Open Access Article
Open Access ArticleRole of a “surface wettability switch” in inter-fiber bonding properties
Jinglei Xiea,
Hongjie Zhang *abc,
Shuai Ana,
Xuejun Qianb,
Hongshun Chengb,
Fengshan Zhangc and
Xiaoliang Lic
*abc,
Shuai Ana,
Xuejun Qianb,
Hongshun Chengb,
Fengshan Zhangc and
Xiaoliang Lic
aTianjin Key Lab of Pulp & Paper, Tianjin University of Science & Technology, Tianjin, 300457 China. E-mail: hongjiezhang@tust.edu.cn; Fax: +86-22-6060-2510; Tel: +86-22-6060-2199
bHebei Huatai Paper Industry Co. Ltd., Huatai Group, Zhaoxian, 051530 China
cShandong Huatai Paper Industry Co. Ltd., Huatai Group, Dongying, 257335 China
First published on 15th January 2018
Abstract
The fiber surface wettability is one of the most important lignocellulosic fiber characteristics affecting the inter-fiber bonding properties of final bio-products. In this study, the surface wettability (evaluated by the surface free energy, surface lignin and surface charge) of mechanically refined fibers and the bonding properties of the fiber matrix (handsheets) were measured and correlated to each other. The results showed that the fiber surface charge increased from 48.38 mmol kg−1 to 60.38 mmol kg−1 and the surface lignin decreased from 87.1% to 77.5% during the fiber mechanical treatment, leading to the improvement of the fiber surface free energy from 46.63 mJ m−2 to 54.45 mJ m−2. As a result, the bonding strength index increased from 2.60 N m g−1 to 9.73 N m g−1 without significant loss of bulk properties. In a word, the fiber surface wettability could be adjusted to facilitate the inter-fiber bonding properties of the paper or paperboard products using lignin-rich fibers as raw materials.
1. Introduction
Lignocellulosic fiber, being degradable and renewable, has been widely used in many areas, such as paper and paperboard products,1 high-temperature fiber composites2 and so on. The fiber surface wettability is thought to be essential for fiber swelling, the ability of the fiber volume to become larger during the wetting process, which is good for the interface contact of two fibers so that the bonding properties could be improved.3 Further, the fiber surface wettability can have an influence on the bonding strength between two fibers in a fiber network (paper and paperboard products) by affecting fiber surface composition and functional groups. However, the lignocellulosic fiber surface wettability has seldom been seriously taken into consideration.Fiber surface wettability is defined as the ability for a kind of liquid to spread onto the fiber surface, which is consisted of surface composites, surface charge, surface free energy and many other kinds of surface properties related to fiber surface wetting process.4 Lignocellulosic fiber is composed of hydrophilic part (carbohydrates, including cellulose and hemicelluloses) and hydrophobic part (lignin and some of the extractives). During the separation from natural plants, fibers were treated with different kinds of methods, either mechanical or chemical, mainly to compromise the relationship between lignin and carbohydrates so that the fiber surface wettability could be improved.
The inter-fiber bonding strength of the fiber network, originating from the hydrogen bonding and the van der Waals' force,5 is one of the most important characteristics influencing the final products. The inter-fiber bonding, especially the hydrogen bonding, is largely dependent on the surface wettability.6 The fiber surface composition, including cellulose, hemicellulose, lignin and some extractives, has a strong influence on the inter-fiber bonding properties.7 It has been proved that the lignin on the fiber surface would have a bad effect on the inter-fiber bonding because the lignin is hydrophobic compared to carbohydrates. However, the phenolic group associated with lignin is proved to be one of the resources of the fiber surface charge,8 which has a non-ignorable influence on the inter-fiber bonding strength.9 The improvement of surface charge can increase the inter-fiber bonding mainly by influencing the inter-fiber hydrogen bonding according to Aracri et al.10 Furthermore, the decrease of the surface lignin can also lead to the final yield sacrificing of the lignocellulosic fibers. As a result, a large amount of studies have been done focusing on the modification of lignin instead of removing it,11,12 which can improve the wettability of the fiber surface so that the inter-fiber bonding could be enhanced. Fiber surface free energy and contact angle are always used as the evaluation of the fiber surface wettability.13 The fiber surface free energy has a vital influence on fiber swelling, assistance, and inter-fiber contact. However, there are very few literatures concerning about lignocellulosic fiber surface free energy.
