 ± 0.38 | 0.38 | 100.48 ± 0.41 | 0.41 | |
| Method II | 0.1 | 101.67 ± 0.81 | 0.80 | 101.32 ± 1.48 | 1.46 |
| 0.5 | 100.63 ± 0.30 | 0.30 | 100.40 ± 0.62 | 0.62 | |
| 0.7 | 100.20 ± 0.12 | 0.12 | 100.23 ± 0.36 | 0.36 | |
| Parameter | Parameter value | % recoverya ± RSD | |
|---|---|---|---|
| Method I | Method II | ||
| a The value is the mean of three determinations and RSD is the relative standard deviation.b The recommended reagent volume was 1.0 mL for erythrosine B (1.7 × 10−4 mol L−1) and 1.4 mL for lanthanum chloride (5.1 × 10−5 mol L−1). | |||
| Buffer pH | +0.2 | 100.91 ± 1.80 | 99.62 ± 0.40 |
| −0.2 | 99.70 ± 1.93 | 99.99 ± 0.73 | |
| Buffer volume | +0.2 mL | 98.52 ± 0.58 | 99.78 ± 0.88 |
| −0.2 mL | 100.01 ± 1.36 | 99.03 ± 1.00 | |
| Reagent volumeb | +0.2 mL | 101.97 ± 1.35 | 99.94 ± 0.40 |
| −0.2 mL | 99.50 ± 1.78 | 99.51 ± 1.21 | |
| Reaction time | +2 min | 98.41 ± 1.25 | 99.40 ± 0.40 |
| −2 min | 98.60 ± 1.36 | 98.98 ± 1.20 | |
3.6. Application of the proposed methods
| Dosage forms | % recoverya ± SD | ||
|---|---|---|---|
| Reported method | Method I | Method II | |
| a The value is the average of five determinations for both the proposed and reported methods.b Tabulated values at 95% confidence limit are t = 2.306, F = 6.338. | |||
| Alzental tablets (200 mg per tablet) | 98.96 ± 1.65 | 100.34 ± 1.37 (t = 1.44, F = 1.46)b | 100.23 ± 1.38 (t = 1.33, F = 2.71)b |
| Alzental suspension (20 mg mL−1) | 99.78 ± 1.36 | 100.54 ± 1.79 (t = 0.76, F = 1.73)b | 100.34 ± 1.33 (t = 0.65, F = 1.84)b |
4. Conclusion
In the current article, the formation of complexes of albendazole was applied for the development of two sensitive and simple spectrofluorimetric methods. The methods were validated according to ICH guidelines and successfully applied for the analysis of dosage forms and spiked human plasma containing the drug. The proposed methods have the virtues of simpler and shorter reaction times, more environmentally safe solvents are reagents, easier operation procedures and lower cost than the reported methods. The thermodynamic study reveals the feasibility of the undergoing reactions at room temperature. All these advantages make the method suitable for the use in quality control laboratories for routine work.Conflicts of interest
There are no conflicts to declare.References
- K. Parfitt and W. Martindale, The complete drug reference, 1999 Search PubMed
.
- E.-M. Bennet and C. Bryant, Mol. Biochem. Parasitol., 1984, 10, 335–346 CrossRef CAS PubMed
.
- N. Swamy and K. Basavaiah, Braz. J. Pharm. Sci., 2014, 50, 839–850 CrossRef
.
- N. Swamy and K. Basavaiah, ISRN Anal. Chem., 2013, 2013, 1–11 CrossRef
.
- A. C. Tella, O. M. Olabemiwo, M. O. Salawu and G. K. Obiyenwa, Int. J. Phys. Sci., 2010, 5, 379–382 CAS
.
- C. P. Sastry, V. N. Sarma, U. Prasad and C. R. Lakshmi, Indian J. Pharm. Sci., 1997, 59, 161 CAS
.
- C. Sastry, A. Sarma Varahabhatia, U. Prasad and C. Lakshmi, Indian Drugs, 1997, 34, 102–104 CAS
.
- M. S. Kamel, B. Barsoum and R. Sayed, J. Appl. Sci. Res., 2008, 4, 1242–1248 CAS
.
- S. M. Derayea, H. R. H. Ali, A. A. Hamad and R. Ali, J. Appl. Pharm. Sci., 2017, 7, 076–082 Search PubMed
.
- S. Küçükkolbaşı, B. Gündüz and E. Kılıç, Anal. Lett., 2008, 41, 104–118 CrossRef
.
