Open Access Article
Open Access Article“Less Blue, More Clean”: Cu2O nano-cubic functionalized hydrogel for the energy transformation of light-emitting screens†
Zhuo Xianga,
Miaoxing Liub,
Fanrong Aic,
Xingwei Dinga,
Ping Qiu b,
Tingtao Chena,
Yisha Yanga,
Huan Wua,
Hongbo Xina and
Xiaolei Wang
b,
Tingtao Chena,
Yisha Yanga,
Huan Wua,
Hongbo Xina and
Xiaolei Wang *ab
*ab
aInstitute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330088, China. E-mail: wangxiaolei@ncu.edu.cn
bCollege of Chemistry, Nanchang University, Nanchang, Jiangxi 330006, China
cSchool of Mechanical & Electrical Engineering, Nanchang University, Nanchang, Jiangxi 330031, China
First published on 31st January 2018
Abstract
Cubic Cu2O nanoparticle modified hydrogel (CMHG) was synthesized to address two major problems (harmful irradiation and high density pathogens) of the current light-emitting screens simultaneously. The as-prepared hydrogel could conveniently form an adjustable semitransparent film over various shaped screens to provide enhanced antibacterial activity and longer Cu2O service life. More importantly, with the aid of cubic Cu2O nanoparticles, the energy of the harmful blue light irradiation could be effectively transformed into a photocatalysed power source to sterilize the surface of the screen. This low toxic CMHG showed no significant influence on the touch control experience, and had obvious eye protective effects, which thus offered a convenient manner to protect vision and sterilize touch screens at the same time.
Introduction
As the core components of mobile phones, tablet computers and automobile navigation, various light emitting screens have been intensively used in our daily life.1,2 Despite considerable progress being made, two common and serious problems still threaten the safety use of these devices. One is the formation of pathogenic biofilm on the surfaces of these frequently touched screens.3–5 The other is the harmful irradiation, especially blue color ranged light (between 400 nm to 500 nm), which had been demonstrated to cause some skin diseases and eye diseases.6–9Aiming at above problems, a low toxic antibacterial material coupled with blue light attenuation property urgently needs to be developed for screens. Although numerous antibacterial agents have been developed, such as Ag2S/Ag heterodimers,10,11 silver nanoparticles (NPs),12–14 copper NPs.15–17 Although these NPs had been used in the fields of drug delivery, antibacterium and photocatalysis.18–21 Unfortunately, very few research explored their applications in the area of touch screen. However, these Cu2O powders were difficult to cover the surface of the screen directly. Besides, exposed Cu2O powders were also easy to be oxidated, which has greatly limited their practical applications.22,23
Results and discussion
In order to solve this problem, pure Cu2O nano-cubic was first synthesized through a facile hydrothermal process. The obtained Cu2O NPs were then coated by specialized hydrogel (HG) which was formed of hyaluronic acid (HA) crosslinked polyvinyl alcohol (PVA). The as-prepared hydrogel was a viscous and transparent materials, it was thus suitable as the coating membrane of various shaped screen. Meanwhile, hydrogel also prevented Cu2O NPs from being oxidized (ESI Fig. S1†). On the other hand, with the aid of Cu2O NPs, most of the harmful blue light irradiation could be absorbed and transform to the photocatalysed power source to sterilize the surface of screen. One feature should be mentioned was that, after drying, CMHG did not affect the touch control experience of the screen (an interesting movie was provided in the ESI Movie S1†).As schematic illustration in Fig. 1, CMHG was composed of PVA, HA and polymer-functionalized cubic Cu2O NPs (Fig. 1a). The PVA served as cross-link agent in the three-dimensional network.24–26 HA was a natural moisturizing factor, which modulated the time of drying.27,28 And, as the core material, polymer-functionalized cubic Cu2O NPs provided dual harmful blue light attenuation and antimicrobial functions (Fig. 1b). The image of HG film and similar sized CMHG film were provided in ESI Fig. S2a and b† respectively. The relative SEM observation indicated some cubic sized Cu2O NPs were dispersed in the film (Fig. S2c and d†). Since Cu2O NPs wrapped in HG was not clear to be observed, the insert image in Fig. S2d† offered a view of naked Cu2O NPs. The average diameter of these uniform cubic Cu2O NPs was around 500 nm. Apart from Energy Dispersive Spectrometer (EDS) characterization (Fig. S2e and f†), XRD (X-ray diffraction) was also used in this study. Fig. S2g† reported a series of XRD patterns for HG and polymer-functionalized cubic Cu2O NPs in two different concentrations (12.5 mg ml−1, 25 mg ml−1), respectively. The diffraction peaks of the CMHG film samples agreed well with the cubic Cu2O (JCPDS card no. 34-1354). The mechanical property of this CMHG was also evaluated (ESI Fig. S3†).