In this study, the lignin-rich fiber will be subjected to mechanical refining in order to modify the fiber surface wettability, and the fiber surface lignin, surface charge and surface free energy would be determined respectively to evaluate the fiber surface wettability. Also, the inter-fiber bonding capability, as well as physical performances of handsheets, would be measured. The main objective of this study is to improve the bonding capability of fiber matrix by improving the fiber surface wettability. The relationship between fiber surface wettability and the inter-fiber bonding strength will also be investigated so that the final product properties could be better adjusted without much sacrifice of the energy and materials during the papermaking process.
2. Materials and methods
2.1 Materials
The pine thermo-mechanical pulp (TMP) fiber, provided by a paper mill in Hebei province in China, was classified by using Bauer-McNett fiber classifier (TMI 8901-5, USA) into different fiber fractions. The R30 fiber fraction with the length of 2.4–2.6 mm and width of 40.1–40.5 μm was chosen as the raw material to be treated by mechanical refining. A PFI mill (Frank-PTI, Germany) was used with a fiber consistency of 30%. The revolution for refining was 0 r, 5000 r, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r, 13
000 r, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r and 15
000 r and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r, respectively. After refining, the fiber was classified and the R30 fraction was collected for further experiments.
000 r, respectively. After refining, the fiber was classified and the R30 fraction was collected for further experiments.
2.2 Fiber surface wettability
The fiber surface lignin content was analyzed by using a PHI-1600 X-ray photoelectron spectrometer (XPS), in accordance with Li and Reeve.14The fiber surface charge was determined through polyelectrolyte adsorption method by using high molecular weight poly-DADMAC (2–3.5 × 105 g mol−1), as reported by Zhang et al.15
The surface free energy of the fiber was detected based on the contact angle measurement.16 Two fibers of a similar diameter were put in a parallel way onto a manmade slide. And the contact angle measurement of the fibers was manufactured by putting a liquid drop onto the gap of 0.1–0.3 mm between them. Two kinds of liquids were applied in this study (water and glycerol).
The surface free energy was calculated according to eqn (1) and (2).17
| γS = γDS + γdS | (1) |
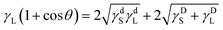 | (2) |
2.3 Fiber characterization
| WRV% = [(wet weight − dry weight)/dry weight]/100 | (3) |
| F = 72d/q/L4 | (4) |
2.4 Physical properties and inter-fiber bonding capability
The fibers were then made into handsheets according to the TAPPI Test Method T205 sp-95 for the determination of physical properties. The tensile strength was tested with a tensile tester (L&W 004, Sweden), and the zero-span tensile strength was detected by a Z-span tester (PULMAC 2400, USA), based on the TAPPI Method T220 sp-96. The bonding strength index (B) was calculated by eqn (5).19| 1/T = 9/8/Z + 1/B | (5) |
2.5 Relative bonded area (RBA) detection
The RBA between fibers were detected through the BET adsorption method according to Soszynski's study.20 The specific surface area of the fiber and the handsheet were tested using the BET method and the RBA was calculated by eqn (6).| RBA = (A0 − A)/A0 | (6) |
3. Results and discusion
Lignocellulosic fiber, a cylinder-shaped material as shown in the SEM image in Fig. 1, is a kind of a “water battery”, where, the “hydrolyte solution” (a solution of surface composition and surface functional groups) plays a major role in the wetting process of fiber surface (in Fig. 1). The “water capacity” is used for the evaluation of bound water the lignocellulosic fiber could adsorb and maintain, which strongly contributes to the fiber swelling and flexibility. The fiber surface wettability, including surface constituents, surface charge, surface free energy and so on, could be seen as a switch accommodating the “water capacity” by getting the fiber “water charged” with different kinds of hydrophilic groups, which further increase the hydrogen bonding between fibers. The process could be expressed by surface contact angle and surface free energy.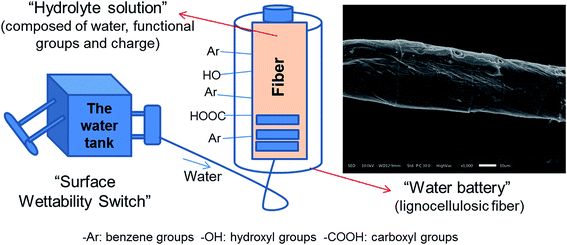 | ||