- G. Y. Zhao, H. Wu, S. L. Dong and L. M. Du, Chin. Chem. Lett., 2008, 19, 951–954 CrossRef CAS
.
- K. A. Attia, A. A. Mohamad and M. S. Emara, Anal. Methods, 2016, 8, 5136–5141 RSC
.
- F. Tian, W. Huang, J. Yang and Q. Li, Spectrochim. Acta, Part A, 2014, 126, 135–141 CrossRef CAS PubMed
.
- G. Balizs, J. Chromatogr. B: Biomed. Sci. Appl., 1999, 727, 167–177 CrossRef CAS
.
- N. Sher, N. Fatima, S. Perveen and F. A. Siddiqui, Instrum. Sci. Technol., 2016, 44, 672–682 CrossRef CAS
.
- M. Whelan, B. Kinsella, A. Furey, M. Moloney, H. Cantwell, S. J. Lehotay and M. Danaher, J. Chromatogr. A, 2010, 1217, 4612–4622 CrossRef CAS PubMed
.
- A. Wojnicz, T. Cabaleiro-Ocampo, M. Román-Martínez, D. Ochoa-Mazarro, F. Abad-Santos and A. Ruiz-Nuño, Clin. Chim. Acta, 2013, 426, 58–63 CrossRef CAS PubMed
.
- J. J. Pandya, M. Sanyal and P. S. Shrivastav, Biomed. Chromatogr., 2017, 31, e3947 CrossRef PubMed
.
- J. Moyano, J. Liro, J. Pérez, M. Arias and P. Sánchez-Soto, Microscopy, 2014, 4, 1043–1050 Search PubMed
.
- J. I. Gowda, R. B. Kantikar, D. G. Harakuni, K. Y. Jadhav, V. C. Chanagoudar and S. T. Nandibewoor, J. AOAC Int., 2016, 99, 1522–1526 CrossRef CAS PubMed
.
- B. C. Lourencao, M. Baccarin, R. A. Medeiros, R. C. Rocha-Filho and O. Fatibello-Filho, J. Electroanal. Chem., 2013, 707, 15–19 CrossRef CAS
.
- J. Wang, Z. Liu, J. Liu, S. Liu and W. Shen, Spectrochim. Acta, Part A, 2008, 69, 956–963 CrossRef PubMed
.
- T. Pérez-Ruiz, C. Martínez-Lozano, V. Tomás and C. Sidrach, Analyst, 1995, 120, 1103–1106 RSC
.
- X. Zhu, J. Sun and Y. Hu, Anal. Chim. Acta, 2007, 596, 298–302 CrossRef CAS PubMed
.
- M. Pesez, J. Bartos, Colorimetric and fluorimetric analysis of organic compounds and drugs, Marcel Dekker, New York, 1974 Search PubMed
.
- C. Y. Huang, Methods Enzymol., 1982, 87, 509–525 CAS
.
- K. Semahat, G. Beniz and K. Esma, Gazi Üniversitesi Gazi Eğitim Fakültesi Dergisi, 2004, 24, 159–174 Search PubMed
.
- M. S. Refat and G. A. M. Mersal, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2012, 42, 786–791 CAS
.
- J. G. Xu and Z. B. Wang, Fluorimetric Analysis Method, Science Press, Beijing, 3rd edn, 2006 Search PubMed
.
- N. Boens, W. Qin, N. Basarić, J. Hofkens, M. Ameloot, J. Pouget, J.-P. Lefèvre, B. Valeur, E. Gratton and M. VandeVen, Anal. Chem., 2007, 79, 2137–2149 CrossRef CAS PubMed
.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer-Verlag, Berlin, Heidelberg, 3rd edn, 2006 Search PubMed
.
- S. Bi, L. Ding, Y. Tian, D. Song, X. Zhou, X. Liu and H. Zhang, J. Mol. Struct., 2004, 703, 37–45 CrossRef CAS
.
- I. Ravikumar and P. Ghosh, Inorg. Chem., 2011, 50, 4229–4231 CrossRef CAS PubMed
.
- ICH Harmonized Tripartite Guideline, Validation of analytical procedures: text and methodology Q2 (R1), 2005 Search PubMed
.
- S. Marriner, D. Morris, B. Dickson and J. Bogan, Eur. J. Clin. Pharmacol., 1986, 30, 705–708 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2018 |