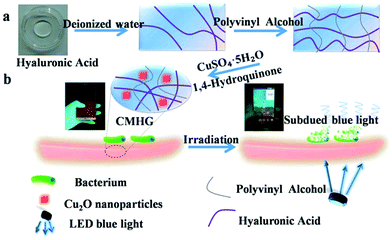 | ||
| Fig. 1 The preparation of CMHG film (a) to sterilize the surface of screen under blue light irradiation (b). | ||
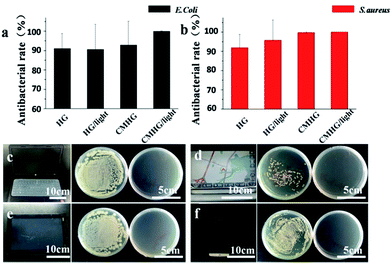
To test the antibacterial property of HG film and CMHG film, Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) were chosen as the model bacteria and evaluated by Spread Plate Method.29,30 For E. coli (Fig. 2a), the HG film owned a good bacteriostatic rate, reached to 91.15%. When under the irradiation of blue light, the bacteriostatic rate had no apparent different. As a comparison, the CMHG film expressed a better bacteriostatic rates (92.88%). More importantly, once blue light was used to “activate” CMHG film, bacteriostatic rate reached to 100% (n = 3) (ESI Fig. S4†). For S. aureus (Fig. 2b), bacteriostatic rate of CMHG film could reach 99.75%. Under blue light, the antibacterial rate also improved to 100%. In totally, CMHG film cooperated with blue light irradiation had the best antibacterial ability. An unexpected discovery was that, pure HG film also showed certain antibacterial effects. We concluded that HG film induced an anaerobic condition which could suppress the growth of Escherichia coli and Staphylococcus aureus. Moreover, the subsequent tearing of HG film also provided a strong physical cleaning of the bacteria.
The screens of four different electronic products were then selected for further practical applications, including notebook computer (Fig. 2c), automobile navigation (Fig. 2d), tablet computer (Fig. 2e) and mobile phone (Fig. 2f). Through colony counting method, the left plate represented the original bacterial number on each screen surface. The right plate exhibited the bacterial number after sterilized by CMHG, which was activated by the emitting of blue light from the screen itself. After 1 hour usage, the obtained results showed that CMHG film had a remarkable antibacterial ability for all the tested light emitting screens.
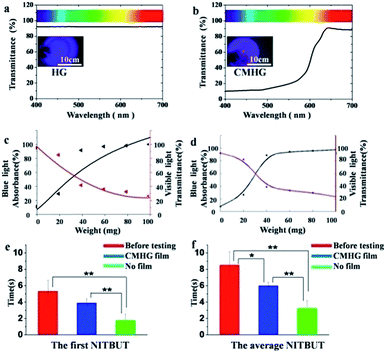
The capability of CMHG film on blue light attenuation was subsequently investigated. For the convenience of calculation, blue light wavelength was defined ranging from 400 nm to 500 nm and visible light wavelength was defined ranging from 400 nm to 700 nm. Through the UV-Vis spectrometer, pure HG film absorbed about 10% in full spectrum of visible light, with no apparent difference between 400 nm and 700 nm wavelength (Fig. 3a), and the inset of Fig. 3a also reported this phenomenon. In the case of CMHG film, the strong absorption of blue light was appeared in 400–500 nm wavelength (Fig. 3b) and the inset of Fig. 3b also approved they had significant color shift between inside and outside of circle. Therefore, CMHG film had a strong absorbing of blue light, filtered around 90% of the blue light irradiation.
With the increasing of polymer-functionalized cubic Cu2O NPs, the blue light absorbance would be enhanced, but the visible light transmittance would be decreased also. In order to understand polymer-functionalized cubic Cu2O NPs based relationship between blue light absorbance and visible light transmittance, we had tested six kinds of CMHG, photos of CMHG film with different concentration of polymer-functionalized cubic Cu2O NPs (0 mg, 20 mg, 40 mg, 60 mg, 80 mg, 100 mg in 2 ml HG) (ESI Fig. S5†) the obtained the average results were summarized in ESI Table S1.† Next the average value of blue light absorbance and visible light transmittance were plotted in the graph to get twelve points, so two series of fitting curves (eqn (1) and (2)) were generated. Then, the black point was fitted by S-curve equation and the red point was fitted by quadration equation (Fig. 3c), details was shown in ESI Table S2.†
What's more, Savitzky–Golay was also used for calculated for smooth curve, by using MATLAB (Fig. 3d). Collectively, blue light absorbance was rising with the adding of polymer-functionalized cubic Cu2O NPs. On the contrary, visible light transmittance was decreasing with the adding of Cu2O NPs. Based on the results from the algorithms, CMHG could reach a good balance between blue light absorption and visible light transmitting, when the content of polymer-functionalized cubic Cu2O NPs was ranging from 20 mg to 40 mg.