| Fig. 1 Schematic diagram for the “water-charged” degree of lignocellulosic fiber (water battery) switched by the fiber surface wettability. | ||
3.1 Surface lignin and surface wettability
The fiber surface composition is the main solute of the “hydrolyte solution”. Fiber surface wettability is mainly dominated by the surface composition, including the hydrophilic carbohydrates and hydrophobic lignin.It's known that there're large amounts of hydroxyl and carboxyl groups in cellulose and hemicellulose structures,21,22 which makes carbohydrates hydrophilic. By contrast, lignin, with a considerable quantity of benzene structures, is more hydrophobic than carbohydrates and the fiber wettability is restricted by surface lignin to a great extent. However, thanks to its structure, lignin has a large rigidity and is known as the support of the lignocellulosic fiber, spacing among other kinds of compositions, which contributes to the high bulk of paper or paperboard products made from lignin-rich fibers. The amount of the surface lignin during the refining process is shown in Table 1.
| Treatment (rev.) | O/C (%) | Surface lignin (%) |
|---|---|---|
| 0 | 39.7 ± 0.1 | 87.1 ± 0.1 |
| 5000 | 40.0 ± 0.1 | 86.7 ± 0.1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42.4 ± 0.2 | 81.9 ± 0.1 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42.5 ± 0.1 | 81.5 ± 0.1 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
44.6 ± 0.3 | 77.5 ± 0.1 |
It can be seen in Table 1 that the surface lignin decreased from 87.1% to 77.5% with the increase of the PFI revolutions. That may be caused by the peeling effect of the refining process.5,23 The decrease of the surface lignin will lead to a better explosion of carbohydrates, resulting in a more hydrophilic surface and larger surface wettability. In addition, the decrease of surface lignin may have a positive effect on the inter-fiber bonding because of the improvement of the fiber softness and swelling. However, the phenolic groups associated with lignin are one of the resources of the fiber surface charge. As a result, the surface charge might be negatively influenced by the decrease of surface lignin.
3.2 Surface charge and surface wettability
Fibers are charged when suspended into water because of their anionic groups from different kinds of surface compositions, such as carboxyl groups. Fiber charge includes surface charge and total charge. The total charge consists of surface and inner charge.20 The surface charge is one of the most important parameters for fiber, especially for its swelling, bonding and the properties of the final products. The surface charge is mainly caused by the anionic groups on fiber surface,24 including the carboxyl groups from hemicellulose and phenolic groups associated with lignin and many other kinds of functional groups. These functional groups existing in the “hydrolyte solution” are the major ingredients to retain water molecules. The comparison of the polarity of some different kinds of functional groups is listed in Table 2.| a Ar is the benzene group. | |||
|---|---|---|---|
| Polarity |  |
||
| Functional groups | Hydroxyl | Phenolic hydroxyl | Carboxyl |
| Chemical formula | –OH | Ar–OH | –COOH |
The surface charge of the treated fibers was shown in Table 3. It can be seen in Table 3 that during the mechanical treatment, the surface charge increased from 48.38 mmol kg−1 to 60.38 mmol kg−1. That might be caused by the increase of the fiber specific surface area, which was due to the fiber fibrillation.25 Further, the increase of the specific surface area can also result in the increase of the accessibility of the polyelectrolyte during the surface charge determination. Moreover, the decrease of the surface lignin could improve the exposure of the carbohydrates on the fiber surface so that many other anionic groups on the carbohydrates would be exposed, which may have a positive effect on the increase of the surface charge. As shown in Table 2, carboxyl groups have a higher polarity compared with many other functional groups, which can better constrain the water26 and increase the fiber surface wettability. Further, the fiber surface functional groups and surface charge may be good for the inter-fiber bonding strength by influencing the inter-fiber bonding force, especially the hydrogen bonding strength.
| Treatment (rev.) | Samples | Surface charge (mmol kg−1) |
|---|---|---|
| 0 | 0# | 48.38 ± 0.8 |
| 5000 | 1# | 51.04 ± 0.6 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2# | 54.69 ± 0.4 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3# | 56.60 ± 0.3 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4# | 60.38 ± 0.5 |
3.3 Surface free energy and surface wettability
The surface contact angle and surface free energy of different kinds of mechanically treated fibers were listed in Table 4, which was calculated based on the eqn (2).| Treatment (rev.) | WCA (degree) | GCA (degree) | γdS (mJ m−2) | γDS (mJ m−2) | γS (mJ m−2) |
|---|---|---|---|---|---|
| a WCA – water contact angle; GCA – glycerol contact angle. | |||||
| 0 | 60.30 ± 0.50 | 50.00 ± 0.40 | 16.66 ± 0.10 | 29.97 ± 0.24 | 46.63 ± 0.33 |
| 5000 | 52.53 ± 0.30 | 42.49 ± 0.30 | 28.07 ± 0.02 | 19.51 ± 0.09 | 47.58 ± 0.11 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
56.32 ± 0.30 | 45.43 ± 0.30 | 19.59 ± 0.03 | 29.09 ± 0.11 | 48.69 ± 0.13 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48.86 ± 0.20 | 38.52 ± 0.20 | 32.78 ± 0.00 | 17.02 ± 0.05 | 49.80 ± 0.04 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
43.24 ± 0.10 | 33.26 ± 0.10 | 44.23 ± 0.01 | 10.22 ± 0.02 | 54.45 ± 0.01 |
Table 4 shows that the fiber surface free energy has an enhancement of nearly 10 mJ m−2 with the PFI revolutions ranging from 0 r to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r, indicating the increase of the surface wettability. That increase may be caused by the improvement of the surface charge and the decrease of the surface lignin, which can have a positive effect on the surface wettability since lignin is more hydrophobic than carbohydrates.27 The increase of surface charge is mainly caused by the fibrillation of the fibers, which exposes more carbohydrates,28 leading to the improvement of fiber surface wettability.
000 r, indicating the increase of the surface wettability. That increase may be caused by the improvement of the surface charge and the decrease of the surface lignin, which can have a positive effect on the surface wettability since lignin is more hydrophobic than carbohydrates.27 The increase of surface charge is mainly caused by the fibrillation of the fibers, which exposes more carbohydrates,28 leading to the improvement of fiber surface wettability.
3.4 Surface wettability and the inter-fiber bonding properties
Fiber surface wettability has a strong influence on the inter-fiber bonding strength. The inter-fiber bonding strength has been conducted by Page as bonding strength index (B), which is decided by a group of parameters. The calculation of B is shown in eqn (7).| B = P × l × b × RBA/12/g/C | (7) |
According to eqn (7), when the fibers are fixed, the inter-fiber bonding strength mainly lies on the RBA and the b of two fibers.
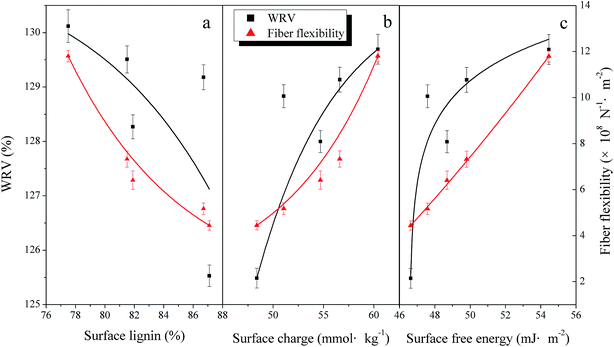
The fiber flexibility is the fiber deflection of unit length under unit load.32 During the papermaking process, the fiber deflection will make the fibers bond more tightly and both the RBA and the hydrogen bonding between two fibers will increase.33 Improving the fiber WRV makes for the fiber swelling, which is also good for the fiber flexibility increasing. The relationships between fiber surface wettability and the fiber flexibility and the WRV are shown in Fig. 2.