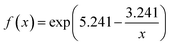 | (1) |
| f(x) = 3.205x2 − 36.958x + 130.471 | (2) |
Some interesting practical applications of CMHG on fashion electronics were also tested in this study. It has been demonstrated that retinal pigment was sensitive to blue light and caused some eye disease.31 Xerophthalmia was a common eye disease in clinic. It is proper way to evaluated the degree of xerophthalmia by testing the first NITBUT (noninvasive tear break-up time) and the average NITBUT.32 So xerophthalmia patients watched CMHG coated VR (virtual reality) film for 3 h movie. Regular VR film without CMHG modification was used as a control.
An obvious decreasing on NITBUT was discovered after watching movies for 3 hours without CMHG (p < 0.01), which meant a 3 h-watching can cause a significant damage to eyes. After coated with CMHG, this damage could be minimized effectively (p > 0.05). The subsequent NITBUT results further demonstrated that CMHG could protect eyes from blue light irradiation (Fig. 3f). According to the features of CMHG, it offered a convenient manner to protect vision and sterilize the surface of various formed electronics.
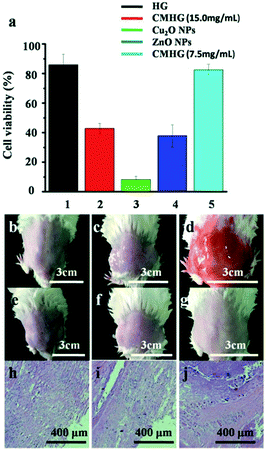
To evaluate toxicity of CMHG, two methods were adopted seperately. HG, CMHG (15.0 mg ml−1), Cu2O NPs (15.0 mg ml−1), ZnO NPs (15.0 mg ml−1) and CMHG NPs (7.5 mg ml−1), four kinds of materials had been used in the cytotoxicity experiment (Fig. 4a). HG showed a lowest cytotoxicity and Cu2O NPs could damage almost cells in a certain concentration. Once Cu2O NPs was coated with HG, there were nearly a half cells surviving.
For further research, CMHG (15.0 mg ml−1) was also evaluated by using a mouse skin model. In this study, the mouse back skin was shaved and then washed by PBS buffer before the experiments. One group of mouse was only treated with PBS buffer, which served as the blank control (Fig. 4b). Then HG and CMHG were applied onto the skins of the other two groups separately. After a 3 day treatment, mouse skin treated with CMHG maintained its normal structure without any indications of toxicity such as erythema or edema (Fig. 4e–g).
Following the skin morphology examination, some skin biopsy were collected at the end of the treatment and further investigated by hematoxylin and eosin (H&E).33 The HG and CMHG treated skin maintained an undisturbed structure with a clear layer of healthy epidermal cells on top of the dermis layer, which was identical to the PBS treated skin sample (Fig. 4h–j). The absence of any skin reaction and toxicity within a 3 day treatment suggested that topically applying CMHG was safe to the skin. However, long-term toxicity test is still recommended, before CMHG based products becomes available to the general public.
Conclusions
Along with the development of high-tech and the arrival of the electronic age, various light emitting touch screens had been intensively used in our daily life. In this study, we developed a CMHG for the current touch screen age. That contains Cu2O NPs for topical antibacterium and eye-protection. By which means, harmful blue light irradiation could be absorbed and transformed to the photocatalysed power source to sterilize the surface of screen. Comparing with the pure Cu2O NPs, the living time and safety of Cu2O was further improved in the form of CMHG. Taken together, CMHG provides a simple and high efficient hybrid formulation for eye-protection and topical sterilization. However, some copper(II) penetrated from CMHG may threaten our health. The ongoing research interests will focus on the systematical optimization of CMHG, including a series of long-term toxicology tests.Experimental section
Ethical statement
15 Kunming mice (18–22 g) were purchased from Animal Center of Nanchang University (Nanchang, China). The animals were maintained in a room temperature (20–22 °C) and 40–50% humidity. Mice were provided with water and a 12 h light/dark cycle. All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee at Institute of Translational Medicine, Nanchang University (no. 2016NC-020-02) and animal handling followed the dictates of the National Animal Welfare Law of China. All the procedures performed involving human participants were in accordance with the ethical standards of the Affiliated Eye Hospital of Nanchang University. The research has been approved by the Bioethics Committee of Translational Medicine of Nanchang University (permission no. YK/92/2016). Informed consent was obtained from all the participants before the study began. No personal patient data were analysed or included in this work.Synthesis of CMHG
To synthesize the Cu2O NPs, 5.000 g CuSO4·5H2O and 2.923 g EDTA were dissolved in 30 ml deionized water and stirred for 30 min at 55 °C. Then, the solution was reduced by 250 ml NaOH solution (0.6 M) and 5.0 g 1,4-hydroquinone (1,4-C6H4(OH)2), stirring occasionally until the solution fell to room temperature. Following the reaction, the solution was repeatedly washed with deionized water and anhydrous alcohol to remove impurities and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 8 min. Finally, the precipitates were obtained and dried in vacuum oven under 50 °C. The cubic Cu2O nanoparticles with a mean diameter of 500 nm.