Fig. 2 shows that both fiber flexibility and WRV increased (4.44 × 108 N−1 m−2 to 1.18 × 109 N−1 m−2 and 125.53% to 130.12%, respectively) with the improvement of surface wettability, surface lignin (87.1% to 77.5%), surface charge (48.38 mmol kg−1 to 60.38 mmol kg−1) and surface free energy (46.63 mJ m−2 to 54.45 mJ m−2). The increase of WRV was due to the fact that the fiber surface became more hydrophilic during the mechanical treatment in accordance with the improvement of the surface free energy. The decrease of surface lignin could lead to the explosion of carbohydrates, which are much more hydrophilic so that the surface wettability increased to an extent.33 The improvement of the pore size and surface charge during the refining process also led to the WRV increase34 by facilitating fiber swelling. The fiber surface charge mainly comes from the functional groups on the fiber surface, such as the carboxyl groups, which are hydrophilic with a high polarity as seen in Table 2 and can strengthen the fiber swelling ability.
In addition, fiber swelling can also promote the fiber flexibility since swelled fiber is much softer than before. During the surface free energy increasing, the fiber swelling could happen more easily, which increased the flexibility of the fibers.35 Lignin is one of the most important factors restricting the fiber flexibility with a stiff structure.36 The inter-fiber bonding strength is limited accordingly. It's reported8 that the surface lignin of TMP fibers accounted for over 25–35% of the total lignin, indicating that the surface lignin plays an especially important role in the fiber flexibility. As a result, the decrease of surface lignin could be beneficial for the fiber flexibility increase.
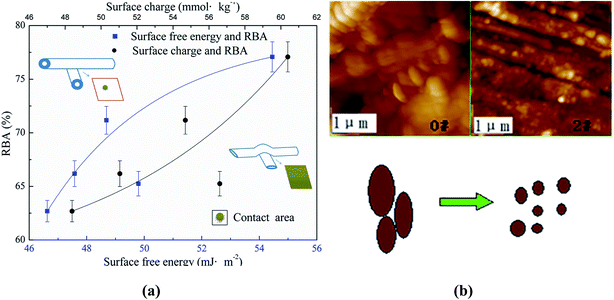
Above all, it can be concluded that the fiber surface wettability could contribute to the improvement of the RBA between two fibers, which can be seen more clearly in Fig. 3.
It's obvious in Fig. 3(a) that the RBA rose from 62.69% to 77.08% with the improvement of both the surface charge and free energy. As discussed before, when the fiber surface charge and surface free energy increased, the fiber became more flexible and more easily wetted, which gained a tighter inter-fiber bonding during the papermaking process. This can also be seen in Fig. 3(a). In Fig. 3(b), the lignin fragment changed into smaller ones during the mechanical treatment, which exposed more carbohydrates with more anionic groups, whose high polarity facilitated the inter-fiber bonding. In addition, it could be seen in Fig. 3(b) that the fiber surface roughness increased after the mechanical treatment, which was caused by the fragmentation of lignin structure and the explosion of micro fibrils.37 This means that the fiber specific surface area enlarged38 so that there were more chances for fibers to contact with each other. It can be concluded that fiber surface wettability is a group of valuable characteristics in adjusting the fiber contact area of the final products.
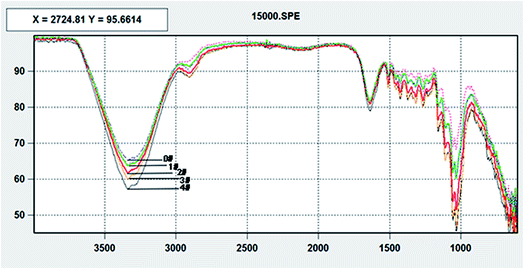
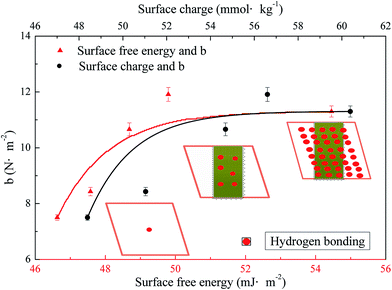
According to Fig. 4, the difference among five kinds of fibers is mainly the difference of the group consistency. The band at around 3422 cm−1 that changed most during the refining process is proved to be the hydroxyl groups,40 which can form more hydrogen bonds between fibers. This might be due to the explosion of the carbohydrates during the refining process, which has a peeling effect on the fiber surface lignin.41 Furthermore, it has been discussed before that the explosion of the surface carbohydrates, especially the hemicellulose, could also induce the increase of the surface carboxyl groups. Hydrogen bonds can also be formed between carboxyl and hydroxyl (or carboxyl) groups.42,43 The relationship between fiber surface wettability and b is shown in Fig. 5.
It could be seen in Fig. 5 that the b rose from 7.5 N m−2 to 11.3 N m−2 with the improvement of both the surface charge and surface free energy. The improvement of the surface charge contributes to the hydrogen bonding between fibers own to the increase of surface functional groups such as hydroxyl groups and carboxyl groups. The increase of the surface free energy implies the increase of the surface hydrophilic groups, the main components of the hydrogen bonding between fibers. The increase of b with the improvement of surface charge and free energy points out that the surface wettability has a positive effect on the inter-fiber bonding of the final products.
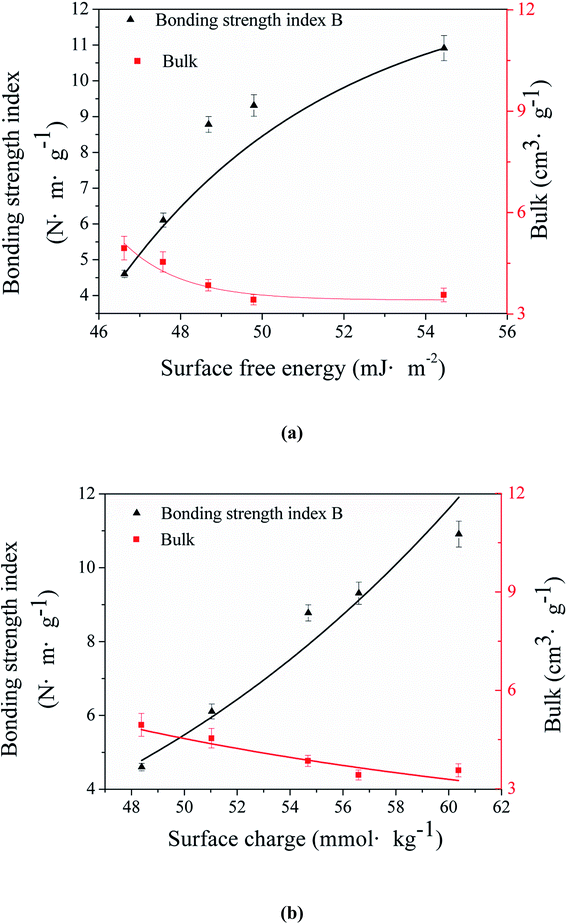
It could be seen in Fig. 6(a) that the bonding strength index of the mechanically treated fibers increased from 4.60 N m g−1 to 10.91 N m g−1 with the improvement of the surface free energy, as the respect of the surface wettability. The fibers gain a better inter bonding through the mechanical treatment mainly by increasing the fiber flexibility and specific surface area,26,28,44 which is mainly caused by the decrease of the surface lignin.
Also, the relationship between the bonding strength index and the surface charge shows a similar result in Fig. 6(b). It's been confirmed that the improvement of the surface charge had a positive effect on the forming of the hydrogen bonding between fibers so that the bonding strength increased.
It's well-known that the bonding strength and the bulk are always a pair of contradiction, hard to compromise. However, in Fig. 6, the bulk only decreased a small deal (from 4.95 cm3 g−1 to 3.56 cm3 g−1) when the bonding strength index was increasing. This is because that most of the lignin was still retained in the fibers, which makes the fiber network hard enough and difficult to be squashed.45 Above all, the improvement of the surface wettability can be useful for the balance between the bonding strength and the bulk of paper or paperboard products using lignin-rich lignin as raw materials.