000 rpm for 8 min. Finally, the precipitates were obtained and dried in vacuum oven under 50 °C. The cubic Cu2O nanoparticles with a mean diameter of 500 nm.
A given weight (2.0 g) of PVA was dissolved in a amount of distilled water (4 ml). Then the solution was heated to 90 °C regulated with an oil bath. The solution was added to 6 ml HA (10%) one time to crosslink HA. Subsequently, Cu2O NPs were functionalized with the crosslinked blend by using the crosslinked blend to embed Cu2O NPs (30.0 mg ml−1). The polymer was selected to modify Cu2O NPs in order to enable the electrostatic interaction of Cu2O NPs with the slightly negatively charged (zeta potential, ζ: −7.9 mV). The solution was purified by precipitating into pured water and centrifuged at 6000 rpm for 30 min, the supernatant was removed and washed by pured water three times to remove the excess polymer.
Characterization of CMHG
The crystalline phases of the samples were characterized by the X-ray diffractometer (XD-3, Beijing) with Cu Kα radiation; the morphologies of the as-prepared Cu2O NPs were investigated by field-emission scanning electron microscopy (JSM-6701F, Japan); CMHG film was tested by UV-Vis spectrometer (UV-2550, Shimadzu).Preparation for HG film and CMHG film
HG film were prepared using heat-drying process. Briefly, 2.0 g PVA was diluted by 8 ml deionized water at room temperature and stirred for 6 h. Then 2 ml HG was added into completely. Finally, HG film were obtained by vacuum drying under 60 °C. A certain amount of polymer-functionalized cubic Cu2O NPs was added into HG. After being stirred for 30 min, CMHG film was obtained by vacuum drying under 60 °C.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21461015 to Wang Xiaolei); The Science Foundation of Jiangxi Provincial Department of Education (No. KJLD14010 and 20153BCB23035 to Wang Xiaolei); The Major Program of Natural Science Foundation of Jiangxi Province (No. 20161ACB21002 to Wang Xiaolei); Nanchang University Seed Grant for Biomedicine; National Key Basic Research Program of China (2013CB531103 to Xin Hongbo); Open Funds of the State Key Laboratory of Electroanalytical Chemistry (No. SKLEAC201602 to Qiu Ping); Natural Science Foundation of Jiangxi Province (20161BAB215203 and GJJ150194 to Ding Xingwei). The National Natural Science Foundation of China (81503364 and 31560264 to Chen Tingtao); China Postdoctoral Science Foundation (No. 2017M610402 to Ai Fanrong); Postdoctoral Science Foundation of Jiangxi Province (No. 2017KY06 to Ai Fanrong).Notes and references
- C. P. Gerba, A. L. Wuollet, P. Raisanen and G. U. Lopez, Am. J. Infect. Control, 2016, 44, 358–360 CrossRef PubMed.
- Y. S. Kim, Light emitting device and system providing white light with various color temperatures, US Pat., 8297783, 2012.
- Y. J. Zhang, S. Li, R. Y. Gan, T. Zhou, D. P. Xu and H. B. Li, Int. J. Mol. Sci., 2015, 16, 7493–7519 CrossRef CAS PubMed.
- H. Wu, M. Claus, H. Z. Wang, H. Niels and Z. J. Song, Int. J. Oral Sci., 2015, 7, 1 CrossRef CAS PubMed.
- l. F. C. De, F. Reffuveille, L. Fernández and R. E. Hancock, Curr. Opin. Microbiol., 2013, 16, 580–589 CrossRef PubMed.
- D. Bléger and S. Hecht, Angew. Chem., Int. Ed., 2015, 54, 11338–11349 CrossRef PubMed.
- H. P. Iseli, N. Körber, A. Karl, C. Koch, C. Schuldt, A. Penk, Q. Liu, D. Huster, J. Käs and A. Reichenbach, Exp. Eye Res., 2015, 139, 37–47 CrossRef CAS PubMed.
- R. Zhou, D. Li, B. Qu, X. Sun, B. Zhang and X. C. Zeng, ACS Appl. Mater. Interfaces, 2017, 8, 27403–27410 Search PubMed.
- J. Krutmann, A. Bouloc, G. Sore, B. A. Bernard and T. Passeron, J. Dermatol. Sci., 2016, 85, 152–161 CrossRef PubMed.
- M. Pang, J. Hu and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 10771–10785 CrossRef CAS PubMed.
- B. G. Kumar, B. Srinivas, M. D. Prasad and K. Muralidharan, J. Nanopart. Res., 2015, 17, 1–11 CrossRef CAS.
- N. Chaubey, A. Sahoo, A. Chattopadhyay and S. Ghosh, Biomater. Sci., 2014, 2, 1080–1089 RSC.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- Z. Xiu, Q. Zhang, H. L. Puppala, V. L. Colvin and P. J. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- H. Pang, F. Gao and Q. Lu, Chem. Commun., 2009, 9, 1076–1078 RSC.
- X. Wang, D. Liu, J. Li, J. Zhen and H. Zhang, NPG Asia Mater., 2015, 7, 7–19 Search PubMed.
- G. Ren, D. Hu, E. W. C. Cheng, M. A. Vargas-Reus, P. Reip and R. P. Allaker, Int. J. Antimicrob. Agents, 2009, 33, 587–590 CrossRef CAS PubMed.
- J. Chen, Z. Guo, H. B. Wang, M. Gong, X. K. Kong, P. Xia and Q. W. Chen, Biomaterials, 2013, 34, 571–581 CrossRef CAS PubMed.
- Z. Zheng, B. Huang, Z. Wang, M. Guo, X. Qin, X. Zhang, P. Wang and Y. Dai, J. Phys. Chem. C, 2009, 113, 462–470 Search PubMed.
- B. L. Ouay and F. Stellacci, Nano Today, 2015, 10, 339–354 CrossRef.
- C. Ramesh, M. Hariprasad and V. Ragunathan, Curr. Nanosci., 2011, 7, 770–775 CrossRef CAS.
- Y. H. Tsai, K. Chanda, Y. T. Chu, C. Y. Chiu and M. H. Huang, Nanoscale, 2014, 6, 8704 RSC.
- M. D. Susman, Y. Feldman, A. Vaskevich and I. Rubinstein, ACS Nano, 2014, 8, 162–174 CrossRef CAS PubMed.
- J. H. Kim, B. R. Min, K. B. Lee, J. Won and Y. S. Kang, Chem. Commun., 2002, 22, 2732–2733 RSC.
- J. Tang, L. Bao, X. Li, L. Chen and F. F. Hong, J. Mater. Chem. B, 2015, 3, 8537–8547 RSC.
- C. Boyer, M. R. Whittaker, V. Bulmus, J. Liu and T. P. Davis, NPG Asia Mater., 2010, 2, 23–30 CrossRef.
- H. S. Jung, W. H. Kong, D. K. Sung, M. Y. Lee, S. E. Beack, d. H. Keum, K. S. Kim, S. H. Yun and S. K. Hahn, ACS Nano, 2014, 8, 260–268 CrossRef CAS PubMed.
- J. C. Angello and S. D. Hauschka, Dev. Biol., 2016, 73, 322–337 CrossRef.
- O. Karabay, E. Koçoglu and M. Tahtaci, J. Infect. Dev. Countries, 2007, 1, 72–73 Search PubMed.
- L. A. Savas and M. Hancer, Appl. Clay Sci., 2015, 108, 40–44 CrossRef CAS.
- S. E. Yanni, J. Wang, C. S. Cheng, K. I. Locke, Y. Wen, D. G. Birch and E. E. Birch, Am. J. Ophthalmol., 2013, 155, 354 CrossRef PubMed.
- J. Wang, J. R. Palakuru and J. V. Aquavella, Am. J. Ophthalmol., 2008, 145, 795 CrossRef PubMed.
- W. Gao, V. Drew, J. Li, J. Zhu, Q. Zhang, V. Fu, J. Li, T. Soracha, D. Lu and L. Zhang, ACS Nano, 2014, 8, 2900–2907 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12331k |
| This journal is © The Royal Society of Chemistry 2018 |