4. Conclusion
Fiber surface wettability properties are vital for the inter-fiber bonding capability. During the mechanical treatment, the surface lignin decreased from 87.1% to 77.5%, so that the surface wettability was dramatically improved, including the surface charge increasing from 48.38 mmol kg−1 to 60.38 mmol kg−1 and the surface free energy rising from 46.63 mJ m−2 to 54.45 mJ m−2.Owing to the improvement of surface wettability, the fiber became more easily swelled and soften with more hydrophilic functional groups on the fiber surface, enhancing the RBA (from 125.53% to 130.12%) and b (from 7.5 N m−2 to 11.3 N m−2). However, the bulk of the lignin-rich fiber network decreased with the increase of the strength properties, which is not so obvious (from 4.95 cm3 g−1 to 3.56 cm3 g−1) with the increase of the surface wettability. It could be concluded that the modification of the surface wettability had a good effect on compromising the contradiction between the strength properties and the bulk property of the resulting products. Above all, with a good knowledge of surface wettability, both adjustably and measurably, the inter-fiber bonding strength can be positively changed and so can the bonding abilities between fibers and other materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC Grant No. 31670588; 31370577), and the China Postdoctoral Science Foundation (Grant No. 2016M600516).References
- R. Gaudreault, N. D. Cesare, T. G. M. V. D. Ven and D. A. Weitz, Ind. Eng. Chem. Res., 2015, 54, 6234–6246 CrossRef CAS.
- X. Li and W. Strieder, Ind. Eng. Chem. Res., 2009, 48, 2236–2244 CrossRef CAS.
- W. T. Tze and D. J. Gardner, Wood Fiber Sci., 2001, 33, 364–376 CAS.
- R. Yu, C. Wang and Y. Qiu, Appl. Surf. Sci., 2007, 253, 9283–9289 CrossRef.
- B. Wang, R. Li, B. He and J. Li, BioResources, 2011, 6, 4356–4369 CAS.
- K. Kulasinski, D. Derome and J. Carmeliet, J. Mech. Phys. Solids, 2017, 103, 221–235 CrossRef CAS.
- T. Tabarsa, S. Sheykhnazari, A. Ashori, M. Mashkour and A. Khazaeian, Int. J. Biol. Macromol., 2017, 101, 334–340 CrossRef CAS PubMed.
- C. Zhao, H. Zhang, X. Zeng, H. Li and D. Sun, Cellulose, 2016, 23, 1617–1628 CrossRef CAS.
- R. S. Ampulski, Nord. Pulp Pap. Res. J., 1989, 4, 155–163 CAS.
- E. Aracri, A. G. Barneto and T. Vidal, Ind. Eng. Chem. Res., 2012, 51, 3895–3902 CrossRef CAS.
- M. Castellano, A. Gandini, P. Fabbri and M. N. Belgacem, J. Colloid Interface Sci., 2004, 273, 505–511 CrossRef CAS PubMed.
- B. Ramalingam, B. Sana, J. Seayad, F. J. Ghadessy and M. B. Sullivan, RSC Adv., 2017, 7, 11951–11958 RSC.
- D. E. Rollings and J. G. C. Veinot, Langmuir, 2008, 24, 13653–13662 CrossRef CAS PubMed.
- K. Li and D. W. Reeve, J. Pulp Pap. Sci., 2002, 28, 369–373 CAS.
- H. Zhang, C. Zhao, Z. Li and J. Li, Cellulose, 2016, 23, 163–173 CrossRef CAS.
- S. L. Schellbach, S. N. Monteiro and J. W. Drelich, Mater. Lett., 2016, 164, 599–604 CrossRef CAS.
- D. K. Owens and R. C. Wendt, J. Appl. Polym. Sci., 1969, 13, 1741–1747 CrossRef CAS.
- Z. Li, H. Zhang, X. Wang, F. Zhang and X. Li, RSC Adv., 2016, 6, 109211–109217 RSC.
- B. Y. Wang and L. I. Rong, China Pulp Pap. Ind., 2013, 12, 33–36 Search PubMed.
- I. Soszynski, Nord. Pulp Pap. Res. J., 1995, 10, 150–151 Search PubMed.
- H. Hu, H. Li, Y. Zhang, Y. Chen, Z. Huang, A. Huang, Y. Zhu, X. Qin and B. Lin, RSC Adv., 2015, 5, 20656–20662 RSC.
- A. I. Adeogun, M. A. Idowu, K. O. Akiode and S. A. Ahmed, Bioresources and Bioprocessing, 2016, 3, 1–16 CrossRef.
- P. Fardim and N. Durán, Colloids Surf., A, 2003, 223, 263–276 CrossRef CAS.
- N. K. Bhardwaj, V. Hoang and K. L. Nguyen, Bioresour. Technol., 2007, 98, 1647–1654 CrossRef CAS PubMed.
- J. Li, Y. Liu, C. Duan, H. Zhang and Y. Ni, Bioresour. Technol., 2015, 192, 501–506 CrossRef CAS PubMed.
- H. N. Banavath, N. K. Bhardwaj and A. K. Ray, Bioresour. Technol., 2011, 102, 4544–4551 CrossRef CAS PubMed.
- Q. Miao, C. Tian, L. Chen, L. Huang, L. Zheng and Y. Ni, Cellulose, 2015, 22, 803–809 CrossRef CAS.
- L. O. Öhman, L. Wågberg, K. Malmgren and Å. Tjernström, J. Pulp Pap. Sci., 1997, 23, J467–J474 Search PubMed.
- L. Paavilainen, Pap. Puu, 1993, 75, 689–702 CAS.
- G. V. Duarte, B. V. Ramarao, T. E. Amidon and P. T. Ferreira, Ind. Eng. Chem. Res., 2011, 50, 9949–9959 CrossRef CAS.
- J. Tao, H. Liu, J. Li, Z. Wu and W. Lv, International Conference on Computer Engineering and Technology, 2010, vol. 4, pp. 621–625 Search PubMed.
- L. Ming, H. Zhang, J. Li and J. Duan, Ind. Eng. Chem. Res., 2013, 52, 4083–4088 CrossRef.
- A. Klash, E. Ncube, B. D. Toit and M. Meincken, Eur. J. For. Res., 2010, 129, 741–748 CrossRef CAS.
- M. A. Hubbe, R. A. Venditti and O. J. Rojas, BioResources, 2007, 2, 739–788 CAS.
- F. Fadavi, H. Kermanian and H. Resalati, Lignocellulose, 2012, 1, 153–163 Search PubMed.
- X. Tang, X. Guo and K. Sun, Advanced Textile Technology, 2013, 21, 57–59 CAS.
- J. Simola, P. Malkavaara, R. Alén and J. Peltonen, Polymer, 2000, 41, 2121–2126 CrossRef CAS.
- A. Haridas, C. Sharma, V. Sritharan and T. Rao, RSC Adv., 2014, 4, 12188–12197 RSC.
- S. Y. Yoon and Y. Deng, Ind. Eng. Chem. Res., 2007, 46, 4883–4890 CrossRef CAS.
- G. Wang, A. M. Huang, X. X. Hu and F. M. Chen, Guangpuxue Yu Guangpu Fenxi, 2010, 30, 2365–2367 CAS.
- J. Tao, H. Liu, X. Chen, W. Shen and X. Zhu, Paper Science & Technology, 2007, 26, 1–5 Search PubMed.
- K. L. Kato and R. E. Cameron, Cellulose, 1999, 6, 23–40 CrossRef CAS.
- S. Fujisawa, Y. Okita and H. Fukuzumi, Carbohydr. Polym., 2011, 84, 579–583 CrossRef CAS.
- K. Grundke, K. Pöschel, A. Synytska, R. Frenzel, A. Drechsler, M. Nitschke, A. L. Cordeiro, P. Uhlmann and P. B. Welzel, Adv. Colloid Interface Sci., 2015, 222, 350–376 CrossRef CAS PubMed.
- D. Yan and K. Li, J. Mater. Sci., 2008, 43, 2869–2878 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |